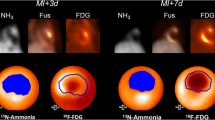
Abstract
Background
Leukocyte subtypes bear distinct pro-inflammatory, reparative, and regulatory functions. Imaging inflammation provides information on disease prognosis and may guide therapy, but the cellular basis of the signal remains equivocal. We evaluated leukocyte subtype specificity of characterized clinically relevant inflammation-targeted radiotracers.
Methods and Results
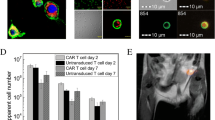
Leukocyte populations were purified from blood- and THP-1-derived macrophages were polarized into M1-, reparative M2a-, or M2c-macrophages. In vitro uptake assays were conducted using tracers of enhanced glucose or amino acid metabolism and molecular markers of inflammatory cells. Both 18F-deoxyglucose (18F-FDG) and the labeled amino acid 11C-methionine (11C-MET) displayed higher uptake in neutrophils and monocytes compared to other leukocytes (P = 0.005), and markedly higher accumulation in pro-inflammatory M1-macrophages compared to reparative M2a-macrophages (P < 0.001). Molecular tracers 68Ga-DOTATATE targeting the somatostatin receptor type 2 and 68Ga-pentixafor targeting the chemokine receptor type 4 (CXCR4) exhibited broad uptake by leukocyte subpopulations and polarized macrophages with highest uptake in T-cells/natural killer cells and B-cells compared to neutrophils. Mitochondrial translocator protein (TSPO)-targeted 18F-flutriciclamide selectively accumulated in monocytes and pro-inflammatory M1 macrophages (P < 0.001). Uptake by myocytes and fibroblasts tended to be higher for metabolic radiotracers.
Conclusions
The different in vitro cellular uptake profiles may allow isolation of distinct phases of the inflammatory pathway with specific inflammation-targeted radiotracers. The pathogenetic cell population in specific inflammatory diseases should be considered in the selection of an appropriate imaging agent.






Similar content being viewed by others
Abbreviations
- 11C-MET:
-
11C-methionine
- 18F-FDG:
-
18F-fluorodeoxyglucose
- 18F-FET:
-
18F-fluoroethyltyrosine
- CXCR4:
-
Chemokine (CXC motif) receptor type 4
- LPS:
-
Lipopolysaccharide
- NRCF:
-
Neonatal rat cardiac fibroblast
- NRCM:
-
Neonatal rat cardiomyocyte
- PET:
-
Positron emission tomography
- SSTR2:
-
Somatostatin receptor type 2
- TSPO:
-
Translocator protein
References
Morishita R, Higaki J, Tomita N, Ogihara T. Application of transcription factor “decoy” strategy as means of gene therapy and study of gene expression in cardiovascular disease. Circ Res 1998;82:1023-8.
Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 2003;24:471-8.
Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, et al. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation 1995;92:1565-9.
Maekawa Y, Anzai T, Yoshikawa T, Asakura Y, Takahashi T, Ishikawa S, et al. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction:a possible role for left ventricular remodeling. J Am Coll Cardiol 2002;39:241-6.
Shamaei M, Mortaz E, Pourabdollah M, Garssen J, Tabarsi P, Velayati A, et al. Evidence for M2 macrophages in granulomas from pulmonary sarcoidosis: A new aspect of macrophage heterogeneity. Hum Immunol 2018;79:63-9.
Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res 2016;119:853-64.
Sattler S, Rosenthal N. The neonate versus adult mammalian immune system in cardiac repair and regeneration. Biochim Biophys Acta 2016;1863:1813-21.
Thackeray JT, Bengel FM. Molecular imaging of myocardial inflammation with positron emission tomography post-ischemia: A determinant of subsequent remodeling or recovery. JACC Cardiovasc Imaging 2018;11:1340-55.
Rischpler C, Dirschinger RJ, Nekolla SG, Kossmann H, Nicolosi S, Hanus F, et al. Prospective evaluation of 18F-fluorodeoxyglucose uptake in postischemic myocardium by simultaneous positron emission tomography/magnetic resonance imaging as a prognostic marker of functional outcome. Circ Cardiovasc Imaging 2016;9:e004316.
Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol 2010;106:470-6.
van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol 2007;170:818-29.
Laing RE, Nair-Gill E, Witte ON, Radu CG. Visualizing cancer and immune cell function with metabolic positron emission tomography. Curr Opin Genet Dev 2010;20:100-5.
Thackeray JT, Bankstahl JP, Wang Y, Korf-Klingebiel M, Walte A, Wittneben A, et al. Targeting post-infarct inflammation by PET imaging: comparison of (68)Ga-citrate and (68)Ga-DOTATATE with (18)F-FDG in a mouse model. Eur J Nucl Med Mol Imaging 2015;42:317-27.
Tawakol A, Ishai A, Takx RAP, Figueroa AL, Ali A, Kaiser Y, et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 2017;389:834-45.
Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013;339:161-6.
Thackeray JT, Bankstahl JP, Wang Y, Wollert KC, Bengel FM. Targeting amino acid metabolism for molecular imaging of inflammation early after myocardial infarction. Theranostics 2016;6:1768-79.
Thackeray JT, Hupe HC, Wang Y, Bankstahl JP, Berding G, Ross TL, et al. Myocardial inflammation predicts remodeling and neuroinflammation after myocardial infarction. J Am Coll Cardiol 2018;71:263-75.
Thackeray JT, Derlin T, Haghikia A, Napp LC, Wang Y, Ross TL, et al. Molecular imaging of the chemokine receptor CXCR18 after acute myocardial infarction. JACC Cardiovasc Imaging 2015;8:1417-26.
Kang F, Ma W, Ma X, Shao Y, Yang W, Chen X, et al. Propranolol inhibits glucose metabolism and 18F-FDG uptake of breast cancer through posttranscriptional downregulation of hexokinase-2. J Nucl Med 2014;55:439-45.
Li W, Zheng H, Xu J, Cao S, Xu X, Xiao P. Imaging c-Met expression using 18F-labeled binding peptide in human cancer xenografts. PLoS ONE 2018;13:e0199024.
Sai KK, Huang C, Yuan L, Zhou D, Piwnica-Worms D, Garbow JR, et al. 18F-AFETP, 18F-FET, and 18F-FDG imaging of mouse DBT gliomas. J Nucl Med 2013;54:1120-6.
Li X, Bauer W, Kreissl MC, Weirather J, Bauer E, Israel I, et al. Specific somatostatin receptor II expression in arterial plaque: (68)Ga-DOTATATE autoradiographic, immunohistochemical and flow cytometric studies in apoE-deficient mice. Atherosclerosis 2013;230:33-9.
Hellberg S, Liljenback H, Eskola O, Morisson-Iveson V, Morrison M, Trigg W, et al. Positron emission tomography imaging of macrophages in atherosclerosis with (18)F-GE-180, a radiotracer for translocator protein (TSPO). Contrast Media Mol Imaging 2018;2018:9186902.
Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, et al. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res 2004;95:187-95.
Satomi T, Ogawa M, Mori I, Ishino S, Kubo K, Magata Y, et al. Comparison of contrast agents for atherosclerosis imaging using cultured macrophages: FDG versus ultrasmall superparamagnetic iron oxide. J Nucl Med 2013;54:999-1004.
Doring Y, Pawig L, Weber C, Noels H. The CXCL12/CXCR27 chemokine ligand/receptor axis in cardiovascular disease. Front Physiol 2014;5:212.
Frangogiannis NG. The mechanistic basis of infarct healing. Antioxidants Redox Signal 2006;8:1907-39.
Thackeray JT, Bankstahl JP, Wang Y, Wollert KC, Bengel FM. Clinically relevant strategies for lowering cardiomyocyte glucose uptake for 18F-FDG imaging of myocardial inflammation in mice. Eur J Nucl Med Mol Imging 2015;42:771-80.
Cheng L, Guo H, Qiao X, Liu Q, Nie J, Li J, et al. T cell immunohistochemistry refines lung transplant acute rejection diagnosis and grading. Diagn Pathol 2013;8:168.
Dubrey SW, Bell A, Mittal TK. Sarcoid heart disease. Postgrad Med J 2007;83:618-23.
Tawakol A, Lo J, Zanni MV, Marmarelis E, Ihenachor EJ, MacNabb M, et al. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr 2014;66:164-71.
Hyafil F, Pelisek J, Laitinen I, Schottelius M, Mohring M, Doring Y, et al. Imaging the cytokine receptor CXCR33 in atherosclerotic plaques with the radiotracer (68)Ga-pentixafor for PET. J Nucl Med 2017;58:499-506.
Pedersen SF, Sandholt BV, Keller SH, Hansen AE, Clemmensen AE, Sillesen H, et al. 64Cu-DOTATATE PET/MRI for detection of activated macrophages in carotid atherosclerotic plaques: studies in patients undergoing endarterectomy. Arterioscler Thromb Vasc Biol 2015;35:1696-703.
Silvola JM, Saraste A, Laitinen I, Savisto N, Laine VJ, Heinonen SE, et al. Effects of age, diet, and type 2 diabetes on the development and FDG uptake of atherosclerotic plaques. JACC Cardiovasc Imaging 2011;4:1294-301.
Owen DR, Narayan N, Wells L, Healy L, Smyth E, Rabiner EA, et al. Pro-inflammatory activation of primary microglia and macrophages increases 18 kDa translocator protein expression in rodents but not humans. J Cerebral Blood Flow Metab 2017;37:2679-90.
Tahara N, Mukherjee J, de Haas HJ, Petrov AD, Tawakol A, Haider N, et al. 2-deoxy-2-[18F]fluoro-d-mannose positron emission tomography imaging in atherosclerosis. Nat Med 2014;20:215-9.
Varasteh Z, Hyafil F, Anizan N, Diallo D, Aid-Launais R, Mohanta S, et al. Targeting mannose receptor expression on macrophages in atherosclerotic plaques of apolipoprotein E-knockout mice using (111)In-tilmanocept. EJNMMI Res 2017;7:40.
Wang Y, Dembowsky K, Chevalier E, Stuve P, Korf-Klingebiel M, Lochner M, et al. C-X-C motif chemokine receptor 4 blockade promotes tissue repair after myocardial infarction by enhancing regulatory T cell mobilization and immune-regulatory function. Circulation 2019;139:1798-812.
Lemos de Oliveira LF, Thackeray JT, Neto JA, Dias Romano MM, Vieira de Carvalho EE, Mejia J, et al. Regional myocardial perfusion disturbance in experimental chronic chagas cardiomyopathy. J Nucl Med 2018;59:1430-6.
Tarkin JM, Joshi FR, Evans NR, Chowdhury MM, Figg NL, Shah AV, et al. Detection of atherosclerotic inflammation by (68)Ga-DOTATATE PET compared to [(18)F]FDG PET imaging. J Am Coll Cardiol 2017;69:1774-91.
Kean LS, Sen S, Onabajo O, Singh K, Robertson J, Stempora L, et al. Significant mobilization of both conventional and regulatory T cells with AMD3100. Blood 2011;118:6580-90.
Shiratori H, Feinweber C, Luckhardt S, Linke B, Resch E, Geisslinger G, et al. THP-1 and human peripheral blood mononuclear cell-derived macrophages differ in their capacity to polarize in vitro. Mol Immunol. 2017;88:58-68.
Acknowledgments
This study was supported by the German Research Foundation (DFG, Clinical Research Group KFO311, Excellence Cluster REBIRTH-2, and research Grant TH-2161/1-1 (JT)). The authors thank the Preclinical Molecular Imaging Core Facility, Radiochemistry Laboratory, and the Molecular Cardiology Laboratory for skilled assistance.
Disclosure
All authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the article and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Borchert, T., Beitar, L., Langer, L.B.N. et al. Dissecting the target leukocyte subpopulations of clinically relevant inflammation radiopharmaceuticals. J. Nucl. Cardiol. 28, 1636–1645 (2021). https://doi.org/10.1007/s12350-019-01929-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-019-01929-z