Abstract
Quantitative analysis has been applied extensively to image processing and interpretation in nuclear cardiology to improve disease diagnosis and risk stratification. This is Part 2 of a two-part continuing medical education article, which will review the potential clinical role for emerging quantitative analysis tools. The article will describe advanced methods for quantifying dyssynchrony, ventricular function and perfusion, and hybrid imaging analysis. This article discusses evolving methods to measure myocardial blood flow with positron emission tomography and single-photon emission computed tomography. Novel quantitative assessments of myocardial viability, microcalcification and in patients with cardiac sarcoidosis and cardiac amyloidosis will also be described. Lastly, we will review the potential role for artificial intelligence to improve image analysis, disease diagnosis, and risk prediction. The potential clinical role for all these novel techniques will be highlighted as well as methods to optimize their implementation.







Similar content being viewed by others
References
Garcia EV, Slomka P, Moody JB, Germano G, Ficaro EP. Quantitative clinical nuclear cardiology, part 1: Established applications. J Nucl Med 2019;60:1507-16.
Garcia EV, Slomka P, Moody JB, Germano G, Ficaro EP. Quantitative clinical nuclear cardiology, Part 1: Established applications. J Nucl Cardiol 2020;27:189-201.
Xu Y, Hayes S, Ali I, Ruddy TD, Wells RG, Berman DS, et al. Automatic and visual reproducibility of perfusion and function measures for myocardial perfusion SPECT. J Nucl Cardiol 2010;17:1050-7.
Otaki Y, Betancur J, Sharir T, Hu L-H, Gransar H, Liang JX, et al. 5-Year prognostic value of quantitative versus visual MPI in subtle perfusion defects. JACC Cardiovasc Imaging 2020;13:774-85.
Slomka PJ, Nishina H, Berman DS, Akincioglu C, Abidov A, Friedman JD, et al. Automatic quantification of myocardial perfusion stress-rest change: A new measure of ischemia. J Nucl Med 2004;45:183-91.
Azadani P, Miller RJH, Sharir T, Diniz MA, Hu L-H, Otaki Y, et al. Impact of early revascularization on major adverse cardiovascular events in relation to automatically quantified ischemia. JACC Cardiovasc Imaging 2020. https://doi.org/10.1016/j.jcmg.2020.05.039.
Slomka PJ, Cheng VY, Dey D, Woo J, Ramesh A, Kriekinge SV, et al. Quantitative analysis of myocardial perfusion SPECT anatomically guided by coregistered 64-slice coronary CT angiography. J Nucl Med 2009;50:1621-30.
Yoneyama H, Nakajima K, Taki J, Wakabayashi H, Matsuo S, Konishi T, et al. Ability of artificial intelligence to diagnose coronary artery stenosis using hybrid images of coronary computed tomography angiography and myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging 2019;46:S103-4.
Piccinelli M, Santana C, Sirineni GKR, Folks RD, Cooke CD, Arepalli CD, et al. Diagnostic performance of the quantification of myocardium at risk from MPI SPECT/CTA 2G fusion for detecting obstructive coronary disease: A multicenter trial. J Nucl Cardiol 2018;25:1376-86.
Piccinelli M, Cho SG, Garcia EV, Alexanderson E, Myung Lee J, Cooke CD, et al. Vessel-specific quantification of absolute myocardial blood flow, myocardial flow reserve and relative flow reserve by means of fused dynamic (13)NH3 PET and CCTA: Ranges in a low-risk population and abnormality criteria. J Nucl Cardiol 2018. https://doi.org/10.1007/s12350-018-01472-3.
Schaap J, de Groot JA, Nieman K, Meijboom WB, Boekholdt SM, Post MC, et al. Hybrid myocardial perfusion SPECT/CT coronary angiography and invasive coronary angiography in patients with stable angina pectoris lead to similar treatment decisions. Heart 2013;99:188-94.
Murthy VL, Bateman TM, Beanlands RS, Berman DS, Borges-Neto S, Chareonthaitawee P, et al. Clinical quantification of myocardial blood flow using PET: Joint position paper of the SNMMI Cardiovascular Council and the ASNC. J Nucl Cardiol 2018;25:269-97.
Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol 2013;62:1639-53.
Schindler TH, Dilsizian V. Coronary microvascular dysfunction: Clinical considerations and noninvasive diagnosis. JACC Cardiovasc Imaging 2020;13:140-55.
Moody JB, Lee BC, Corbett JR, Ficaro EP, Murthy VL. Precision and accuracy of clinical quantification of myocardial blood flow by dynamic PET: A technical perspective. J Nucl Cardiol 2015;22:935-51.
Lee BC, Moody JB, Poitrasson-Riviere A, Melvin AC, Weinberg RL, Corbett JR, et al. Blood pool and tissue phase patient motion effects on (82)rubidium PET myocardial blood flow quantification. J Nucl Cardiol 2019;26:1918-29.
Lee BC, Moody JB, Poitrasson-Riviere A, Melvin AC, Weinberg RL, Corbett JR, et al. Automated dynamic motion correction using normalized gradient fields for (82)rubidium PET myocardial blood flow quantification. J Nucl Cardiol 2018. https://doi.org/10.1007/s12350-018-01471.
Armstrong IS, Memmott MJ, Saint KJ, Saillant A, Hayden C, Arumugam P. Assessment of motion correction in dynamic rubidium-82 cardiac PET with and without frame-by-frame adjustment of attenuation maps for calculation of myocardial blood flow. J Nucl Cardiol 2019. https://doi.org/10.1007/s12350-019-01834-5.
Poitrasson-Riviere A, Moody JB, Hagio T, Weinberg RL, Corbett JR, Murthy VL, et al. Reducing motion-correction-induced variability in (82)rubidium myocardial blood-flow quantification. J Nucl Cardiol 2019;27:1104-13.
Klein R, Ocneanu A, Renaud JM, Ziadi MC, Beanlands RSB, deKemp RA. Consistent tracer administration profile improves test-retest repeatability of myocardial blood flow quantification with (82)Rb dynamic PET imaging. J Nucl Cardiol 2018;25:929-41.
Pieper J, Patel VN, Escolero S, Nelson JR, Poitrasson-Riviere A, Shreves CK, et al. Initial clinical experience of N13-ammonia myocardial perfusion PET/CT using a compact superconducting production system. J Nucl Cardiol 2019. https://doi.org/10.1007/s12350-019-01886-7.
Moody JB, Poitrasson-Rivière A, Hagio T, Buckley C, Weinberg RL, Corbett JR, et al. Added value of myocardial blood flow using 18F-flurpiridaz PET to diagnose coronary artery disease: The flurpiridaz 301 trial. J Nucl Cardiol 2020. https://doi.org/10.1007/s12350-020-02034-2.
Harms HJ, Knaapen P, de Haan S, Halbmeijer R, Lammertsma AA, Lubberink M. Automatic generation of absolute myocardial blood flow images using [15O]H2O and a clinical PET/CT scanner. Eur J Nucl Med Mol Imaging 2011;38:930-9.
Moody JB, Hiller KM, Lee BC, Poitrasson-Riviere A, Corbett JR, Weinberg RL, et al. The utility of (82)Rb PET for myocardial viability assessment: Comparison with perfusion-metabolism (82)Rb-(18)F-FDG PET. J Nucl Cardiol 2019;26:374-86.
Slomka P, Miller RJ, Hu L-H, Germano G, Berman D. Solid-state detector SPECT myocardial perfusion imaging. J Nucl Med 2019;60:1194-204.
Ben-Haim S, Murthy VL, Breault C, Allie R, Sitek A, Roth N, et al. Quantification of myocardial perfusion reserve using dynamic SPECT imaging in humans: A feasibility study. J Nucl Med 2013;54:873-9.
Otaki Y, Manabe O, Miller RJH, Manrique A, Nganoa C, Roth N, et al. Quantification of myocardial blood flow by CZT-SPECT with motion correction and comparison with (15)O-water PET. J Nucl Cardiol 2019. https://doi.org/10.1007/s12350-019-01854-1.
Wells RG, Marvin B, Poirier M, Renaud J, deKemp RA, Ruddy TD. Optimization of SPECT measurement of myocardial blood flow with corrections for attenuation, motion, and blood binding compared with PET. J Nucl Med 2017;58:2013-9.
Agostini D, Roule V, Nganoa C, Roth N, Baavour R, Parienti J-J, et al. First validation of myocardial flow reserve assessed by dynamic (99 m)Tc-sestamibi CZT-SPECT camera: head to head comparison with (15)O-water PET and fractional flow reserve in patients with suspected coronary artery disease. The WATERDAY study. Eur J Nucl Med Mol Imaging 2018;45:1079-90.
Giubbini R, Bertoli M, Durmo R, Bonacina M, Peli A, Faggiano I, et al. Comparison between N(13)NH(3)-PET and (99m)Tc-Tetrofosmin-CZT SPECT in the evaluation of absolute myocardial blood flow and flow reserve. J Nucl Cardiol 2019. https://doi.org/10.1007/s12350-019-01939-x.
Bailly M, Thibault F, Courtehoux M, Metrard G, Ribeiro MJ. Impact of attenuation correction for CZT-SPECT measurement of myocardial blood flow. J Nucl Cardiol 2020. https://doi.org/10.1007/s12350-020-02075-7.
Do J, Ruddy TD, Wells RG. Reduced acquisition times for measurement of myocardial blood flow with 99mTc-tetrofosmin and solid-state detector SPECT. J Nucl Cardiol 2020. https://doi.org/10.1007/s12350-020-02048-w.
Wells RG, Radonjic I, Clackdoyle D, Do J, Marvin B, Carey C, et al. Test-retest precision of myocardial blood flow measurements with 99mTc-tetrofosmin and solid-state detector single photon emission computed tomography. Circ Cardiovasc Imaging 2020;13:e009769.
Trimble MA, Borges-Neto S, Honeycutt EF, Shaw LK, Pagnanelli R, Chen J, et al. Evaluation of mechanical dyssynchrony and myocardial perfusion using phase analysis of gated SPECT imaging in patients with left ventricular dysfunction. J Nucl Cardiol 2008;15:663-70.
Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol 2005;12:687-95.
Nakajima K, Okuda K, Matsuo S, Kiso K, Kinuya S, Garcia EV. Comparison of phase dyssynchrony analysis using gated myocardial perfusion imaging with four software programs: Based on the Japanese Society of Nuclear Medicine working group normal database. J Nucl Cardiol 2017;24:611-21.
Folks RD, Cooke CD, Garcia EV. Optimizing gated myocardial perfusion imaging processing for phase analysis. J Nucl Cardiol 2016;23:1348-54.
Trimble MA, Velazquez EJ, Adams GL, Honeycutt EF, Pagnanelli RA, Barnhart HX, et al. Repeatability and reproducibility of phase analysis of gated single-photon emission computed tomography myocardial perfusion imaging used to quantify cardiac dyssynchrony. Nucl Med Commun 2008;29:374-81.
Lin X, Xu H, Zhao X, Folks RD, Garcia EV, Soman P, et al. Repeatability of left ventricular dyssynchrony and function parameters in serial gated myocardial perfusion SPECT studies. J Nucl Cardiol 2010;17:811-6.
Henneman MM, Chen J, Ypenburg C, Dibbets P, Bleeker GB, Boersma E, et al. Phase analysis of gated myocardial perfusion single-photon emission computed tomography compared with tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol 2007;49:1708-14.
Henneman MM, Chen J, Dibbets-Schneider P, Stokkel MP, Bleeker GB, Ypenburg C, et al. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med 2007;48:1104-11.
Friehling M, Chen J, Saba S, Bazaz R, Schwartzman D, Adelstein EC, et al. A prospective pilot study to evaluate the relationship between acute change in left ventricular synchrony after cardiac resynchronization therapy and patient outcome using a single-injection gated SPECT protocol. Circ Cardiovasc Imaging 2011;4:532-9.
Peix A, Karthikeyan G, Massardo T, Kalaivani M, Patel C, Pabon LM, et al. Value of intraventricular dyssynchrony assessment by gated-SPECT myocardial perfusion imaging in the management of heart failure patients undergoing cardiac resynchronization therapy (VISION-CRT). J Nucl Cardiol 2019. https://doi.org/10.1007/s12350-018-01589-5.
Boogers MJ, Chen J, van Bommel RJ, Borleggs CJW, Dibbets-Schneider P, van der Hiel B, et al. Optimal left ventricular lead position assessed with phase analysis on gated myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging 2011;38:230-8.
Muchtar E, Blauwet LA, Gertz MA. Restrictive cardiomyopathy: Genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res 2017;121:819-37.
Slart R, Glaudemans A, Lancellotti P, Hyafil F, Blankstein R, Schwartz RG, et al. A joint procedural position statement on imaging in cardiac sarcoidosis: From the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. J Nucl Cardiol 2018;25:298-319.
Youssef G, Leung E, Mylonas I, Nery P, Williams K, Wisenberg G, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: A systematic review and metaanalysis including the Ontario experience. J Nucl Med 2012;53:241--8.
Miller RJH, Cadet S, Pournazari P, Pope A, Kransdorf E, Hamilton MA, et al. Quantitative assessment of cardiac hypermetabolism and perfusion for diagnosis of cardiac sarcoidosis. J Nucl Cardiol 2020. https://doi.org/10.1007/s12350-020-02201-5.
Birnie D, Beanlands RSB, Nery P, Aaron SD, Culver DA, DeKemp RA, et al. Cardiac Sarcoidosis multi-center randomized controlled trial (CHASM CS- RCT). Am Heart J 2020;220:246-52.
Ahmadian A, Brogan A, Berman J, Sverdlov AL, Mercier G, Mazzini M, et al. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol 2014;21:925-39.
Osborne MT, Hulten EA, Singh A, Waller AH, Bittencourt MS, Stewart GC, et al. Reduction in (1)(8)F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol 2014;21:166-74.
Sperry BW, Tamarappoo BK, Oldan JD, Javed O, Culver DA, Brunken R, et al. Prognostic impact of extent, severity, and heterogeneity of abnormalities on (18)F-FDG PET scans for suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 2018;11:336-45.
Bokhari S, Morgenstern R, Weinberg R, Kinkhabwala M, Panagiotou D, Castano A, et al. Standardization of (99m)Technetium pyrophosphate imaging methodology to diagnose TTR cardiac amyloidosis. J Nucl Cardiol 2018;25:181-90.
Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R, et al. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: Predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol. 2016;1:880-9.
Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404-12.
Sperry BW, Vranian MN, Tower-Rader A, Hachamovitch R, Hanna M, Brunken R, et al. Regional variation in technetium pyrophosphate uptake in transthyretin cardiac amyloidosis and impact on mortality. JACC Cardiovasc Imaging 2018;11:234-42.
Morgenstern R, Yeh R, Castano A, Maurer MS, Bokhari S. (18)Fluorine sodium fluoride positron emission tomography, a potential biomarker of transthyretin cardiac amyloidosis. J Nucl Cardiol 2018;25:1559-67.
Dweck MR, Chow MW, Joshi NV, Williams MC, Jones C, Fletcher AM, et al. Coronary arterial 18F-sodium fluoride uptake: A novel marker of plaque biology. J Am Coll Cardiol 2012;59:1539-48.
Kwiecinski J, Adamson PD, Lassen ML, Doris MK, Moss AJ, Cadet S, et al. Feasibility of coronary (18)F-sodium fluoride positron-emission tomography assessment with the utilization of previously acquired computed tomography angiography. Circ Cardiovasc Imaging 2018;11:e008325.
Lassen ML, Kwiecinski J, Dey D, Cadet S, Germano G, Berman DS, et al. Triple-gated motion and blood pool clearance corrections improve reproducibility of coronary (18)F-NaF PET. Eur J Nucl Med Mol Imaging 2019;46:2610-20.
Lassen ML, Kwiecinski J, Cadet S, Dey D, Wang C, Dweck MR, et al. Data-driven gross patient motion detection and compensation: implications for coronary (18)F-NaF PET imaging. J Nucl Med 2019;60:830-6.
Kwiecinski J, Tzolos E, Cadet S, Adamson PD, Moss A, Joshi NV, et al. 18F-sodium fluoride coronary uptake predicts myocardial infarctions in patients with known coronary artery disease. J Am Coll Cardiol 2020;75:3667.
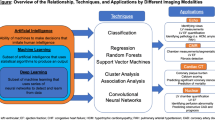
Slomka PJ, Miller RJ, Isgum I, Dey D. Application and translation of artificial intelligence to cardiovascular imaging in nuclear medicine and noncontrast CT. Semin Nucl Med 2020;50:357-66.
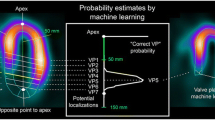
Betancur J, Rubeaux M, Fuchs TA, Otaki Y, Arnson Y, Slipczuk L, et al. Automatic valve plane localization in myocardial perfusion SPECT/CT by machine learning: Anatomic and clinical validation. J Nucl Med 2017;58:961-7.
Wang T, Lei Y, Tang H, He Z, Castillo R, Wang C, et al. A learning-based automatic segmentation and quantification method on left ventricle in gated myocardial perfusion SPECT imaging: A feasibility study. J Nucl Cardiol 2019;27:976-87.
Shiri I, Amir Mozafari Sabet K, Arabi H, Pourkeshavarz M, Teimourian B, Ay MR, et al. Standard SPECT myocardial perfusion estimation from half-time acquisitions using deep convolutional residual neural networks. J Nucl Cardiol 2020. https://doi.org/10.1007/s12350-020-02119-y.
Arsanjani R, Xu Y, Dey D, Vahistha V, Shalev A, Nakanishi R, et al. Improved accuracy of myocardial perfusion SPECT for the detection of coronary artery disease using a support vector machine algorithm. J Nucl Med 2013;54:549-55.
Slomka PJ, Betancur J, Liang JX, Otaki Y, Hu L-H, Sharir T, et al. Rationale and design of the REgistry of fast myocardial perfusion imaging with NExt generation SPECT (REFINE SPECT). J Nucl Cardiol 2018. https://doi.org/10.1007/s12350-018-1326-4.
Betancur J, Commandeur F, Motlagh M, Sharir T, Einstein AJ, Bokhari S, et al. Deep learning for prediction of obstructive disease from fast myocardial perfusion SPECT: A multicenter study. JACC Cardiovasc Imaging 2018;11:1654-63.
Betancur J, Hu L-H, Commandeur F, Sharir T, Einstein AJ, Fish MB, et al. deep learning analysis of upright-supine high-efficiency SPECT myocardial perfusion imaging for prediction of obstructive coronary artery disease: A multicenter study. J Nucl Med 2019;60:664-70.
Spier N, Nekolla S, Rupprecht C, Mustafa M, Navab N, Baust M. Classification of polar maps from cardiac perfusion imaging with graph-convolutional neural networks. Sci Rep 2019;9:7569.
Betancur J, Otaki Y, Motwani M, Fish MB, Lemley M, Dey D, et al. Prognostic value of combined clinical and myocardial perfusion imaging data using machine learning. JACC Cardiovasc Imaging 2018;11:1000-9.
Arsanjani R, Dey D, Khachatryan T, Shalev A, Hayes SW, Fish M, et al. Prediction of revascularization after myocardial perfusion SPECT by machine learning in a large population. J Nucl Cardiol 2015;22:877-84.
Hu LH, Betancur J, Sharir T, Einstein AJ, Bokhari S, Fish MB, et al. Machine learning predicts per-vessel early coronary revascularization after fast myocardial perfusion SPECT. Eur Heart J Cardiovasc Imaging 2020;21:549-59.
Garcia EV, Klein JL, Moncayo V, Cooke CD, Del’Aune C, Folks R, et al. Diagnostic performance of an artificial intelligence-driven cardiac-structured reporting system for myocardial perfusion SPECT imaging. J Nucl Cardiol 2018. https://doi.org/10.1007/s12350-018-1432-3.
Hu LH, Miller RJH, Sharir T, Commandeur F, Rios R, Einstein AJ, et al. Prognostically safe stress-only SPECT myocardial perfusion imaging guided by machine learning: Report from REFINE SPECT. Eur Heart J Cardiovasc Imaging 2020. https://doi.org/10.1093/ehjci/jeaa134.
Prasad M, Slomka PJ, Fish M, Kavanagh P, Gerlach J, Hayes S, et al. Improved quantification and normal limits for myocardial perfusion stress-rest change. J Nucl Med 2010;51:204-9.
Kwiecinski J, Cadet S, Daghem M, Lassen ML, Dey D, Dweck MR, et al. Whole-vessel coronary (18)F-sodium fluoride PET for assessment of the global coronary microcalcification burden. Eur J Nucl Med Mol Imaging 2020;47:1736-45.
Disclosures
Dr. Slomka receives grants from the National Institutes of Health and Siemens Medical Systems and receives software royalties from Cedars-Sinai Medical Center. Dr. Moody, Ms. Renaud, and Dr. Ficaro are employees of INVIA. Ms. Renaud is a consultant to Jubilant DraxImage Inc. and receives royalties from the sales of FlowQuant® software. Dr. Miller has no relevant disclosures. Dr. Garcia has a grant from the National Institutes of Health and receives royalties from and owns an equity position with Syntermed. He is also a consultant for GE Healthcare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is being jointly published in The Journal of Nuclear Medicine (https://doi.org/10.2967/jnumed.120.242537) and the Journal of Nuclear Cardiology (https://doi.org/10.1007/s12350-020-02337-4).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Slomka, P.J., Moody, J.B., Miller, R.J.H. et al. Quantitative clinical nuclear cardiology, part 2: Evolving/emerging applications. J. Nucl. Cardiol. 28, 115–127 (2021). https://doi.org/10.1007/s12350-020-02337-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-020-02337-4