Abstract
Background
Pulsed-field ablation (PFA) is a novel ablation modality for atrial fibrillation (AF) ablating myocardium by electroporation without tissue-heating. With its different mechanism of tissue ablation, it is assumed that lesion creation is divergent to thermal energy sources. 68Ga-fibroblast-activation protein inhibitor (FAPI) PET/CT targets FAP-alpha expressed by activated fibroblasts. We aimed to assess 68Ga-FAPI uptake in pulmonary veins as surrogate for ablation damage after PFA and cryoballoon ablation (CBA).
Methods
26 patients (15 PFA, 11 CBA) underwent 68Ga-FAPI-PET/CT after ablation. Standardized uptake values (SUV) and fibroblast-activation volumes of localized tracer uptake were assessed.
Results
Patient characteristics were comparable between groups. In PFA, focal FAPI uptake was only observed in 3/15 (20%) patients, whereas in the CBA cohort, 10/11 (90.9%) patients showed atrial visual uptake. We observed lower values of SUVmax (2.85 ± 0.56 vs 4.71 ± 2.06, P = 0.025) and FAV (1.13 ± 0.84 cm3 vs 3.91 ± 2.74 cm3, P = 0.014) along with a trend towards lower SUVpeak and SUVmean in PFA vs CBA patients, respectively.
Conclusion
Tissue response with respect to fibroblast activation seems to be less pronounced in PFA compared to established thermal ablation systems. This functional assessment might contribute to a better understanding of lesion formation in thermal and PFA ablation potentially contributing to better safety outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary vein isolation (PVI) is the interventional cornerstone therapy for atrial fibrillation (AF).1 Cryoballoon ablation (CBA) has become an established single-shot-ablation technique achieving PVI by circumferential ablation lesions.2 Major complications such as atrio-esophageal fistula or phrenic nerve palsy after thermal ablation are rare but still of concern.3 Introduction of pulse field ablation (PFA) as a novel single-shot modality promises an equally effective, yet safer modality due to its tissue specificity and consequently absence of significant collateral damage to nearby structures (particularly the esophagus, vessels and phrenic nerve).4,5 PFA employs trains of short-duration high amplitude electrical pulses that specifically ablate cardiomyocytes within the myocardial tissue by forming irreversible nanoscale pores leading to apoptosis through electroporation without tissue heating.5 With its different mechanism of tissue ablation, it is assumed that tissue damage and consecutive lesion creation is divergent to thermal energy sources.6,7
We have already shown the feasibility of hybrid imaging using 68Ga-Fibroblast-activation protein inhibitor (FAPI) positron-emission tomography (PET) in assessing thermal damage in post PVI patients (cryo and radiofrequency energy) compared to controls.8 As PFA uses non thermal energy to induce cell death, the aim of our study was to assess FAPI uptake in patients after PFA as a surrogate for ablation damage and compare it with uptake after CBA. The hypothesis was that patients treated with PFA will have a different extent of tracer uptake due to its selective mechanism of irreversible electroporation resulting in apoptosis of cardiomyocytes opposed to CBA-induced nonselective tissue necrosis with collateral microvascular obstruction or intramural hemorrhage (Figure 1).
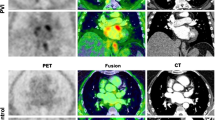
Patient 1 received CBA and showed intense tracer uptake in PVs; Patient 2 underwent PFA 7 days before imaging and showed mildly increased tracer uptake in the ostia of PVs. Of note, this patient had persistent AF, as well as severely dilated left atrium (left atrial volume of 40 mL·cm−2) which might have contributed to FAPI uptake as well. Incidental diffuse uptake in the left ventricle may possibly reflect myocardial remodeling in the setting of DCM; Patient 3 having undergone CBA and PET/MRI revealed moderate tracer uptake with corresponding late gadolinium enhancement (LGE) of the left atrium; Patient 4: received PFA and no visual tracer uptake can be observed in PET nor LGE in MRI. CBA, cryoballoon ablation; PVs, pulmonary veins; PFA, pulse field ablation; PET, positron emission tomography; CT, computer tomography; MRI, magnetic resonance imaging
Methods
Patient population
Twenty-six patients who had undergone 68Ga-FAPI-PET after PVI were included. Five patients matched for age, left ventricular ejection fraction without a history of AF or cardiac ablation having undergone 68Ga-FAPI-PET for tumor staging served as controls. Patients treated with cardio-toxic drugs like anthracyclines or immune checkpoint inhibitors, or chest radiation were excluded to avoid distortion of myocardial FAPI uptake due to confounders.9 We assessed clinical baseline characteristics, imaging parameters from echocardiography and procedural PVI data as well as 6 months follow-up data. The investigations were conducted in accordance with the Declaration of Helsinki and national regulations. All patients gave written informed consent to undergo 68Ga-FAPI PET/computer tomography (CT) or 68Ga-FAPI PET/magnetic resonance (MRI) following the regulations of the German Pharmaceuticals Act §13(2b). Retrospective analysis of PET hybrid imaging and clinical data was approved by the local ethics committee for the purpose of the present study (Permit No. 20-9777-BO).
Catheter ablation
All procedures were performed under uninterrupted oral anticoagulation and under continuous heparin administration during the procedure guided by activated clotting time goal of 300-350 ms. All PVI procedures were performed under 3D high-density mapping guidance (Rhythmia HDx™, Boston Scientific, Massachusetts, United States) with a diagnostic coronary sinus catheter, an additional catheter in right ventricle or right atrium (RA), respectively for phrenic nerve capture if required. Transseptal puncture and 3D mapping were performed as previously described.10
In all patients, the procedural endpoint was complete PVI. In the PFA group, a 12-French over-the-wire PFA ablation catheter (Farawave, Farapulse, Inc., California, United States) was used in either a flower or olive configuration delivering the energy in a set of microsecond-scale biphasic pulses of 1800-2000 Volts in bipolar approach across all electrodes. Each application was made of five pulse packets delivered over a few seconds. Applications were repeated eight times per vein, 4 times in flower, 4 times in olive configuration, with repositioning and/or rotation of the catheter every two applications to ensure circumferential PV ostial and antral coverage. The procedural endpoint of complete PVI was confirmed by high-density mapping after the PFA applications.
In patients undergoing CBA, PVI was performed using a 28-mm cryoballoon catheter (POLARx™, Boston Scientific, Massachusetts, United States) under fluoroscopic guidance. The occlusion of PV was confirmed with the retention/leakage of the contrast agent after injection at the balloon’s distal tip. A minimum of two freezes was delivered to each PV with a targeted duration of 240 seconds. After PVI, the entrance block was confirmed by placing an electrode catheter (Polar map catheter) within the PVs, and the exit block by pacing from the catheter.
Follow-up care after an ablation included physical examination and 24 hours Holter-Monitoring in 3 and 6 month intervals post PVI. AF recurrence was defined as any documented AF or flutter > 30 seconds after blanking period of 3 months.
Radiotracer synthesis
The radiotracer 68Ga-FAPI-46, a recently developed FAPI compound, has substantially improved ratios of tumor-to-blood, liver, muscle, and intestinal uptake compared to other FAPI radiotracers. The synthesis of 68Ga-FAPI-46 was performed as previously described.11
Image acquisition
PET scans were performed on a PET/CT system (Biograph Vision, Siemens Healthineers, Erlangen, Germany). Injected activity of 68Ga-FAPI was 113.5 ± 27.8 MBq [73;165 MBq]. ECG-gated cardiac PET imaging was performed approximately 20 minutes p.i. Low-dose CT was performed for attenuation correction (30 mAs, 120 keV, 512 × 512 matrix, 3 mm slice thickness). In addition, a CT angiography (CTA) was performed (30-40 mL iomeprol; 400 mg iodine per milliliter; Iomeron 400; Bracco, Milan, Italy) with the following parameters: spiral mode, 0.6 seconds gantry rotation; collimation, 64 × 0.6 mm; pitch, 1.375:1; section thickness, 0.6 mm; reconstruction interval, 0.5 mm; tube voltage: 120 kV; current intensity: 300 mA. Three patients underwent PET/MRI (Biograph mMR, Siemens Healthcare, Erlangen, Germany) with PET acquisition in 1 bed position and 3D image reconstruction (2 × 2 × 2 mm voxel size) using ordinary Poisson ordered subset expectation maximization with 3 iterations and 21 subsets, a Gaussian filter with 5.0 mm full width at half-maximum and a 344 × 344 image matrix. For automatic attenuation correction of the acquired PET data, a four-compartment model attenuation map was calculated from fat-only and water-only Dixon-based sequences by segmentation into background, lung, fat, and soft tissue.
Image evaluation
PET data was analyzed by two nuclear medicine specialists (CR and LK) on a consensus decision. Tracer uptake was visually rated as “intense”, “moderate” or “absent” if uptake was clearly higher, slightly higher or comparable to blood pool in RA, respectively. Tracer uptake was quantified as maximum (SUVmax), peak (SUVpeak) and mean (SUVmean) standardized uptake values (SUV) from static images 20 minutes after tracer injection. For this purpose, a region grow algorithm at the PV ostia with patient individual threshold of mean uptake in the RA + 2 standard deviations (Syngo.via software; Siemens Healthineers, Erlangen, Germany) and fibroblast activation volume-of-interest (FAV) for each PV ostium was defined. Background (bloodpool, RA) was quantified using a circular 1 cm diameter sphere.
Statistical analysis
Statistical analysis was performed using SPSS statistics and GraphPad Prism (version 8.4.2; GraphPad Software, San Diego, California USA), with quantitative values expressed as mean ± standard deviation or median and range where appropriate. Comparison of non-parametric data was performed using a Mann–Whitney-U-test or Kruskal–Wallis test for multiple comparisons. All tests were performed two-sided and a P value < 0.05 was considered to indicate statistical significance.
Results
Patient characteristics and follow-up regarding AF recurrence
A total of n = 26 patients (CBA = 11, PFA = 15) were retrospectively analyzed with respect to the tracer uptake in pulmonary veins after PVI. Of those n = 7 CBA have been previously reported.8 Five ablation-naïve patients without AF, matched for age, left ventricular ejection fraction (LVEF), who were scanned as a part of their oncological follow-up served as controls.8 All 11/11 CBA patients, 66.7% (10/15) of PFA patients and 80% (4/5) of controls were male. There was no statistical significance in difference of proportion of patients with paroxysmal AF between CBA (36.4%) and PFA (60.0%). The overall baseline characteristics of the three study groups were balanced for age, LVEF, left atrial volume index, arterial hypertension and chronic heart failure. Only one case of AF recurrence in the CBA cohort was registered in 6 months follow-up compared to n = 0 cases in the PFA cohort. Data on patient characteristics is provided in Table 1.
Procedural characteristics
Fluoroscopy time was significantly higher in the PFA group (PFA = 31.1 ± 9.8 minutes vs CBA = 23.1 ± 7.0 minutes, P = 0.03), while amount of administered contrast agent was higher in CBA cohort (CBA = 167.5 ± 84.4 mL vs PFA = 104.7 ± 40.8 mL, P = 0.01). Total procedural time, incl. 3D mapping before and after ablation was balanced between the two groups (CBA: 177.3.0 ± 56.5 minutes vs PFA: 179.3 ± 49.3 minutes, P = 0.92). Other procedural data are listed in Table 2.
Visual and quantitative tracer uptake after PFA vs CBA procedures
In the PFA group only 3/15 (20%) patients showed visual uptake (intense), whereas 80% of PFA patients showed no visual tracer uptake at all. In the CBA cohort, 9/11 (81.8%) patients showed intense, 1/11 (9.1%) moderate visual uptake in PV ostia, while no uptake was observed in only 1/11 patient.
We observed significantly lower values of SUVmax (2.85 ± 0.56 vs 4.71 ± 2.06, P = 0.025) and FAV (1.13 ± 0.84 cm3 vs 3.91 ± 2.74 cm3, P = 0.014) in PFA vs CBA patients, respectively. Further, there was a strong trend towards lower SUVpeak (2.43 ± 0.42 vs 3.24 ± 1.25, P = 0.25) and SUVmean (2.30 ± 0.46 vs 2.87 ± 1.10, P = 0.45) for PFA compared to CBA patients, respectively. A significantly higher uptake in CBA procedures compared to controls could be shown for all PET parameters. Patients after PFA showed a trend of higher uptake than controls, reaching the boundaries of statistical significance for SUVpeak (2.43 ± 0.42 vs 1.28 ± 0.15 P = 0.02) and SUVmean (2.30 ± 0.46 vs 1.12 ± 0.08 P = 0.014). Figure 2 exemplarily depicts the tracer uptake in the study groups.
The distribution of the specific PET parameters SUVmax (A), SUVpeak (B), SUVmean (C) and FAV (D) between uptake at PV antra in CBA, PFA and ablation-naïve individuals (controls), demonstrating quantitatively significantly lower tracer uptake in PFA patients in comparison to CBA for SUVmax (A) and FAV (D) with a clear, albeit non-significant lower values of SUVpeak (B) and SUVmean (C) for PFA. All PET parameters after CBA were significantly higher than in controls (A-D). PFA patients had significantly higher tracer uptake in comparison to controls for SUVpeak (B) and SUVmean (C) with a trend of higher uptake for SUVmax (A) and FAV (D) CBA, cryoballoon ablation; PFA, pulse field ablation SUV, standardized uptake value; FAV, fibroblast activation volume
Temporal changes of FAPI uptake after PFA and CBA ablation
Further, we sought to evaluate a potential temporal change of FAPI tracer uptake after both single-shot modalities. For this purpose, we stratified both cohorts according to the time of imaging (Figure 3), differentiating between patients that have been scanned within first 20 days after PVI (9/15 and 5/11 patients of PFA and CBA cohorts) and patients that were scanned 21-63 days post ablation. In PFA patients, we observed a trend of higher FAPI uptake in all PET parameters within the early phase (< 20 days) post ablation (SUVmax: 2.97 ± 0.67 vs 2.65 ± 0.29, P = 0.21; FAV: 1.28 ± 0.93 vs 0.92 ± 0.69, P = 0.46). However, none of the PET parameters reached the boundaries of statistical significance.
PET parameters after CBA and PFA stratified for time interval between ablation and FAPI scan: (A) Figure showing a clear tendency of lower uptake of PET parameters in CBA patients that were imaged within first 20 days of CBA ablation, with SUVmean reaching the boundaries of significance. (B) In PFA patients, there was trend of higher uptake of SUVmax, SUVpeak, SUVmean and FAV within the first 20 days of ablation with PFA without reaching statistical significance. CBA, cryoballoon ablation; PFA, pulse field ablation; SUV, standardized uptake value; FAV, fibroblast activation volume
The opposite held true in the CBA cohort, where a trend of lower FAPI uptake was observed within the early phase (SUVmax: 4.24 ± 1.90 vs 5.09 ± 2.28, P = 0.54; SUVpeak: 2.89 ± 0.99 vs 3.36 ± 1.54, P = 0.66; FAV: 2.60 ± 1.62 vs 4.62 ± 3.56, P = 0.43) with SUVmean reaching the boundaries of significance (1.56 ± 0.42 vs 2.84 ± 0.91, P = 0.02).
Discussion
This retrospective study is the first comparing FAPI uptake as surrogate for fibroblast activation in patients after single shot non-thermal ablation with PFA to thermal ablation with CBA. Patients treated with PFA had significantly lower FAPI uptake compared to individuals treated with CBA indicating less pronounced fibroblast activation after non-thermal ablation by electroporation.
Tracer uptake after nonthermal vs thermal ablation
Our study found that visual tracer uptake was significantly lower in patients after PFA compared to CBA procedures, as only 3/15 (20%) of PFA patients had a positive visual uptake compared to 10/11 (90.9%) CBA patients who had intense-to moderate visual uptake. These results were in line with quantitative analyses showing significantly lower quantitative uptake in PVs after PFA in comparison to CBA-treated individuals.
The significantly lower extent of tracer uptake as a surrogate for a lower degree of fibrotic remodeling after PFA in comparison to CBA could be explained through different mechanism of energy delivery. PFA, using a predefined protocol, causes selective electroporation of cardiomyocytes leading to apoptosis, thereby sparing the surrounding vascularized connective tissue, which seems to be less sensitive for the used set-up of electroporation.4,12,13 In contrast, CBA results in a non-selective coagulatory necrosis of both the target area and the surrounding tissue, affecting both cardiomyocytes as well as nearby microvascular structures which may translate in different magnitude/pathways of inflammatory vs fibrotic reactive processes in terms of tissue remodeling/healing.14,15 As PFA has only recently been introduced published data in regard to imaging is scarce. A recent MRI study of patients after ablation with both non-thermal and thermal modalities identified a large area of late-gadolinium enhancement (LGE) in PFA patients directly after ablation, which, however, almost disappeared in the 3-months follow up scan.16 This was in complete contrast to an initially small non-homogenous LGE after CBA which then persisted three months later.16 The authors suggested that the initial (extensive) LGE after PFA was induced by acute disintegration of the sarcolemma, resulting in tissue oedema. The regredient LGE after 3 months was explained by the lack of development of chronic fibrosis.16 The chronic disappearance of LGE after PFA is an interesting finding and in accordance with our results as we observed significantly lower amount of FAPI uptake in PFA patients compared to CBA within the first weeks post PVI suggesting a lower degree of activation of fibroblasts in PFA and in consequence lack of fibrosis or absence of LGE as a surrogate of fibrosis. We hypothesize that cardiomyocyte apoptosis after PFA, opposed to general tissue necrosis after CBA, does not trigger an inflammatory cascade that ultimately causes fibroblast activation by the transformation from inflammation to fibrosis by stimulating collagen synthesis.16,17,18 In contrast, CBA, as a thermal modality causing coagulatory necrosis, is suggested to trigger an inflammatory response, hence activating fibroblasts that may be responsible for LGE persistence as well as significantly increased FAPI uptake in these patients.14,16 Further, the disruption of structural integrity as a consequence of thermal ablation due to microvascular obstruction or intramural hemorrhage may lead to mechanical strain on fibroblasts, stimulating their activation, while after an ablation with PFA the structural integrity of extracellular matrix is preserved, preventing the additional mechanical stress on fibroblasts.7,14,16,19
Fibroblast activation after thermal/non-thermal ablation vs controls
The finding of significantly higher fibroblast activation after CBA, which seems to further increase over time, has been reported and discussed elsewhere.8 Here we report a positive visual uptake in 3/15 (20%) of PFA patients and the finding that FAPI uptake in PFA patients tended to be higher compared to ablation-naïve controls in SUVmean and SUVpeak parameters. This finding suggests divergent extent of fibroblast activation in patients after PVI with a clear increase after CBA and a less pronounced, but still to some degree enhanced level of fibroblast activation after PFA. At the moment one can only speculate about this finding. Preclinical studies have shown that although apoptosis is the dominant pathway of cell death in PFA, some degree of immediate cell necrosis after PFA energy delivery may be present, possibly triggering a low-level inflammation cascade that further results in activation of fibroblasts.5,18,20 Another reason for this finding could be found in the direct contact of the PFA device. Especially the olive configuration where the PV ostium is deeply intubated with the PFA device might lead to the triggering of a local fibroblast activation by mechanical stimulation as a consequence of a direct myocardial contact.21,22
Last, the increased FAPI uptake in PFA vs controls could be suggested in the pathophysiologic characteristics of the AF itself, as chronic inflammation and increased fibrotic atrial burden have been increasingly recognized as an important pathomechanism for AF in terms of an atrial cardiomyopathy and no serial pre-ablation imaging was performed in the PFA patients to determine the pre-ablation/baseline FAPI status in these patients.23,24,25,26,27 Both chronic atrial inflammation as well as atrial fibrosis may explain the increased level of FAPI uptake in PFA patients, opposed to non-AF controls.
As an incidental finding, a diffuse left ventricular FAPI uptake was observed in one PFA patient with dilated cardiomyopathy (DCM) and reduced LVEF, possibly reflecting ongoing cardiac remodeling in the setting of DCM, which is further highlighted by the diffuse pattern of ventricular uptake.28 This would be in line with reports of previous studies which associated decreased LVEF with FAPI uptake as well as recent preclinical study, where in rats with heart failure a positive FAPI uptake was observed as a result of an ongoing myocardial fibrosis development.9,11,29
Temporal relation of FAPI uptake depending on the imaging timepoint after PFA/CBA
The MRI study on PFA patients observed LGE disappearance after 3 months post PFA.16 Analogous to this study we tried to evaluate if there is a trend of lower FAPI uptake after PFA procedures over time. After stratifying the PFA cohort according to the time of the imaging we observed only a trend of higher FAPI uptake within the early phase without significant changes in tracer uptake between the time points of imaging. However, preclinical data do suggest that activation of fibroblasts happens within the first 28 days following myocardial injury due to myocardial infarction,30 and published preclinical data suggest peak FAPI uptake to be 6 days after myocardial injury.31 Although the results of our study might have been hampered by the small patient cohort and/or the overall low uptake, due to the specific tissue response following PFA, there might be a trend of decreasing fibroblast activation after PFA. On the other side in CBA patients there was a clear increase after PVI and even a sign of further increase in FAPI uptake over time in the late vs early CBA cohort further underlying a different effect of the ablation technologies on the cellular level.
Clinical implication and future perspectives
Despite the lack of chronic LGE or fibroblast activation, the follow-up data of PFA studies and the results of our 6-month follow-up suggest that recurrence rate is not inferior to thermal ablation.6,16 This is also underlined by the first reported 1-year outcome data of PFA compared to thermal PVI.6 This is an encouraging finding suggesting that PFA is simultaneously a noninferior modality with respect to AF recurrence while simultaneously being a safer modality with respect to adverse effects of increased fibrosis and its complications observed after thermal ablation including PV stenosis, substrate for macro-reentry arrhythmias or atrial stiffness due to adverse remodeling.5,6,32
Protocols of PFA utilization regarding the use of either monophasic or biphasic waveforms have been modified according to the increasing experience and while results of PFA have been promising in regard to safety and outcomes, we still don´t know its possibilities in regard to overpowering.5 Imaging with FAPI may offer a chance to monitor different PFA protocols in regard to their configuration through level of FAPI uptake as surrogate for fibroblast activation. Further, FAPI imaging, especially in combination with LGE-MRI and functional follow-up parameters of myocardial function may allow to better understand subsequent myocardial remodeling due to both thermal and non-thermal ablation caused lesions as well as mechanisms behind AF recurrence. The question if there could be a direct effect of the electroporation on the fibroblast activity, which could in consequence alter the progress of atrial fibrosis, remains completely speculative and has to be elucidated by future studies.
Limitations
This was an observational study of small cohorts with no histological validation, nor a pre-ablation imaging in the ablation patients. Larger prospective studies with repeat hybrid imaging with PET and LGE-MRI in combination with electrophysiological studies, histological validation, longer follow-up and cardiac biomarkers as well as concentration of fibroblast activation protein in serum are needed to further explore the significance of this tracer in evaluation of structural changes following different PVI modalities.
New Knowledge Gained
Tissue response with respect to fibroblast activation seems to be less pronounced in non-thermal single shot ablation system with PFA compared to established thermal single shot ablation systems.
Timeline of peak fibroblast activation seems to occur earlier in a non-thermal ablation with PFA, compared to a thermal ablation with CBA.
Conclusion
Ablation with PFA led to lower levels of fibroblast activation compared to thermal ablation with CBA, reflecting different cell death mechanism and collateral remodeling processes induced by these ablation techniques. Since PFA is a relatively novel ablation modality, imaging with 68Ga-FAPI-PET may help to understand lesion formation after PFA and how it relates to a long-term outcome or possible complications, that at the moment may not be apparent. 68Ga-FAPI-PET may be used as an imaging modality to monitor atrial remodeling in response to tissue damage.
Data availability
Data available on request from the authors upon reasonable request.
Abbreviations
- PVI:
-
Pulmonary vein isolation
- AF:
-
Atrial fibrillation
- CBA:
-
Cryoballoon ablation
- PFA:
-
Pulse field abaltion
- PET/CT:
-
Positron emission tomography/computed tomography
- FAPI:
-
68 Ga-Fibroblast-activation protein inhibitor
- MRI:
-
Magnetic resonance imaging
- LVEF:
-
Left ventricular ejection fraction
- LGE:
-
Late gadolinium enhancement
- SUV:
-
Standardized uptake value
References
Hindricks G, Potpara T, Dagres N, Bax JJ, Blomström-Lundqvist C, Boriani G, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020;42:373‐498.
Andrade JG, Champagne J, Dubuc M, Deyell M. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: A randomized clinical trial. Circulation 2019;140:1779‐88.
Andrade JG. Cryoballoon ablation for pulmonary vein isolation. J Cardiovasc Electrophysiol 2020;31:2128‐35.
Bradley CJ, Haines DE. Pulsed field ablation for pulmonary vein isolation in the treatment of atrial fibrillation. J Cardiovasc Electrophysiol 2020;31:2136‐47.
Reddy VY, Neuzil P, Koruth JS, Petru P, Funosako M, Cochet H, et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol 2019;74:315‐26.
Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako M, et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol 2021;7:614‐27.
Koruth J, Kuroki K, Iwasawa J, Enomoto Y, Viswanathan R, Brose R, et al. Preclinical evaluation of pulsed field ablation: Electrophysiological and histological assessment of thoracic vein isolation. Circ Arrhythm Electrophysiol 2019;12:e007781.
Kupusovic J, Kessler L, Nekolla SG, Riesinger L, Weber MM, Ferdinandus J, et al. Visualization of thermal damage using (68) Ga-FAPI-PET/CT after pulmonary vein isolation. Eur J Nucl Med Mol Imaging 2022;49:1553‐9.
Siebermair J, Kohler MI, Kupusovic J, Nekolla SG, Kessler L, Ferdinandus J, et al. Cardiac fibroblast activation detected by Ga-68 FAPI PET imaging as a potential novel biomarker of cardiac injury/remodeling. J Nucl Cardiol. 2020;28:812‐21.
Wakili R, Siebermair J, Fichtner S, Sinner MF, Klocker E, Olesch L, et al. One-year clinical outcome after ablation with a novel multipolar irrigated ablation catheter for treatment of atrial fibrillation: Potential implications for clinical use. Europace 2016;18:1170‐8.
Kessler L, Kupusovic J, Ferdinandus J, Hirmas N, Umutlu L, Zarrad F, et al. Visualization of fibroblast activation after myocardial infarction using 68Ga-FAPI PET. Clin Nucl Med 2021;46:807‐13.
Cochet H, Nakatani Y, Sridi-Cheniti S, Cheniti G, Ramirez FD, Nakashima T, et al. Pulsed field ablation selectively spares the oesophagus during pulmonary vein isolation for atrial fibrillation. Europace 2021;23:1391‐9.
Stewart MT, Haines DE, Verma A, Kirchhof N, Barka N, Grassl E, et al. Intracardiac pulsed field ablation: Proof of feasibility in a chronic porcine model. Heart Rhythm 2019;16:754‐64.
Halbfass PM, Mitlacher M, Turschner O, Brachmann J, Mahnkopf C. Lesion formation after pulmonary vein isolation using the advance cryoballoon and the standard cryoballoon: Lessons learned from late gadolinium enhancement magnetic resonance imaging. Europace 2015;17:566‐73.
Andrade JG, Khairy P, Dubuc M. Catheter cryoablation: Biology and clinical uses. Circ Arrhythm Electrophysiol 2013;6:218‐27.
Nakatani Y, Sridi-Cheniti S, Cheniti G, Ramirez FD, Goujeau C, André C, et al. Pulsed field ablation prevents chronic atrial fibrotic changes and restrictive mechanics after catheter ablation for atrial fibrillation. Europace 2021;23:1767‐76.
Okyere AD, Tilley DG. Leukocyte-dependent regulation of cardiac fibrosis. Front Physiol 2020;11:301.
Maor E, Ivorra A, Rubinsky B. Non thermal irreversible electroporation: Novel technology for vascular smooth muscle cells ablation. PLoS ONE 2009;4:e4757.
Kurose J, Kiuchi K, Fukuzawa K, Takami M, Mori S, Suehiro H, et al. Lesion characteristics between cryoballoon ablation and radiofrequency ablation with a contact force-sensing catheter: Late-gadolinium enhancement magnetic resonance imaging assessment. J Cardiovasc Electrophysiol 2020;31:2572‐81.
Van Linthout S, Miteva K, Tschope C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res 2014;102:258‐69.
Haines DE. What is different about pulsed-field ablation … everything? J Cardiovasc Electrophysiol 2022;33:368‐70.
Su W, Aryana A, Passman R, Singh G, Hokanson R, Kowalski M, et al. Cryoballoon Best Practices II: Practical guide to procedural monitoring and dosing during atrial fibrillation ablation from the perspective of experienced users. Heart Rhythm 2018;15:1348‐55.
Scott L Jr, Li N, Dobrev D. Role of inflammatory signaling in atrial fibrillation. Int J Cardiol 2019;287:195‐200.
Wu L, Emmens RW, van Wezenbeek J, Stooker W, Allaart CP, Vonk ABA, et al. Atrial inflammation in different atrial fibrillation subtypes and its relation with clinical risk factors. Clin Res Cardiol 2020;109:1271‐81.
Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: Epidemiology, pathophysiology, and clinical outcomes. Circ Res 2017;120:1501‐17.
Kottkamp H. Fibrotic atrial cardiomyopathy: A specific disease/syndrome supplying substrates for atrial fibrillation, atrial tachycardia, sinus node disease, AV node disease, and thromboembolic complications. J Cardiovasc Electrophysiol 2012;23:797‐9.
Kottkamp H. On the atrial fibrillation substrate: From the “Unknown species” to deeper insights into pathophysiology. J Am Coll Cardiol 2019;74:1348‐51.
Prasad SK, Halliday BP. Myocardial fibrosis in dilated cardiomyopathy: Moving from stratifying risk to improving outcomes. JACC Cardiovasc Imaging 2021;14:1351‐3.
Song W, Zhang X, He S, Gai Y, Qin C, Hu F, et al. (68)Ga-FAPI PET visualize heart failure: From mechanism to clinic. Eur J Nucl Med Mol Imaging 2022;50:475‐85.
Ma Y, Iyer RP, Jung M, Czubryt MP, Lindsey ML. Cardiac fibroblast activation post-myocardial infarction: Current knowledge gaps. Trends Pharmacol Sci 2017;38:448‐58.
Varasteh Z, Mohanta S, Robu S, Braeuer M, Li Y, Omidvari N, et al. Molecular imaging of fibroblast activity after myocardial infarction using a (68)Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J Nucl Med 2019;60:1743‐9.
Petru J, Funasako M, Minami K, Breskovic T, Sikiric I, et al. Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol 2020;76:1068‐80.
Disclosures
J. Kupusovic has no relevant financial or non-financial interests to disclose. L. Kessler has no relevant financial or non-financial interests to disclose. F. Bruns has no relevant financial or non-financial interests to disclose. J. Bohnen has no relevant financial or non-financial interests to disclose. S. Nekolla has no relevant financial or non-financial interests to disclose. M. Weber has no relevant financial or non-financial interests to disclose. A. Lauenroth has no relevant financial or non-financial interests to disclose. M. Rattka has no relevant financial or non-financial interests to disclose. K. Herrmann reports personal fees from Bayer, personal fees and other from Sofie Biosciences, personal fees from SIRTEX, non-financial support from ABX, personal fees from Adacap, personal fees from Curium, personal fees from Endocyte, grants and personal fees from BTG, personal fees from IPSEN, personal fees from Siemens Healthineers, personal fees from GE Healthcare, personal fees from Amgen, personal fees from Novartis, personal fees from ymabs, personal fees from Aktis Oncology, personal fees from Theragnostics, personal fees from Pharma15, personal fees from Debiopharm, personal fees from AstraZeneca, personal fees from Janssen, outside the submitted work. D. Dobrev has no relevant financial or non-financial interests to disclose. T. Rassaf has no relevant financial or non-financial interests to disclose. R. Wakili has received consultant fees, speaking honoraria and travel expenses from Biotronik, Boston Scientific and Medtronic; investigator-initiated funding for research projects (initiated by him) from Bristol-Myers Squibb/Pfizer, and Boston Scientific. R. Wakili was, unrelated to this study, funded by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation – DO637/23-1; Projektnummer 39443325). C. Rischpler reports a research grant from Pfizer, consultancy for Adacap and Pfizer, speaker honoraria from Adacap, Alnylam, BTG, Curium, GE Healthcare, Pfizer and Siemens Healthineers, outside the submitted work. J. Siebermair has no relevant financial or non-financial interests to disclose. The remaining authors have no relevant financial or non-financial interests to disclose.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Funding
The authors have not received funding for the present study.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
All editorial decisions for this article, including selection of reviewers and the final decision, were made by guest editor Nagara Tamaki, MD.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kupusovic, J., Kessler, L., Bruns, F. et al. Visualization of fibroblast activation using 68Ga-FAPI PET/CT after pulmonary vein isolation with pulsed field compared with cryoballoon ablation. J. Nucl. Cardiol. 30, 2018–2028 (2023). https://doi.org/10.1007/s12350-023-03220-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-023-03220-8