Abstract
Calcific aortic valve disease is the most common valvular disease and confers significant morbidity and mortality. There are currently no medical therapies that successfully halt or reverse the disease progression, making surgical replacement the only treatment currently available. The majority of patients will receive a bioprosthetic valve, which themselves are prone to degeneration and may also need replaced, adding to the already substantial healthcare burden of aortic stenosis. Echocardiography and computed tomography can identify late-stage manifestations of the disease process affecting native and bioprosthetic aortic valves but cannot detect or quantify early molecular changes. 18F-fluoride positron emission tomography, on the other hand, can non-invasively and sensitively assess disease activity in the valves. The current review outlines the pivotal role this novel molecular imaging technique has played in improving our understanding of native and bioprosthetic aortic valve disease, as well as providing insights into its feasibility as an important future research and clinical tool.
Similar content being viewed by others
Introduction
Aortic stenosis affects 1-2% of the general population >65 years old, conferring with it increased mortality.1,2 Current international guidance recommends that the aortic valve should be replacement in those with severe, symptomatic aortic stenosis; a procedure which carries morbidity and significant cost.1,3,4,5 Replacement with a bioprosthetic valve is recommended in those over 65-70 years old, with mechanical valves preferred in the minority of younger patients.6 Bioprosthetic valves are beneficial in that they do not require the life-long anticoagulation that comes with mechanical valve implantation; however, they are prone to degradation over a relatively short time-span of ~15 years.6 Medical therapies that prevent progression of native or bioprosthetic aortic valve disease would represent a significant step in the management of these patient groups, but have so far remained elusive. To develop successful targeted medical therapy, identification of the mechanisms driving the disease process is crucial. Conventional imaging with computed tomography (CT) or transthoracic echocardiography (TTE) can identify the structural and haemodynamic manifestations of valvular disease; however, they cannot provide information about the molecular processes underpinning valve disease. Molecular imaging, on the other hand, can identify and quantify disease activity non-invasively and has an emerging role in evaluating the efficacy of medical therapies in randomized controlled trials as well a possible clinical utility in the early detection of aggressive disease.7 The current review outlines the pivotal role molecular imaging has played in our understanding of disease mechanisms, as well as providing insights into its feasibility as an important future research and clinical tool in the setting of native and bioprosthetic aortic valve disease.
Disease of the Native Aortic Valve
The aortic valve is located at the junction between the left ventricle and aorta and functions to prevent backflow of blood into the left ventricle during diastole while allowing unimpaired systolic ejection. Anatomically, the aortic valve consists of three leaflets that are anchored to a crown-like anulus. During diastole, the high aortic pressures force the leaflets to coapt, closing the valve and forming three blood-filled sinuses; two of which contain coronary arteries and facilitate blood flow. The aortic leaflets are highly specialized structures that must be compliant enough to open without resistance to blood flow, while being strong enough to withstand the repeated mechanical stresses applied throughout the cardiac cycle. In aortic stenosis, diseased valve leaflets become stiff and lose compliance, increasing the intraventricular pressure required to generate the same flow across the valve. The current model of native aortic valve pathology is thought to involve an initiation phase, where initial valvular injury leads to inflammation, immune activation and initial calcium deposition, followed by a propagation phase, involving a damaging cycle of increasing calcification activity.8 Increasing haemodynamic resistance across the valve can be identified with transthoracic echocardiography (TTE), while end-stage structural valve disease can be seen as valvular calcification identified on computed tomography (CT). Both TTE and CT calcium scoring have been integrated into international guidelines to determine when to perform aortic valve replacement.3,4 However, by the time haemodynamic changes or overt calcific disease are seen on TTE or CT, these disease processes are already well under way. The development of a successful medical therapy will depend on identifying and understanding the details of the active disease processes leading to these structural and haemodynamic changes.
Calcification as the Driver of Disease in Native Aortic Valve Stenosis
Due to a strong overlap between risk factors for developing atherosclerosis and aortic stenosis, the lipid-lowering effect of statins was initially investigated as a means of slowing aortic stenosis progression. Frustratingly, multiple randomized controlled trials did not demonstrate a reduction in aortic valve velocity or calcium score progression over medium-term follow-up.7,9 While disappointing, the results also questioned the understood inflammatory mechanisms driving the process of aortic stenosis.
To explore the relative contributions of inflammatory and calcific processes to aortic stenosis in vivo, researchers performed hybrid positron emission tomography combined with computed tomography (PET/CT), comparing 18F-fluorodeoxygloucose, (18F-FGD), a non-specific marker of inflammation, and 18F-sodium fluoride (18F-NaF), which preferentially binds to developing microcalcification, in a cohort of patients with varying degrees of aortic stenosis. The results demonstrated that calcification activity dominated over inflammation in aortic stenosis, particularly in the latter stages of moderate or severe stenosis (Fig. 1).10 The reverse situation was observed in concomitant regions of atheroma, with inflammation predominating: an observation that perhaps explains the differential effects of statins in these two conditions.11 Importantly, the anatomical pattern of 18F-NaF uptake at baseline was different to the presence of baseline calcium on CT, confirming that these two modalities provide different information. However, this baseline 18F-NaF PET activity did predict where new regions of calcium on CT would develop after 1-2 years of follow-up (Fig. 2). Similar observations have been made in different cardiovascular conditions (e.g., mitral annular calcification and coronary atherosclerosis)12,13 supporting 18F-NaF PET as a marker of newly developing calcification beyond the resolution of CT and as a marker of disease activity in aortic stenosis. Overall in the population of 121 patients, baseline 18F-NaF closely predicted progression of aortic valve calcium score after 2 years (R = .80, P < .001) as well as clinical events (aortic valve replacement or death).14,15
Uptake of 18F-fluorodeoxyglucose (18F-FDG) and 18F-sodium fluoride (18F-NaF) according to the severity of aortic stenosis. Box plots show the median and interquartile ranges of the tissue-to-background ratios (TBR) for 18F-NaF (white boxes) and 18F-FDG (gray boxes) with whiskers to 1.5 interquartile range. Figure reproduced with permission from Dweck et al.10
Two patients with progressive calcific aortic valve disease. (Left) Baseline computed tomography (CT) images. (Middle) Fused positron emission tomography (PET)-CT images showing increased 18F-fluoride valvular uptake (red/yellow areas). (Right) Repeat CT scans after 14 months with progressive aortic valve calcium score and new macroscopic calcium (white areas) in a similar distribution to that of baseline PET uptake. 18F-NaF PET-CT, 18F-sodium fluoride; PET, positron emission tomography; CT, computed tomography; Ca Score, Calcium score in Agatston units; TBRmax, maximum tissue-to-background ratio
Accurately Measuring Aortic Valve 18F-Sodium Fluoride Uptake
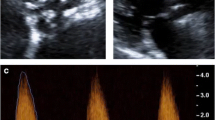
While the early studies described above demonstrate that 18F-NaF is a potentially useful biomarker, recent advances in imaging protocols have improved image quality and reproducibility of measurements, thereby enhancing this technique. A detailed description of standardized analysis techniques have been previously outlined.16,17 Utilization of contrast-enhanced computed tomography was an important advance, allowing accurate co-registration of the PET and CT scans by lining up the relatively high 18F-NaF activity in the blood pool compared to the myocardium with the left ventricular cavity visualized on contrast-enhanced CT in three orthogonal planes (Fig. 3). Moreover, this provides increased anatomical detail of the valve, allowing differentiating of 18F-NaF uptake in the valve from adjacent structures such as the aorta and coronary arteries. Motion correction techniques have proved similarly important. Motion of the valve throughout the cardiac cycle produces a wider distribution of PET signal, therefore, ECG-gating that captured the valve in diastole (50-75% RR interval) was employed to improve reproducibility.16,18 However, ECG-gating in this manner excludes 75% of potential data capture, increasing noise. Researchers have therefore developed a ‘motion correction’ algorithm that tracks the PET signal throughout each ECG gate and then collates all data to a single gate. This corrects for cardiac motion but does not involve data loss and therefore improves the signal-to-noise ratio (Fig. 4).19 Further iterations of this approach now allow additional correction for respiratory motion as well as bulk motion artifact.20 The final important advance has been in the approach to quantification. The ‘most diseased segment’ method, which averages the mean or maximum PET uptake values of the hottest two slices, precludes reproducibility issues associated with identifying the top and bottom slices of the valve.
Improved localization of positron emission tomography (PET) signal within the aortic valve and its leaflets. Paired non-contrast PET-computed tomography (CT) scans (left) and contrast-enhanced PET-CT images (right). Images demonstrate the typical distribution of the tracer uptake within the valve at sites of increased mechanical stress, that is, at the leaflet tips (top, blue arrow) and at the commissures (middle, blue arrows). Contrast enhancement also aids identification of valvular and peri-valvular uptake, for example in the coronary arteries (bottom, blue arrow). Scale bars represent standardized uptake values (SUV). PET, positron emission tomography; CT, computed tomography
Fused 18F-fluoride PET/contrast-enhanced MR angiograms of aortic valve of 60-year old man with aortic stenosis. Shown are original diastolic gate (A), summed image (B), and motion-corrected image (C) with focal 18F-fluoride uptake (arrows). This research was originally published in JNM. Doris et al.19 © SNMMI
When the above advances are combined, scan–rescan measurement error falls from >60% to 10%.16,18 With this degree of reproducibility, 18F-NaF becomes a crucial tool for elucidating novel insights in to the pathology of aortic stenosis and bioprosthetic valve degeneration as well as an efficacy endpoint in randomized controlled trials of novel therapies.
18F-Sodium Fluoride in Clinical Research
Using PET/CT 18F-sodium fluoride activity, the relationship between possible disease instigators and aortic valve disease can be investigated. Both lipoprotein(a) and oxidized phospholipids have been genetically linked to an increased incidence of aortic stenosis in the general population; however, whether these lipid markers are also associated with the propagation phase of aortic stenosis and faster disease progression remained uncertain.21,22,23 In a recent study of patients with calcific aortic valve disease, high levels of lipoprotein(a) or oxidized lipoprotein (the top tertile) were shown to be associated with higher baseline aortic valve 18F-NaF uptake, with this increased PET activity translating in to faster progression of the aortic valve CT calcium score, and more rapid progression of haemodynamic gradients on echocardiography.24 Raised oxidized phospholipids and lipoprotein(a) levels are therefore associated with an increased incidence and rate of progression in aortic stenosis and are therefore important potential therapeutic targets for patients with increased levels.24 Indeed, currently two randomized controlled trials are ongoing which assess the effect of niacin (NCT02109614) or PCSK9 inhibitors (NCT03051360) on lipoprotein(a) lowering and aortic stenosis progression; the latter using 18F-NaF uptake as a primary outcome.22
18F-Sodium Fluoride as a Clinical Tool in Native Aortic Valve Stenosis
Given the ability of 18F-NaF to measure disease activity and predict disease progression and clinical events in aortic stenosis, is there a role for this technique in clinical practice?25 The substantial costs and radiation exposure associated with 18F-NaF PET/CT means that it would need to provide incremental information to established echocardiographic and CT approaches. However, the studies reported to date have suggested that CT calcium scoring provides similar prognostic and predictive information to PET.15 In large part, this is due to the reasonably close association between baseline 18F-NaF PET activity and CT calcium scores. While at present this argues for wider use of CT and against routine 18F-NaF PET imaging, it does provide important pathophysiological insights. In particular, it suggests that calcium in the valve leaflets encourages further calcification activity and faster disease progression, perhaps via the increase in associated mechanical stresses within the valve. The result is a vicious cycle of progressive calcification that drives the propagation phase of aortic stenosis and suggests that calcification should be the predominant target for future therapies. Two ongoing randomized controlled trials are investigating this hypothesis, assessing the effect of denosumab or alendronate (SALTIRE II, NCT02132026) and vitamin K2 (BASIK 2, NCT02917525) on aortic stenosis progression (Table 1).2618F-NaF is being used as an efficacy endpoint in both trials.
Further prospective studies are now ongoing to assess whether 18F-NaF PET imaging using the latest protocols and image analysis approaches can provide the incremental predictive information that would support a more extensive clinical role.
Bioprosthetic Aortic Valve Degeneration
Bioprosthetic aortic valves are preferred in patients >65 years old because of reduced thrombogenicity and no requirement for long term anticoagulation. While there has been significant improvement in longevity since the introduction of bioprosthetic valves in the 1960s, the propensity towards structural deterioration over 10-20 years remains a major limitation to their use in younger patients.27,28 Typically, bioprosthetic valves are made of a covered frame with valve leaflets fashioned from either explanted porcine aortic valve or bovine pericardium. The pathological pathways underpinning bioprosthetic valve degradation are not fully understood, but are thought to involve microthrombus, pannus formation and excess mineralization consequent to plasminogen/fibrinogen, myofibroblast and macrophage-driven responses, respectively, quite different from those affecting the native valve.29,30,31,32 Structural valve degeneration (SVD) is defined as intrinsic leaflet deterioration associated with eventual haemodynamic dysfunction, and is categorized into four major stages: stage 0, no evidence of SVD; stage 1, SVD without significant haemodynamic changes (no/mild stenosis or regurgitation); stage 2, SVD with moderate stenosis or regurgitation; stage 3, SVD with severe stenosis or regurgitation, with re-intervention considered once symptoms develop in patients with severe disease.33 Standard assessment involves TTE (or transesophageal echocardiograph if TTE windows are poor) at baseline, one month after implantation and then annually to assess for changes in haemodynamic gradient.4,33,34 TTE/TEE is ideal for assessing haemodynamic changes and can identify gross leaflet abnormalities such as fluttering, thickening, or abnormal opening; however, echocardiography frequently only identifies end-stage valve degeneration with patients presenting in extremis and misses the earlier stage of its development. Computed tomography has been utilized to identify early and subtle changes on the valve which may not be seen on TTE/TEE, particularly thrombus and pannus formation, with hypoattenuated leaftlet thickening (HALT) seen in 4-7% of surgical bioprosthetic aortic valves at various periods after implantation.35,36 However, this imaging technique is also limited by imaging artifact related to the stent frame and motion in patients that frequently cannot receive beta-blockade. Moreover although CT can identify these changes, the clinical implications are not clear, with no difference in hemodynamic gradient or outcomes at one year in those with and without HALT.37 A biomarker which could sensitively detect the early stages of bioprosthetic degeneration and predict eventual deterioration would, therefore, fill an important area of clinical need.
As bioprosthetic valve leaflet calcification appears central to structural deterioration, researchers investigated the relationship between SVD and calcification ex vivo using explanted surgical bioprosthetic valves and 18F-NaF microPET/CT. Various pathological processes, including overt nodular calcification, thrombus, pannus and non-specific leaflet thickening were all visually highlighted by 18F-NaF, and correlated with pathology on histological staining (Fig. 5), suggesting calcification as a common endpoint for a variety of different triggers to bioprosthetic degeneration.38 In a prospective study, 80 patients recruited 1 month - 20 years after surgical bioprosthetic aortic valve replacement received baseline 18F-NaF PET/CT and TTE, with follow-up at 2 years. The same pathological processes were identified in vivo as seen in the explanted tissue (Fig. 6).38 Seventy one patients who had no echocardiographic evidence of valve dysfunction at baseline, 19% were found have pathological CT change, while a third had evidence of increased 18F-sodium fluoride uptake. Of the 67 who had available follow-up data, 10 (15%) developed new valve dysfunction, with two requiring urgent valve replacement and one death directly related to valve failure. Crucially, in multivariable linear regression analysis, baseline 18F-NaF was the only predictor of bioprosthetic valve dysfunction outperforming age, echocardiographic findings, CT findings, valve age and gender (Fig. 7). The patients who developed overt valve failure all had intense 18F-NaF baseline activity. 18F-NaF PET/CT therefore holds major promise for the early detection of bioprosthetic valve degeneration with potentially important clinical implications on the intensity of follow-up, and timing of replacement surgery for patients. Establishing the clinical role for 18F-NaF PET/CT in these patient groups will require validation in larger multicenter prospective studies.
(Row A) Macroscopic visual appearances of failed and explanted bioprosthetic valves. (Row B) CT en face images of the valves. (Row C) PET en face images demonstrating increased 18F-fluoride uptake in all valves. (Row D) Histology staining of sections taken from valve leaflet as indicated, with von Kossa (top row, calcium appears black), Movat Pentachrome (bottom row, valves 1 and 4), and hematoxylin and eosin (bottom row, valves 2 and 3) stains. All 4 degenerate bioprostheses demonstrate increased 18F-fluoride uptake in the valve leaflets. In valve 1, this uptake corresponds to gross leaflet calcification observed macroscopically and on CT images with confirmation on histology (extensive black staining). In valve 2, increased 18F-fluoride uptake is observed in association with fibrotic leaflet thickening and pannus (red arrows) with associated calcification (black arrows) observed macroscopically and on CT with confirmation on histology. In valve 3, increased 18F-fluoride uptake is observed at the site of valve leaflet thrombus (red arrow) observed macroscopically at the base of leaflet 1, with confirmation of thrombus (red arrow) and colocalized calcification (black arrow) on histology. In valve 4, extensive 18F-fluoride uptake is observed in the absence of calcification on CT and histology but instead in areas of leaflet thickening, marked fluid insudation, and disrupted collagen architecture. CT, computed tomography; PET, positron emission tomography. Adapted from Timothy et al.38 Under creative commons licence
Baseline CT (left) and 18F-fluoride PET (right) images from patients with bioprosthetic aortic valves. En face CT images of aortic bioprosthetic valves showing spotty calcification and large calcification (top left), circumferential pannus (bottom left), and noncalcific leaflet thickening suggestive of thrombus (top right) (all abnormalities identified by red arrows). Hybrid en face PET-CT images in the same patients: increased bioprosthetic 18F-fluoride activity (red/yellow areas) is observed in each patient colocalizing with the CT abnormalities. 18F-fluoride activity was also commonly observed remote from leaflet changes on CT (bottom right). Scale bars in the center of each pair of images represent standardized uptake values (SUV). Target-to-background (TBR) values are annotated on the hybrid PET-CT images in white text. Adapted from Timothy et al.38 Under creative commons licence
(A) A strong correlation was observed between baseline 18F-fluoride uptake in the bioprosthetic valves (TBR) and subsequent progression in bioprosthetic valve peak velocity (log transformation applied; r = 0.72; P < .001). Orange dots signify patients who developed new bioprosthetic valve regurgitation during follow-up. (B) 18F-fluoride uptake (dashed orange line represents threshold for increased 18F-fluoride uptake; TBR 1.3) in patients with different stages of structural valve degeneration after 2-year follow-up (stage 0: no significant change from post-implantation [n = 54]; stage 1: morphological abnormalities without significant hemodynamic changes [n = 9]; stage 2: new moderate stenosis and/or regurgitation [n = 5]; stage 3: new severe stenosis and/or severe regurgitation [n = 2]) demonstrating incrementally higher uptake values with increasing severity of structural valve degeneration. (C and D) Forest plots of unstandardized coefficients (95% confidence intervals) from a multivariable linear regression analysis predicting change in bioprosthetic valve function (annualized change in peak velocity) during follow-up. When examining all relevant baseline characteristics, 18F-fluoride uptake was the only independent predictor of hemodynamic deterioration in valve function when used both as a dichotomous variable (PET, TBR > 1.3) (C) and as a continuous variable (TBR) (D). CI, confidence interval. Adapted from Timothy et al.38 Under creative commons licence
Conclusions
Aortic stenosis and bioprosthetic aortic valve degeneration represent major health problems that, despite considerable research effort, currently lack effective preventative medical therapies. Calcification activity, as quantified non-invasively using 18F-NaF PET/CT, has been identified as the primary driver of both types of disease. Recent optimization of 18F-NaF PET has made it a sensitive and reproducible marker of aortic stenosis disease activity, providing important pathological insights and an efficacy endpoint in multiple ongoing randomized controlled trials. However, perhaps the most promising clinical translation for 18F-NaF PET lies in bioprosthetic aortic valve degeneration, where it can provide an early assessment of valve degeneration not currently offered by other imaging techniques.
Abbreviations
- 18F-NaF:
-
18F-sodium fluoride
- HALT:
-
Hypoattenuated leaflet thickening
- PET:
-
Positron emission tomography
- CT:
-
Computed tomography
- TAVI:
-
Transcatheter aortic valve implantation
- TTE:
-
Transthoracic echocardiography
- TEE:
-
Transesophageal echocardiography
References
Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: A population-based study. Lancet. 2006;368:1005-11.
Rezzoug N, Vaes B, de Meester C, Degryse J, Van Pottelbergh G, Mathei C, Adriaensen W, Pasquet A, Vanoverschelde JL. The clinical impact of valvular heart disease in a population-based cohort of subjects aged 80 and older. BMC Cardiovasc Disord. 2016;16:7.
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL and Group ESD. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739-91.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD, Members AATF. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440-92.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB and Subcommittee AHASCaSS. Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation. 2014;129:e28-e292.
Head SJ, Çelik M, Kappetein AP. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J. 2017;38:2183-91.
Thiago L, Tsuji SR, Nyong J, Puga ME, Gois AF, Macedo CR, Valente O and Atallah Á. Statins for aortic valve stenosis. Cochrane Database Syst Rev. 2016;9:CD009571.
Pawade TA, Newby DE, Dweck MR. Calcification in aortic stenosis: The skeleton key. J Am Coll Cardiol. 2015;66:561-77.
Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA and Scottish Aortic Stenosis and Lipid Lowering Trial IpoRSI. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389-97.
Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, Marsden M, Pessotto R, Clark JC, Wallace WA, Salter DM, McKillop G, van Beek EJ, Boon NA, Rudd JH, Newby DE. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125:76-86.
Dweck MR, Khaw HJ, Sng GK, Luo EL, Baird A, Williams MC, Makiello P, Mirsadraee S, Joshi NV, van Beek EJ, Boon NA, Rudd JH, Newby DE. Aortic stenosis, atherosclerosis, and skeletal bone: Is there a common link with calcification and inflammation? Eur Heart J. 2013;34:1567-74.
Massera D, Trivieri MG, Andrews JPM, Sartori S, Abgral R, Chapman AR, Jenkins WSA, Vesey AT, Doris MK, Pawade TA, Zheng KH, Kizer JR, Newby DE, Dweck MR. Disease activity in mitral annular calcification. Circ Cardiovasc Imaging. 2019;12:e008513.
Doris M, Moss AJ, Andrews JPM, Williams M, Van Beek EJR, Forsyth R, Dweck MR, Newby DE, Adamson PD. Coronary 18F-sodium fluoride uptake predicts progression of coronary arterial calcification. European Heart Journal. 2019. https://doi.org/10.1093/eurheartj/ehz747.0051.
Dweck MR, Jenkins WS, Vesey AT, Pringle MA, Chin CW, Malley TS, Cowie WJ, Tsampasian V, Richardson H, Fletcher A, Wallace WA, Pessotto R, van Beek EJ, Boon NA, Rudd JH, Newby DE. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging. 2014;7:371-8.
Jenkins WS, Vesey AT, Shah AS, Pawade TA, Chin CW, White AC, Fletcher A, Cartlidge TR, Mitchell AJ, Pringle MA, Brown OS, Pessotto R, McKillop G, Van Beek EJ, Boon NA, Rudd JH, Newby DE, Dweck MR. Valvular (18)F-fluoride and (18)F-fluorodeoxyglucose uptake predict disease progression and clinical outcome in patients with aortic stenosis. J Am Coll Cardiol. 2015;66:1200-1.
Pawade TA, Cartlidge TR, Jenkins WS, Adamson PD, Robson P, Lucatelli C, Van Beek EJ, Prendergast B, Denison AR, Forsyth L, Rudd JH, Fayad ZA, Fletcher A, Tuck S, Newby DE, Dweck MR. Optimization and reproducibility of aortic valve 18F-fluoride positron emission tomography in patients with aortic stenosis. Circ Cardiovasc Imaging. 2016;9(10):e005131.
Fletcher AJ, Singh T, Syed MBJ, Dweck MR. Imaging aortic valve calcification: Significance, approach and implications. Clin Radiol. 2020. https://doi.org/10.1016/j.crad.2020.04.007.
Massera D, Doris MK, Cadet S, Kwiecinski J, Pawade TA, Peeters FECM, Dey D, Newby DE, Dweck MR, Slomka PJ. Analytical quantification of aortic valve 18F-sodium fluoride PET uptake. J Nucl Cardiol. 2018;25(4):1148-55.
Doris MK, Rubeaux M, Pawade T, Otaki Y, Xie Y, Li D, Tamarappoo BK, Newby DE, Berman DS, Dweck MR, Slomka PJ, Dey D. Motion-corrected imaging of the aortic valve with 18F-sodium fluoride PET/CT and PET/MRI: A feasibility study. J Nucl Med. 2017;58:1811-4.
Lassen ML, Kwiecinski J, Dey D, Cadet S, Germano G, Berman DS, Adamson PD, Moss AJ, Dweck MR, Newby DE, Slomka PJ. Triple-gated motion and blood pool clearance corrections improve reproducibility of coronary. Eur J Nucl Med Mol Imaging. 2019;46:2610-20.
Capoulade R, Chan KL, Yeang C, Mathieu P, Bossé Y, Dumesnil JG, Tam JW, Teo KK, Mahmut A, Yang X, Witztum JL, Arsenault BJ, Després JP, Pibarot P, Tsimikas S. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66:1236-46.
Tsimikas S. Potential causality and emerging medical therapies for lipoprotein(a) and its associated oxidized phospholipids in calcific aortic valve stenosis. Circ Res. 2019;124:405-15.
Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, Malhotra R, O’Brien KD, Kamstrup PR, Nordestgaard BG, Tybjaerg-Hansen A, Allison MA, Aspelund T, Criqui MH, Heckbert SR, Hwang SJ, Liu Y, Sjogren M, van der Pals J, Kälsch H, Mühleisen TW, Nöthen MM, Cupples LA, Caslake M, Di Angelantonio E, Danesh J, Rotter JI, Sigurdsson S, Wong Q, Erbel R, Kathiresan S, Melander O, Gudnason V, O’Donnell CJ, Post WS and Group CECW. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503-12.
Zheng KH, Tsimikas S, Pawade T, Kroon J, Jenkins WSA, Doris MK, White AC, Timmers NKLM, Hjortnaes J, Rogers MA, Aikawa E, Arsenault BJ, Witztum JL, Newby DE, Koschinsky ML, Fayad ZA, Stroes ESG, Boekholdt SM, Dweck MR. Lipoprotein(a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J Am Coll Cardiol. 2019;73:2150-62.
Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, Joshi FR, Gallagher FA, Warburton EA, Bennett MR, Brindle KM, Newby DE, Rudd JH, Davenport AP. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495.
Doris MK, Everett RJ, Shun-Shin M, Clavel MA, Dweck MR. The role of imaging in measuring disease progression and assessing novel therapies in aortic stenosis. JACC Cardiovasc Imaging. 2019;12:185-97.
Nitsche C, Kammerlander AA, Knechtelsdorfer K, Kraiger JA, Goliasch G, Dona C, Schachner L, Öztürk B, Binder C, Duca F, Aschauer S, Zimpfer D, Bonderman D, Hengstenberg C, Mascherbauer J. Determinants of bioprosthetic aortic valve degeneration. JACC Cardiovasc Imaging. 2020;13:345-53.
Stassano P, Di Tommaso L, Monaco M, Iorio F, Pepino P, Spampinato N, Vosa C. Aortic valve replacement: A prospective randomized evaluation of mechanical versus biological valves in patients ages 55 to 70 years. J Am Coll Cardiol. 2009;54:1862-8.
Sakaue T, Nakaoka H, Shikata F, Aono J, Kurata M, Uetani T, Hamaguchi M, Kojima A, Uchita S, Yasugi T, Higashi H, Suzuki J, Ikeda S, Higaki J, Higashiyama S, Izutani H. Biochemical and histological evidence of deteriorated bioprosthetic valve leaflets: The accumulation of fibrinogen and plasminogen. Biol Open. 2018. https://doi.org/10.1242/bio.034009.
Siddiqui RF, Abraham JR, Butany J. Bioprosthetic heart valves: Modes of failure. Histopathology. 2009;55:135-44.
Rosseel L, De Backer O, Søndergaard L. Clinical valve thrombosis and subclinical leaflet thrombosis following transcatheter aortic valve replacement: Is there a need for a patient-tailored antithrombotic therapy? Front Cardiovasc Med. 2019;6:44.
Vasin SL, Rosanova IB, Sevastianov VI. The role of proteins in the nucleation and formation of calcium-containing deposits on biomaterial surfaces. J Biomed Mater Res. 1998;39:491-7.
Dvir D, Bourguignon T, Otto CM, Hahn RT, Rosenhek R, Webb JG, Treede H, Sarano ME, Feldman T, Wijeysundera HC, Topilsky Y, Aupart M, Reardon MJ, Mackensen GB, Szeto WY, Kornowski R, Gammie JS, Yoganathan AP, Arbel Y, Borger MA, Simonato M, Reisman M, Makkar RR, Abizaid A, McCabe JM, Dahle G, Aldea GS, Leipsic J, Pibarot P, Moat NE, Mack MJ, Kappetein AP, Leon MB and Investigators VViVID. Standardized Definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018;137:388-399.
Lancellotti P, Pibarot P, Chambers J, Edvardsen T, Delgado V, Dulgheru R, Pepi M, Cosyns B, Dweck MR, Garbi M, Magne J, Nieman K, Rosenhek R, Bernard A, Lowenstein J, Vieira ML, Rabischoffsky A, Vyhmeister RH, Zhou X, Zhang Y, Zamorano JL, Habib G. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:589-90.
Makkar RR, Fontana G, Jilaihawi H, Chakravarty T, Kofoed KF, De Backer O, Asch FM, Ruiz CE, Olsen NT, Trento A, Friedman J, Berman D, Cheng W, Kashif M, Jelnin V, Kliger CA, Guo H, Pichard AD, Weissman NJ, Kapadia S, Manasse E, Bhatt DL, Leon MB, Søndergaard L. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med. 2015;373:2015-24.
Leetmaa T, Hansson NC, Leipsic J, Jensen K, Poulsen SH, Andersen HR, Jensen JM, Webb J, Blanke P, Tang M, Nørgaard BL. Early aortic transcatheter heart valve thrombosis: Diagnostic value of contrast-enhanced multidetector computed tomography. Circ Cardiovasc Interv. 2015;8(4):e001596.
Khan JM, Rogers T, Waksman R, Torguson R, Weissman G, Medvedofsky D, Craig PE, Zhang C, Gordon P, Ehsan A, Wilson SR, Goncalves J, Levitt R, Hahn C, Parikh P, Bilfinger T, Butzel D, Buchanan S, Hanna N, Garrett R, Shults C, Garcia-Garcia HM, Kolm P, Satler LF, Buchbinder M, Ben-Dor I, Asch FM. Hemodynamics and subclinical leaflet thrombosis in low-risk patients undergoing transcatheter aortic valve replacement. Circ Cardiovasc Imaging. 2019;12:e009608.
Cartlidge TRG, Doris MK, Sellers SL, Pawade TA, White AC, Pessotto R, Kwiecinski J, Fletcher A, Alcaide C, Lucatelli C, Densem C, Rudd JHF, van Beek EJR, Tavares A, Virmani R, Berman D, Leipsic JA, Newby DE, Dweck MR. Detection and prediction of bioprosthetic aortic valve degeneration. J Am Coll Cardiol. 2019;73:1107-19.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fletcher, A.J., Dweck, M.R. Detecting native and bioprosthetic aortic valve disease using 18F-sodium fluoride: Clinical implications. J. Nucl. Cardiol. 28, 481–491 (2021). https://doi.org/10.1007/s12350-020-02411-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-020-02411-x