Abstract
Aim
The aim of the study was to evaluate the changes in central vascular inflammation measured by FDG PET and myocardial blood flow reserve (MFR) determined by 82Rb PET following therapy with biologic agents for 6 months in patients with psoriatic arthritis (PsA) and/or cutaneous psoriasis (PsO) (group 1) and compare with PsO subjects receiving non-biologic therapy (group 2) and controls (group 3).
Methods and Results
Target-to-background ratio (TBR) by FDG PET in the most diseased segment of the ascending aorta (TBRmax) was measured to assess vascular inflammation. 82Rb PET studies were used to assess changes in left ventricular MFR. A total of 34 participants were enrolled in the study (11 in group 1, 13 in group 2, and 10 controls). A significant drop in the thoracic aorta uptake was observed in the biologic-treated group (ΔTBRmax: − .46 ± .55) compared to the PsO group treated with non-biologic therapy (ΔTBRmax: .23 ± .67). Those showing response to biologic agents maintained MFR compared to who showed no response.
Conclusion
In a cohort of psoriasis patients treated with biologics, FDG uptake in the thoracic aorta decreased over the study period. Patients who demonstrated a significant anti-inflammatory response on FDG PET imaging maintained their MFR compared to non-responders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Psoriatic arthritis (PsA) is a disease of chronic inflammation and occurs in 14-30% of patients with skin or nail psoriasis (PsO).1,2 Similar to other diseases of chronic inflammation, patients with PsA or PsO have higher than expected rates of cardiovascular (CV) disease and an increased risk of major adverse cardiovascular events (MACE).3,4 Indeed, CV disease is the single leading cause of death in patients with PsA and dermatologic psoriasis and may account for > 50% of all deaths in these populations.5
Systemic inflammation is thought to be the key mediator in the link between psoriasis and CV disease.6 Patients with psoriasis are in a state of chronic immune activation and have increased levels of inflammatory cytokines, such as tumor necrosis factor (TNF)-α.7 Interestingly, data have linked psoriasis severity with the degree of systemic inflammation.8 Chronic inflammation has a negative effect on the vasculature and plays a pivotal role in the mediation of the peripheral microvascular dysfunction that is seen in patients with psoriasis.9 A better understanding of the pathophysiology and potential treatment options behind the accelerated atherosclerosis and inflammation that is seen in patients with psoriasis remains a priority.
F-18-fluorodeoxyglucose positron emission tomography (FDG PET) provides an attractive and pragmatic means to measure and follow vascular inflammation in these patients, as it is the most widely validated and applied imaging probe in this regard.10,11,12 Previous studies have shown that its uptake predicts MACE;13,14,15 and it is also highly sensitive to short-term treatment effects.16,17 Furthermore, PET myocardial perfusion imaging in conjunction with rubidium-82 (82Rb) affords the ability to assess regional myocardial blood flow to the left ventricle in absolute terms (mL·min−1·g−1).18 Rubidium-82 PET has been established as a nuclear imaging method for accurate, reproducible, and routine quantification of myocardial blood flow and myocardial blood flow reserve [MFR (stress/rest perfusion)] in humans and in animal models of disease.19,20,21,22
Patients with psoriasis, especially those requiring systemic biologic therapy, have increased vascular inflammation.8 We hypothesized that it is increased central vascular inflammation in these patients that is contributing to the development of accelerated atherosclerosis. We sought to use FDG PET to quantify vascular inflammation in the ascending aorta in patients with PsA and PsO and hypothesized that thoracic aortic vascular inflammation would improve in these patients following therapy with biologic agents compared to psoriasis patients receiving non-systemic therapies and control patients with non-inflammatory skin/joint disease. Secondly, we sought to explore changes in MFR over time after treatment with biologic agents and explore the relationship between changes in vascular inflammation, as measured by FDG PET, to changes in MFR determined by 82Rb PET.
Methods
This was a prospective cohort clinical study designed to determine the effect of biologic therapy on vascular inflammation and MFR in patients with PsA compared to cohorts not treated with biologic therapies. Study approval was obtained from the University of Ottawa Heart Institute’s Research Ethics Board in accordance with the principles of Declaration of Helsinki. All study participants provided written informed consent.
We studied 3 patient groups which were consecutively enrolled: (i) patients with PsA and/or PsO who were to be started on anti-TNF-α, anti-interleukin (IL)-17, or anti-IL-12/23 therapy biologic agents, (ii) patients with psoriasis managed on non-biologic therapies, and (iii) control patients with non-inflammatory skin or joint conditions. Participants were enrolled from the Rheumatology and Dermatology Clinics at the Ottawa Hospital, Ottawa, Ontario, Canada. A convenience sample was used, and all participants underwent 82Rb PET and FDG PET examinations at baseline and at 6 months. Demographic and anthropometric data including age, sex, ethnicity, height, weight, waist circumference, and blood pressure were recorded as well as current medications, medical history, smoking status, and family history of CV disease.
Group 1 consisted of subjects with PsA and/or PsO scheduled for initiation of anti-TNF-α therapy, anti-IL-17, or anti-IL-12/23 therapy with prior failed response to traditional disease-modifying anti-rheumatic drugs (DMARDs). After inclusion in the study, subjects in Group 1 received 6-month treatment with a biologic therapy following the baseline study investigations. Patients with PsA were included if they met the classification criteria for PsA21 and had persistent joint inflammation measured as 5 or more swollen joints, despite therapy for at least three months, each with 2 or 3 conventional DMARDs (methotrexate, leflunomide, or sulfasalazine). To minimize the effect that duration of the disease could play on the results, only patients with a recent diagnosis of PsA were included (i.e., patients that had a diagnosis of PsA for > 3 months and < 2 years).23 The treatment method chosen for patients was decided by the patient’s treating rheumatologist.
Group 2 included subjects with psoriasis on non-biologic treatment (i.e., topical medications, acitretin, or phototherapy only). Psoriasis Area and Severity Index (PASI) score was used for the assessment of severity of dermatological psoriasis.24
Group 3 included patients with an established diagnosis of non-inflammatory joint diseases, such as osteoarthritis. These control patients were identified by a detailed history and physical examination, with normal baseline blood tests (full blood count, renal function, fasting glucose, and lipid profile).
Primary outcome: FDG PET imaging of ascending aortic inflammation
The primary outcome of our study was the change in vascular inflammation in the ascending aorta, which was measured according to standardized methods16 as the target-to-background ratio (TBR) in the most diseased segment of the ascending aorta at baseline compared to 6-month follow-up (TBRmax). All patients underwent FDG PET with low-dose computed tomography at baseline and then following 6-month follow-up. Patients in group 1 started on biologic agents following their baseline FDG PET. FDG PET images were analyzed to determine TBRmax values by utilizing previously published and validated methodology.16 In short, we first obtained the maximum standardized uptake value (SUV) for the most diseased segment of the ascending aorta by averaging the SUV for 3 consecutive axial slices centered on the highest uptake slice and the adjacent slices superior and inferior to it, providing approximately 1 cm of the most inflamed section of the aortic wall. We then calculated the TBRmax by determining the ratio of this SUVmax for the most inflamed region of the ascending aorta to background venous activity, derived from an image region in the superior vena cava. On the follow-up scan in the treatment group, the same 3 slice locations were used to calculate the follow-up TBRmax. Of note, the FDG PET analysis was performed by an independent reader blinded to clinical details.
Secondary outcome: FDG PET imaging of other aortic inflammation
As a secondary analysis, we also evaluated the change in vascular inflammation in other areas of the aorta, including the aortic arch and descending aorta. Similar to the methods outlined above for the ascending aorta, we measured this as the TBR in the most diseased segment of the respective portion of the aorta (arch or descending aorta) at baseline compared to 6-month follow-up (TBRmax).
Secondary outcome: 82Rb PET for MFR
Dynamic 82Rb PET was performed according to previously published and validated methodology.25,26 In short, absolute myocardial blood flow was calculated at rest and during dipyridamole stress using our FlowQuant© analysis software. The 82Rb analysis was performed by an independent reader blinded to clinical details.
Post hoc analysis: MFR
To better understand the relationship of changes in vascular inflammation to microvascular function, in a post hoc analysis we evaluated the change in MFR values in the subgroup of PsA patients treated with biologic agents. We compared the MFR of patients in the biologic group that had a response in their TBRmax (that was greater or equal to the median delta TBRmax of this group) to those that had a response that was less than the median (i.e., post hoc analysis for effect modification).
Statistical methods
Descriptive statistics (such as median with interquartile range (IQR) for continuous variables, frequencies with percentages for categorical variables) are used to summarize the three study groups’ baseline demographic and clinical variables. Continuous variables are presented as medians with IQR and categorical variables are presented as frequencies and percentages (unless otherwise stated). Demographic characteristics are presented as median (IQR) or percentage as appropriate. Differences between patient groups were evaluated by the Fisher exact test for discrete clinical variables and by the Kruskal–Wallis test for continuous variables. Follow-up data were collected as scheduled. All the tests were two-sided and a P value of < .05 was considered statistically significant. Within-group testing of changes in baseline and 6-month TBRmax and left ventricle (LV) MFR values were conducted with Wilcoxon Signed Rank testing. The relative changes in TBRmax and LV MFR over the study period were compared between groups using a generalized linear mixed effects model (GLMM) for repeated measures analysis. Variables included in the model were age, body mass index, sex, and baseline aortic TBRmax. Missing data were considered missing at random and complete case analysis was used to handle the missingness. We also performed multiple imputation as a sensitivity analysis to assess the effect of missingness on our results using a GLMM with baseline and follow-up TBRmax included in the model (thereby adjusting for differences in baseline aortic inflammation across groups). Statistical analyses were performed using MedCalc for Windows version 12.0 (MedCalc Software, Ostend, Belgium) and Stata/IC for Windows version 16.1 (StataCorp LLC, TX, USA).
Results
A total of 42 participants were enrolled in the study (12 PsA and/or PsO patients who were started on biologic agents, 18 PsO patients treated with non-biologic therapies, and 12 patients in the non-inflammatory control group with osteoarthritis). In the biologics group, 5 patients were started on an anti-TNF-α agent (4 adalimumab and 1 etanercept), 4 on an IL-17 inhibitor (secukinumab), and 2 on an IL-12/23 inhibitor (ustekinumab). One patient in the biologic group dropped out after having an abnormal imaging study that required further intervention, 5 patients in the PsO group not on biologic therapies withdrew after baseline imaging studies, and 2 patients in the control group withdrew after baseline imaging studies. This resulted in a total of 34 patients having complete baseline and follow-up imaging data for analysis: 11 PsA and/or PsO patients started on biologic agents, 13 PsO patients treated with non-biologic therapies, and 10 patients in the non-inflammatory control group with osteoarthritis. Additionally, all patients (including patients that withdrew/were lost to follow-up) were included in the sensitivity analysis with multiple imputation.
Participant characteristics are described in Table 1. In summary, 64.7% of participants were men, and the median age was 62 years (IQR: 48, 69), which was similar between the three groups. Median BMI was 28.7 kg·m−2 (IQR: 27.1, 36.3). More patients in Group 1 had diabetes (P = .032), were more commonly treated with Methotrexate (P = .002), or on medications, such as oral steroids (P < .001) and non-steroidal anti-inflammatory drugs (NSAIDs) (P = .002).
Inflammation imaging results
Baseline ascending aortic TBRmax was not statistically different between the three groups: 2.84 (IQR 2.51, 3.37), 2.73 (IQR 2.54, 3.28), versus 2.70 (IQR 2.68, 2.76) in the biologics, non-biologic, and control groups, respectively (P = .725). Similarly, baseline aortic arch TBRmax did not differ across the groups (P = .565) and nor did baseline descending aortic TBRmax (P = .190). For the primary outcome, analysis within groups with Wilcoxon Rank Sum testing demonstrated a statistically significant reduction in vascular inflammation measured as FDG TBRmax within the ascending aorta, in the biologic group only [baseline 2.84 (IQR 2.51, 3.37), follow-up 2.50 (IQR 2.27, 2.94) (P = .033)]. There were no significant changes in either the non-biologic [baseline 2.73 (IQR 2.54, 3.28), follow-up 2.95 (IQR 2.64, 3.63) (P = .279)] or control groups [baseline 2.70 (IQR 2.68, 2.76), follow-up 2.94 (IQR 2.67, 3.28) (P = .114)] (Table 2 and Fig. 1). Similarly, there were statistically significant decreases in measured FDG TBRmax within the aortic arch (P = .002) and descending aorta (P = .007) within the biologic group, but not the other groups (P > .05). Table 2 illustrates the change in aortic TBR uptake in the ascending aorta as well as change in MFR of the three groups. GLMM for repeated measures analysis revealed the change in FDG TBRmax over the study period in Group 1 differed significantly from Group 2 (β ± SE: .697 ± .245, P = .004) and Group 3 (β ± SE: .670 ± .259, P = .010). This was true for the aortic arch and descending aorta as well. Sensitivity analysis with multiple imputation for missing FDG TBRmax values showed the results remained significant after imputation (biologic group versus non-biologic group: β ± SE: .651 ± .230, P = .005); biologic group versus control group: β ± SE: .631 ± .261, P = .016). The change in FDG uptake from baseline to follow-up is shown in Fig. 2.
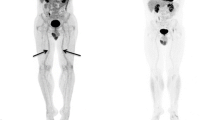
Change in ascending aorta FDG uptake from baseline to 6-month follow-up in Group 1 (patients with PsA and/or PsO on biologics), Group 2 (patients with PsO on non-biologics), and group 3 (control group). FDG, F-18-fluorodeoxyglucose; PsA, psoriatic arthritis; PsO, psoriatic disease; TBR, target-to-background ratio
FDG images showing change in ascending aorta FDG uptake from baseline to 6-month follow-up in (A) patients with PsA and/or PsO on biologic agents, (B) patients with PsO on non-biologic agents, and (C) control patients with non-inflammatory arthritis. FDG, F-18-fluorodeoxyglucose; PsA, psoriatic arthritis; PsO, psoriatic disease
Regarding the exploratory outcome, baseline MFR was not statistically different between the three groups: Baseline MFR was 2.96 (IQR 2.38, 3.46), 3.14 (IQR 2.54, 3.95), and 3.43 (IQR 2.84, 4.34) in the biologic, non-biologic, and control groups, respectively (P = .528). Analysis within groups with Wilcoxon Rank Sum testing demonstrated a statistically significant reduction in MFR in the biologic group only [baseline 2.96 (IQR 2.38, 3.46), follow-up 2.85 (IQR 2.20, 3.06) (P = .037)]. There were no significant changes in either the non-biologic [baseline: 3.14 (IQR 2.54, 3.95), follow-up: 3.58 (IQR 3.08, 3.94) (P = .279)] or control groups [baseline: 3.43 (IQR 2.84, 4.34), follow-up: 3.92 (IQR 2.97, 4.38) (P = .114)] (Table 2). GLMM for repeated measures analysis revealed no statistically significant differences in the change in MFR over the study period between the biologic group and non-biologic group (β ± SE: .477 ± .370, P = .198) and control group (β ± SE: .467 ± .395, P = .237), respectively.
Sensitivity analysis with multiple imputation for missing ascending aortic FDG TBRmax values showed that results remained non-significant even after imputation (biologic group versus non-biologic group: β ± SE: .522 ± .407, P = .201; biologic group versus control group: β ± SE: .384 ± .456, P = .400).
While GLMM for repeated measures analysis revealed no significant difference in the change in MFR between the three groups over the study period, to better understand the relationship of changes in vascular inflammation to microvascular function, we decided to further evaluate the change in MFR in the subgroup of PsA patients treated with biologic agents in a post hoc analysis. We compared the MFR of patients in the biologic group who had a response in their TBRmax that was greater or equal to the median of this group to those who had a response that was less than the median. We found a significant difference in these groups of patients. The group with a clinical response to biologic agents as measured by a change in TBRmax that was greater or equal to the median change (7%) maintained MFR (3.40 ± 1.23 MFR to 3.5 ± 1.2 MFR over 6 months) when compared to the group with a below median response on TBRmax which had a drop in MFR (2.9 ± .8 MFR to 2.2 ± .6 over 6 months; P = .03). Spearman’s coefficient of rank correlation (rho) between change in TBRmax and change in MFR was .709 (P = .0146) (Fig. 3). Finally, as an additional exploratory analysis we looked at whether there were any differences in vascular inflammation change between patients in Group 1 on different types of biologics (TNF inhibitor, IL-17 inhibitor, and IL-12/23 inhibitor). We found no difference, likely due to the small number of patients.
Correlation between change in TBRmax and MFR in patients with PsA and/or PsO on biologic agents. Patients with a clinically significant response to biologic agents are colored red. Delta TBRmax, target-to-background ratio in the most diseased segment of the ascending aorta at baseline compared to 6-month follow-up; Delta MFR, myocardial blood flow reserve at baseline compared to 6-month follow-up
Discussion
We conducted a prospective cohort study to determine the impact of systemic anti-cytokine treatment on vascular inflammation and MFR in patients with psoriasis, an inflammatory disease that is well known to be associated with vascular inflammation.8 In a cohort of psoriasis patients that were started on biologic therapy, representative of clinical practice, we found that FDG uptake in the thoracic aorta decreased over the 6-month study period compared to no change in psoriasis patients treated with non-systemic therapies or in a cohort of control patients with non-inflammatory joint and skin disease. Additionally, we found that in those psoriasis patients treated with biologics who had imaging evidence of a significant anti-inflammatory response to the biologics, MFR was maintained compared to the group that did not have a significant response to the biologic therapy. The present study helps fill a knowledge gap by suggesting that anti-cytokine therapy may have vascular anti-inflammatory effects. These changes may be linked to changes in the coronary microvasculature in patients with psoriasis and support the notion that anti-inflammatory biologics may have a role in mitigating CV disease in this patient population.
Psoriasis disease is a chronic inflammatory skin condition that affects over 125 million people worldwide.27 Patients with psoriasis have been found to have an increased incidence of CV disease and increased risk of MACE.3,28,29,30 A key link between psoriasis and heart disease is mediated through inflammation. Patients with psoriasis have increased vascular inflammation measured by FDG PET, and it has been shown that increasing severity of skin disease is associated with increased vascular inflammation.8,31 However, at this point it is not yet known whether targeting inflammatory pathways could lead to downstream effects in reducing CV morbidity and mortality in these patients.
In our jurisdiction, PsA patients progressing to a biologic agent need to have ongoing arthritis (5 or more swollen joints) despite treatment with methotrexate for 3 months (usually at doses between 20 and 25 mg·week−1) and an additional 3 months on leflunomide (20 mg·day−1) or sulfasalazine (2000 mg·day−1). Patients with skin disease need to demonstrate at least 3% of total body skin involvement despite 3 months of treatment with systemic therapy, usually methotrexate or cyclosporine. In the present study, treatment with anti-cytokine therapy was associated with a clinically significant reduction in aortic inflammation both when compared within the biologic group and also when compared to the non-biologic and control groups. Our data provide insights into the potential linkage of atherosclerosis with inflammation and further suggests that treatment of psoriasis patients with biologic agents may help to mitigate vascular inflammation. Additionally, while the overall change in MFR did not differ between the psoriasis group treated with anti-cytokine therapy and the other groups, when we looked specifically at patients in the psoriasis group that were treated with biologic agents who had a response in their TBRmax that was greater than the median (compared to the patients treated with biologic agents with a response less than the median), MFR was maintained in the responders compared to the non-responders (where it significantly dropped).
Our results are consistent with a noncontrolled study in rheumatoid arthritis of anti-TNF-α therapy, which demonstrated a reduction in vascular inflammation on FDG PET imaging after 8 weeks of treatment.32 They are also consistent with an observational cohort from Kim et al. evaluating the anti-inflammatory effect of 25 patients with PsO treated with Ustekinumab.33 Furthermore, an observational study from Dey et al. that evaluated a cohort of psoriasis patients being treated systemically had similar findings.34
Our results, however, are not consistent with a randomized placebo-controlled trial from Mehta et al., which showed no change in vascular inflammation on FDG PET in patients randomized to adalimumab, phototherapy, or placebo for a period of 12 weeks, with an open-label extension of the adalimumab arm for a period of 52 weeks.35 There are several possible reasons for the discrepancy of our results with that observed by Mehta et al. Firstly, our study had 64% of the PsA/PsO population with PsA (while only 10% in the Mehta et al., study had PsA). Thus, there are underlying differences in the composition of the study populations which could theoretically influence the vascular response to biologic therapy. Secondly, while there was no difference in baseline inflammatory levels between our three study groups, it should be noted that our biologic therapy group had a higher proportion of patients pretreated with NSAIDS, methotrexate, and steroids. This difference in anti-inflammatory pretreatment across study groups could again theoretically alter the vascular response to biologic agents. Thirdly, our sample size was very small, increasing the risk for our results to be influenced by statistical chance. However, the consistent response of different anatomical sections of the aorta to the biologic agents certainly argues against this explanation. Lastly, ours was a non-randomized study, which could result in potential unmeasured confounding of our results. It should be noted, however, that these possible explanations are purely speculative.
Our study has several important limitations. Firstly, this was a non-randomized prospective observational study with a modest sample size, and limited clinical follow-up. Thus, our results should be interpreted with caution, and while they are largely hypothesis generating, they should certainly fuel further randomized clinical trials to investigate these findings. Conversely, we employed rigorous methodological analyses as well as a sensitivity analysis with multiple imputation to attempt to overcome some of these limitations and strengthen our results. Secondly, we utilized imaging outcomes to measure inflammatory response to biologic therapy rather than clinical outcomes. While clinical assessment of joint response to biologic therapy involves subjective measures, the imaging data of vascular inflammation that we utilized for our primary outcome are a completely objective measure of treatment response. Both vascular inflammation and MFR have been shown to be robust indices, which can give important insights into disease activity over shorter periods of clinical observation. Therefore, both vascular inflammation and MFR represent important surrogate markers for imaging trials that allow for the assessment of therapeutic benefit of interventions in a timely manner. In this regard, while FDG PET has been evaluated utilized extensively for the non-invasive detection of inflammation related to atherosclerosis, it does have several limitations which should be highlighted. While FDG PET imaging targets activated macrophages, it is still a non-specific probe that can accumulate in other metabolically active tissues which can therefore introduce interfering background signal.36,37 Imaging of the ascending aorta requires strict dietary preparation to suppress FDG-myocardium uptake (with a high-fat, low-carbohydrate diet prior to PET imaging), given the significant background myocardial FDG uptake which can make it more difficult to differentiate vascular inflammation from background uptake.38,39 FDG PET imaging is also affected by glucose levels, so particular caution in imaging patients with diabetes and hyperglycemia must be employed.40 From a technical perspective, the partial volume effect and motion artifacts from cardiac and organ motion during imaging all limit the spatial resolution and specificity of the test.41 Additionally, given the limited spatial resolution of current PET imaging systems, we are currently unable to directly quantify “vulnerable plaque” in smaller-sized vessels, such as the coronary arteries meaning we are limited to assessing larger caliber vessels, such as the aorta.42 Finally, while baseline demographic factors were not statistically different between groups, it is possible that the numeric difference in the proportion of participants with cardiac risk factors like hypertension, diabetes or current smoking, or differences in baseline BMI could have influenced the differences we saw in baseline TBRmax and response to biologics between the groups.
In conclusion, our study suggests that anti-cytokine therapy is associated with decreasing vascular inflammation in patients with PsA as assessed by FDG PET in contrast to non-biologics as well as a non-inflammatory control group. While our study should be interpreted with caution given the small number of participants and limited generalizability, our results do suggest an association between these biologic agents and preserved MFR in patients who respond favorably in terms of their vascular inflammation. This study supports the notion that there is a positive impact of immune therapies on vascular inflammation and microvascular disease in patients with chronic inflammation; however, larger prospective clinical outcome trials investigating this are warranted.
New knowledge gained
Anti-cytokine therapy is associated with decreasing vascular inflammation in patients with psoriatic arthritis as assessed by FDG PET.
Data availability
Data is available upon request.
Change history
27 February 2023
Missing Open Access funding information has been added in the Funding Note
References
Sankowski AJ, Lebkowska UM, Cwikla J, Walecka I, Walecki J. Psoriatic arthritis. Pol J Radiol 2013;78:7‐17.
Peluso R, Caso F, Tasso M, Ambrosino P, Dario Di Minno MN, Lupoli R. Cardiovascular risk markers and major adverse cardiovascular events in psoriatic arthritis patients. Rev Recent Clin Trials 2018;13:199‐209.
Yeung H, Takeshita J, Mehta NN, Kimmel SE, Ogdie A, Margolis DJ, et al. Psoriasis severity and the prevalence of major medical comorbidity: A population-based study. JAMA Dermatol 2013;149:1173‐9.
Gelfand JM, Troxel AB, Lewis JD, Kurd SK, Shin DB, Wang X, et al. The risk of mortality in patients with psoriasis: Results from a population-based study. Arch Dermatol 2007;143:1493‐9.
McHugh NJ, Balachrishnan C, Jones SM. Progression of peripheral joint disease in psoriatic arthritis: A 5-yr prospective study. Rheumatology (Oxford) 2003;42:778‐83.
Daghem M, Newby D. Psoriasis and inflammation more than skin deep. Circ Cardiovasc Imaging 2018;11:e007849.
Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine 2015;73:342‐50.
Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol 2015;35:2667‐76.
Piaserico S, Osto E, Famoso G, Montisci R, De Michieli L, Zanetti I, et al. Long-term prognostic value of coronary flow reserve in psoriasis patients. Atherosclerosis 2019;289:57‐63.
Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002;105:2708‐11.
Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006;48:1818‐24.
Cocker MS, Spence JD, Hammond R, deKemp RA, Lum C, Wells G, et al. [18F]-Fluorodeoxyglucose PET/CT imaging as a marker of carotid plaque inflammation: Comparison to immunohistology and relationship to acuity of events. Int J Cardiol 2018;271:378‐86.
Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging 2013;6:1250‐9.
Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med 2009;50:1611‐20.
Marnane M, Merwick A, Sheehan OC, Hannon N, Foran P, Grant T, et al. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol 2012;71:709‐18.
Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: Results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol 2013;62:909‐17.
Mizoguchi M, Tahara N, Tahara A, Nitta Y, Kodama N, Oba T, et al. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes a prospective, randomized, comparator-controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc Imaging 2011;4:1110‐8.
Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging 2010;3:623‐40.
deKemp RA, Klein R, Beanlands RS. (82)Rb PET imaging of myocardial blood flow-have we achieved the 4 “R”s to support routine use? EJNMMI Res 2016;6:69.
Germino M, Ropchan J, Mulnix T, Fontaine K, Nabulsi N, Ackah E, et al. Quantification of myocardial blood flow with (82)Rb: Validation with (15)O-water using time-of-flight and point-spread-function modeling. EJNMMI Res 2016;6:68.
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheumatism 2006;54:2665‐73.
Anagnostopoulos C, Almonacid A, El Fakhri G, Curillova Z, Sitek A, Roughton M, et al. Quantitative relationship between coronary vasodilator reserve assessed by 82Rb PET imaging and coronary artery stenosis severity. Eur J Nucl Med Mol Imaging 2008;35:1593‐601.
Khraishi M, MacDonald D, Rampakakis E, Vaillancourt J, Sampalis JS. Prevalence of patient-reported comorbidities in early and established psoriatic arthritis cohorts. Clin Rheumatol 2011;30:877‐85.
Puzenat E, Bronsard V, Prey S, Gourraud PA, Aractingi S, Bagot M, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol 2010;24:10‐6.
Lortie M, Beanlands RS, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging 2007;34:1765‐74.
Chih S, Chong AY, Erthal F, deKemp RA, Davies RA, Stadnick E, et al. PET Assessment of epicardial intimal disease and microvascular dysfunction in cardiac allograft vasculopathy. J Am Coll Cardiol 2018;71:1444‐56.
Goff KL, Karimkhani C, Boyers LN, Weinstock MA, Lott JP, Hay RJ, et al. The global burden of psoriatic skin disease. Br J Dermatol 2015;172:1665‐8.
Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006;296:1735‐41.
Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: Cohort study using the General Practice Research Database. Eur Heart J 2010;31:1000‐6.
Mehta NN, Li R, Krishnamoorthy P, Yu Y, Farver W, Rodrigues A, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis 2012;224:218‐21.
Harrington CL, Dey AK, Yunus R, Joshi AA, Mehta NN. Psoriasis as a human model of disease to study inflammatory atherogenesis. Am J Physiol Heart Circ Physiol 2017;312:H867‐73.
Maki-Petaja KM, Elkhawad M, Cheriyan J, Joshi FR, Ostor AJ, Hall FC, et al. Anti-tumor necrosis factor-alpha therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation 2012;126:2473‐80.
Kim BS, Lee WK, Pak K, Han J, Kim GW, Kim HS, et al. Ustekinumab treatment is associated with decreased systemic and vascular inflammation in patients with moderate-to-severe psoriasis: Feasibility study using (18)F-fluorodeoxyglucose PET/CT. J Am Acad Dermatol 2019;80:1322‐31.
Dey AK, Joshi AA, Chaturvedi A, Lerman JB, Aberra TM, Rodante JA, et al. Association between skin and aortic vascular inflammation in patients with psoriasis: A case-cohort study using positron emission tomography/computed tomography. JAMA Cardiol 2017;2:1013‐8.
Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: A randomized placebo-controlled trial. Circ Cardiovasc Imaging 2018;11:e007394.
Bengel FM. Imaging of post-infarct inflammation: Moving forward toward clinical application. Circ Cardiovasc Imaging 2016;9:e004713.
Li X, Rosenkrans ZT, Wang J, Cai W. PET imaging of macrophages in cardiovascular diseases. Am J Transl Res 2020;12:1491‐514.
Tarkin JM, Joshi FR, Rudd JH. PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol 2014;11:443‐57.
Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014;383:705‐13.
Fox JJ, Strauss HW. One step closer to imaging vulnerable plaque in the coronary arteries. J Nucl Med 2009;50:497‐500.
Wu C, Li F, Niu G, Chen X. PET imaging of inflammation biomarkers. Theranostics 2013;3:448‐66.
Joseph P, Tawakol A. Imaging atherosclerosis with positron emission tomography. Eur Heart J 2016;37:2974‐80.
Acknowledgments
Special thanks to Cathy Kelly, University of Ottawa Heart Institute (UOHI) PET nursing and clinical staff and Linda Garrard for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
JW, WZ, JK, CAF, NCW, and SG have nothing to disclose. KEB is supported by a CIHR Fellowship Award. RdK reports grants, consulting fees, and license revenues from Jubilant DraxImage and uOttawa Heart Institute (UOHI), outside the submitted work. RSB reports grants and honoraria from Lantheus Medical Imaging, grants and honoraria from Jubilant DraxImage, and grants from GE Healthcare, outside the submitted work. RSB is UOHIs Vered Chair in Cardiology and uOttawa’s Distinguished Chair in CV Imaging Research. GD is Wesfarmers Chair in Cardiology at University of Western Australia with an Adjunct Professor appointment at UOHI. He has received honoraria from Amgen, Pfizer, and Artrya Ltd and has an equity interest in Artrya Pty Ltd, all outside the submitted work. The study was performed at UOHI.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Funding Open Access funding enabled and organized by CAUL and its Member Institutions. This study was supported by the Canadian Institutes of Health Research (CIHR) grant. KEB is supported by a CIHR Fellowship Award. RSBB was a Career Investigator supported by the Heart and Stroke Foundation of Ontario, the UOHI Vered Chair of Cardiology, and a University of Ottawa Distinguished Chair of Cardiovascular Imaging Research. GD was supported by a CIHR new investigator salary support award (supported by OHTN) while at UOHI.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boczar, K.E., Beanlands, R.S., Glassman, S.J. et al. Anti-inflammatory effect of biologic therapy in patients with psoriatic disease: A prospective cohort FDG PET study. J. Nucl. Cardiol. 30, 1642–1652 (2023). https://doi.org/10.1007/s12350-023-03204-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-023-03204-8