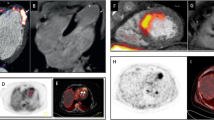
Abstract
Purpose of Review
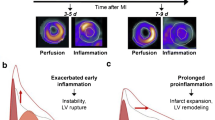
Local inflammation after myocardial infarction (MI) plays a role in subsequent ventricular remodeling, influences cardiac outcome, and has emerged as a therapeutic target. Preclinical and clinical PET imaging studies have employed a variety of radiotracers to target inflammatory leukocytes in the early stages after MI.
Recent Findings
Imaging of enhanced metabolism in activated macrophages with 18F-FDG is feasible and has been associated with cardiac outcome in a small prospective study. Novel targeted PET agents show higher specificity for inflammatory leukocytes and can identify therapeutic response with limited background.
Summary
While PET imaging of acute inflammation after MI has grown in recent years, significant challenges remain to widespread clinical application, including the complex cellular composition of the imaging signal and unclear association with functional outcome. Future studies must address the prognostic value of post-MI inflammation imaging and the ability to discern response to targeted, expensive, and personalized therapies.





Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17(5):581–8. https://doi.org/10.1038/nm.2354.
Jung K, Kim P, Leuschner F, Gorbatov R, Kim JK, Ueno T, et al. Endoscopic time-lapse imaging of immune cells in infarcted mouse hearts. Circ Res. 2013;112(6):891–9. https://doi.org/10.1161/CIRCRESAHA.111.300484.
Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127(20):2038–46. https://doi.org/10.1161/CIRCULATIONAHA.112.000116.
Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339(6116):161–6. https://doi.org/10.1126/science.1230719.
Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–64. https://doi.org/10.1038/nri1733.
Anzai T. Post-infarction inflammation and left ventricular remodeling- a double-edged sword. Circ J. 2013;77(3):580–7. https://doi.org/10.1253/circj.CJ-13-0013.
Gao XM, Xu Q, Kiriazis H, Dart AM, Du XJ. Mouse model of post-infarct ventricular rupture: time course, strain- and gender-dependency, tensile strength, and histopathology. Cardiovasc Res. 2005;65(2):469–77. https://doi.org/10.1016/j.cardiores.2004.10.014.
Kelle S, Roes SD, Klein C, Kokocinski T, de Roos A, Fleck E, et al. Prognostic value of myocardial infarct size and contractile reserve using magnetic resonance imaging. J Am Coll Cardiol. 2009;54(19):1770–7. https://doi.org/10.1016/j.jacc.2009.07.027.
Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55(15):1629–38. https://doi.org/10.1016/j.jacc.2009.08.089.
Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11(5):255–65. https://doi.org/10.1038/nrcardio.2014.28.
Barron HV, Harr SD, Radford MJ, Wang Y, Krumholz HM. The association between white blood cell count and acute myocardial infarction mortality in patients > or =65 years of age: findings from the cooperative cardiovascular project. J Am Coll Cardiol. 2001;38(6):1654–61. https://doi.org/10.1016/S0735-1097(01)01613-8.
Maekawa Y, Anzai T, Yoshikawa T, Asakura Y, Takahashi T, Ishikawa S, et al. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction:a possible role for left ventricular remodeling. J Am Coll Cardiol. 2002;39(2):241–6. https://doi.org/10.1016/S0735-1097(01)01721-1.
Ruparelia N, Digby JE, Jefferson A, Medway DJ, Neubauer S, Lygate CA, et al. Myocardial infarction causes inflammation and leukocyte recruitment at remote sites in the myocardium and in the renal glomerulus. Inflamm Res. 2013;62(5):515–25. https://doi.org/10.1007/s00011-013-0605-4.
Ammirati E, Cannistraci CV, Cristell NA, Vecchio V, Palini AG, Tornvall P, et al. Identification and predictive value of interleukin-6+ interleukin-10+ and interleukin-6- interleukin-10+ cytokine patterns in ST-elevation acute myocardial infarction. Circ Res. 2012;111(10):1336–48. https://doi.org/10.1161/CIRCRESAHA.111.262477.
Vanderheyden M, Kersschot E, Paulus WJ. Pro-inflammatory cytokines and endothelium-dependent vasodilation in the forearm. Serial assessment in patients with congestive heart failure. Eur Heart J. 1998;19(5):747–52. https://doi.org/10.1053/euhj.1997.0828.
Lee WW, Marinelli B, van der Laan AM, Sena BF, Gorbatov R, Leuschner F, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol. 2012;59(2):153–63. https://doi.org/10.1016/j.jacc.2011.08.066.
Thackeray JT, Bankstahl JP, Wang Y, Korf-Klingebiel M, Walte A, Wittneben A, et al. Targeting post-infarct inflammation by PET imaging: comparison of (68)Ga-citrate and (68)Ga-DOTATATE with (18)F-FDG in a mouse model. Eur J Nucl Med Mol Imaging. 2015;42(2):317–27. https://doi.org/10.1007/s00259-014-2884-6.
Wollenweber T, Roentgen P, Schafer A, Schatka I, Zwadlo C, Brunkhorst T, et al. Characterizing the inflammatory tissue response to acute myocardial infarction by clinical multimodality noninvasive imaging. Circ Cardiovasc Imaging. 2014;7(5):811–8. https://doi.org/10.1161/CIRCIMAGING.114.001689.
Morooka M, Moroi M, Uno K, Ito K, Wu J, Nakagawa T, et al. Long fasting is effective in inhibiting physiological myocardial 18F-FDG uptake and for evaluating active lesions of cardiac sarcoidosis. EJNMMI Res. 2014;4(1):1. https://doi.org/10.1186/2191-219X-4-1.
Minamimoto R, Morooka M, Kubota K, Ito K, Masuda-Miyata Y, Mitsumoto T, et al. Value of FDG-PET/CT using unfractionated heparin for managing primary cardiac lymphoma and several key findings. J Nucl Cardiol. 2011;18(3):516–20. https://doi.org/10.1007/s12350-011-9358-z.
Harisankar CN, Mittal BR, Agrawal KL, Abrar ML, Bhattacharya A. Utility of high fat and low carbohydrate diet in suppressing myocardial FDG uptake. J Nucl Cardiol. 2011;18(5):926–36. https://doi.org/10.1007/s12350-011-9422-8.
Prato FS, Butler J, Sykes J, Keenliside L, Blackwood KJ, Thompson RT, et al. Can the inflammatory response be evaluated using 18F-FDG within zones of microvascular obstruction after myocardial infarction? J Nucl Med. 2015;56(2):299–304. https://doi.org/10.2967/jnumed.114.147835.
Taki J, Wakabayashi H, Inaki A, Imanaka-Yoshida K, Hiroe M, Ogawa K, et al. 14C-methionine uptake as a potential marker of inflammatory processes after myocardial ischemia and reperfusion. J Nucl Med. 2013;54(3):431–6. https://doi.org/10.2967/jnumed.112.112060.
Thackeray JT, Bankstahl JP, Wang Y, Wollert KC, Bengel FM. Targeting amino acid metabolism for molecular imaging of inflammation early after myocardial infarction. Theranostics. 2016;6(11):1768–79. https://doi.org/10.7150/thno.15929.
Maya Y, Werner RA, Schutz C, Wakabayashi H, Samnick S, Lapa C, et al. 11C-methionine PET of myocardial inflammation in a rat model of experimental autoimmune myocarditis. J Nucl Med. 2016;57(12):1985–90. https://doi.org/10.2967/jnumed.116.174045.
Morooka M, Kubota K, Kadowaki H, Ito K, Okazaki O, Kashida M, et al. 11C-methionine PET of acute myocardial infarction. J Nucl Med. 2009;50(8):1283–7. https://doi.org/10.2967/jnumed.108.061341.
Tarkin JM, Joshi FR, Evans NR, Chowdhury MM, Figg NL, Shah AV, et al. Detection of atherosclerotic inflammation by 68Ga-DOTATATE PET compared to [18F]FDG PET imaging. J Am Coll Cardiol. 2017;69(14):1774–91. https://doi.org/10.1016/j.jacc.2017.01.060.
Li X, Bauer W, Kreissl MC, Weirather J, Bauer E, Israel I, et al. Specific somatostatin receptor II expression in arterial plaque: (68)Ga-DOTATATE autoradiographic, immunohistochemical and flow cytometric studies in apoE-deficient mice. Atherosclerosis. 2013;230(1):33–9. https://doi.org/10.1016/j.atherosclerosis.2013.06.018.
Lapa C, Reiter T, Li X, Werner RA, Samnick S, Jahns R, et al. Imaging of myocardial inflammation with somatostatin receptor based PET/CT - a comparison to cardiac MRI. Int J Cardiol. 2015;194:44–9. https://doi.org/10.1016/j.ijcard.2015.05.073.
Schatka I, Wollenweber T, Haense C, Brunz F, Gratz KF, Bengel FM. Peptide receptor targeted radionuclide therapy alters inflammation in atherosclerotic plaques. J Am Coll Cardiol. 2013;62(24):2344–5. https://doi.org/10.1016/j.jacc.2013.08.1624.
Jujo K, Hamada H, Iwakura A, Thorne T, Sekiguchi H, Clarke T, et al. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci U S A. 2010;107(24):11008–13. https://doi.org/10.1073/pnas.0914248107.
Herrmann K, Lapa C, Wester HJ, Schottelius M, Schiepers C, Eberlein U, et al. Biodistribution and radiation dosimetry for the chemokine receptor CXCR4-targeting probe 68Ga-pentixafor. J Nucl Med. 2015;56(3):410–6. https://doi.org/10.2967/jnumed.114.151647.
• Thackeray JT, Derlin T, Haghikia A, Napp LC, Wang Y, Ross TL, et al. Molecular Imaging of the Chemokine Receptor CXCR4 After Acute Myocardial Infarction. JACC Cardiovasc Imaging. 2015;8(12):1417–26. This translational study was the first to demonstrate selective accumulation of 68Ga-pentixafor by CXCR4-rich cells in the infarct territory in mice and humans. These results point to CXCR4 as a viable imaging target for a broad base of leukocytes to interrogate myocardial inflammation.
Lapa C, Reiter T, Werner RA, Ertl G, Wester HJ, Buck AK, et al. [(68)Ga]Pentixafor-PET/CT for imaging of chemokine receptor 4 expression after myocardial infarction. JACC Cardiovasc Imaging. 2015;8(12):1466–8. https://doi.org/10.1016/j.jcmg.2015.09.007.
Gaemperli O, Shalhoub J, Owen DR, Lamare F, Johansson S, Fouladi N, et al. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur Heart J. 2012;33(15):1902–10. https://doi.org/10.1093/eurheartj/ehr367.
Hellberg S, Silvola JMU, Kiugel M, Liljenback H, Savisto N, Li XG, et al. 18-kDa translocator protein ligand 18F-FEMPA: biodistribution and uptake into atherosclerotic plaques in mice. J Nucl Cardiol. 2017;24(3):862–71. https://doi.org/10.1007/s12350-016-0527-y.
Kashiyama N, Miyagawa S, Fukushima S, Kawamura T, Kawamura A, Yoshida S, et al. Development of PET imaging to visualize activated macrophages accumulated in the transplanted iPSc-derived cardiac myocytes of allogeneic origin for detecting the immune rejection of allogeneic cell transplants in mice. PLoS One. 2016;11(12):e0165748. https://doi.org/10.1371/journal.pone.0165748.
• Thackeray JT, Hupe HC, Wang Y, Bankstahl JP, Berding G, Ross TL, et al. Molecular imaging of the heart-brain axis: Post-infarct myocardial inflammation predicts subsequent remodelinig and triggers neuroinflammation. J Am Coll Cardiol. 2018;71:263–75. This study demonstrated that imaging of mitochondrial translocator protein early after infarction could positively predict the decline of contractile function in mice 8 weeks later. Moreover, it described the forward connection between heart and brain, whereby myocardial infarction leads to acute and chronic neuroinflammation.
Laitinen I, Saraste A, Weidl E, Poethko T, Weber AW, Nekolla SG, et al. Evaluation of alphavbeta3 integrin-targeted positron emission tomography tracer 18F-galacto-RGD for imaging of vascular inflammation in atherosclerotic mice. Circ Cardiovasc Imaging. 2009;2(4):331–8. https://doi.org/10.1161/CIRCIMAGING.108.846865.
Beer AJ, Pelisek J, Heider P, Saraste A, Reeps C, Metz S, et al. PET/CT imaging of integrin alphavbeta3 expression in human carotid atherosclerosis. JACC Cardiovasc Imaging. 2014;7(2):178–87. https://doi.org/10.1016/j.jcmg.2013.12.003.
van den Borne SW, Isobe S, Verjans JW, Petrov A, Lovhaug D, Li P, et al. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. J Am Coll Cardiol. 2008;52(24):2017–28. https://doi.org/10.1016/j.jacc.2008.07.067.
Wu C, Yue X, Lang L, Kiesewetter DO, Li F, Zhu Z, et al. Longitudinal PET imaging of muscular inflammation using 18F-DPA-714 and 18F-Alfatide II and differentiation with tumors. Theranostics. 2014;4(5):546–55. https://doi.org/10.7150/thno.8159.
Higuchi T, Bengel FM, Seidl S, Watzlowik P, Kessler H, Hegenloh R, et al. Assessment of alphavbeta3 integrin expression after myocardial infarction by positron emission tomography. Cardiovasc Res. 2008;78(2):395–403. https://doi.org/10.1093/cvr/cvn033.
Jenkins WS, Vesey AT, Stirrat C, Connell M, Lucatelli C, Neale A, et al. Cardiac alphaVbeta3 integrin expression following acute myocardial infarction in humans. Heart. 2017;103(8):607–15. https://doi.org/10.1136/heartjnl-2016-310115.
Keliher EJ, Ye YX, Wojtkiewicz GR, Aguirre AD, Tricot B, Senders ML, et al. Polyglucose nanoparticles with renal elimination and macrophage avidity facilitate PET imaging in ischaemic heart disease. Nat Commun. 2017;8:14064. https://doi.org/10.1038/ncomms14064.
Luehmann HP, Pressly ED, Detering L, Wang C, Pierce R, Woodard PK, et al. PET/CT imaging of chemokine receptor CCR5 in vascular injury model using targeted nanoparticle. J Nucl Med. 2014;55(4):629–34. https://doi.org/10.2967/jnumed.113.132001.
Caobelli F, Wollenweber T, Bavendiek U, Kuhn C, Schutze C, Geworski L, et al. Simultaneous dual-isotope solid-state detector SPECT for improved tracking of white blood cells in suspected endocarditis. Eur Heart J. 2017;38(6):436–43. https://doi.org/10.1093/eurheartj/ehw231.
Terrovitis J, Lautamaki R, Bonios M, Fox J, Engles JM, Yu J, et al. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol. 2009;54(17):1619–26. https://doi.org/10.1016/j.jacc.2009.04.097.
Kovacic JC, Fuster V. Cell therapy for patients with acute myocardial infarction: ACCRUEd evidence to date. Circ Res. 2015;116(8):1287–90. https://doi.org/10.1161/CIRCRESAHA.115.306323.
Thunemann M, Schorg BF, Feil S, Lin Y, Voelkl J, Golla M, et al. Cre/lox-assisted non-invasive in vivo tracking of specific cell populations by positron emission tomography. Nat Commun. 2017;8(1):444. https://doi.org/10.1038/s41467-017-00482-y.
•• Rischpler C, Dirschinger RJ, Nekolla SG, Kossmann H, Nicolosi S, Hanus F, et al. Prospective Evaluation of 18F–Fluorodeoxyglucose Uptake in Postischemic Myocardium by Simultaneous Positron Emission Tomography/Magnetic Resonance Imaging as a Prognostic Marker of Functional Outcome. Circ Cardiovasc Imaging. 2016;9(4):e004316. This study established that the size and intensity of 18 F–FDG uptake in the hypoperfused cardiac territory at <5d after first myocardial infarction was predictive of subsequent decline in contractile function. This finding establishes the principle for further studies using more specific imaging markers to determine the value of inflammation imaging to predict functional outcome.
Kim EJ, Kim S, Kang DO, Seo HS. Metabolic activity of the spleen and bone marrow in patients with acute myocardial infarction evaluated by 18f-fluorodeoxyglucose positron emission tomograpic imaging. Circ Cardiovasc Imaging. 2014;7(3):454–60. https://doi.org/10.1161/CIRCIMAGING.113.001093.
Nahrendorf M, Frantz S, Swirski FK, Mulder WJ, Randolph G, Ertl G, et al. Imaging systemic inflammatory networks in ischemic heart disease. J Am Coll Cardiol. 2015;65(15):1583–91. https://doi.org/10.1016/j.jacc.2015.02.034.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
James T. Thackeray declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Nuclear Cardiology
Rights and permissions
About this article
Cite this article
Thackeray, J.T. PET Assessment of Immune Cell Activity and Therapeutic Monitoring Following Myocardial Infarction. Curr Cardiol Rep 20, 13 (2018). https://doi.org/10.1007/s11886-018-0955-1
Published:
DOI: https://doi.org/10.1007/s11886-018-0955-1