Abstract
Objectives
Quantification of myocardial blood flow (MBF) and stress/rest flow reserve is used increasingly to diagnose multi-vessel coronary artery disease and micro-vascular disease with PET imaging. However, variability in the measurements may limit physician confidence to direct revascularization therapies based on specific threshold values. This study evaluated the effects of rubidium-82 (82Rb) tracer injection profile using a constant-activity-rate (CA) vs a constant-flow-rate (CF) infusion to improve test–retest repeatability of MBF measurements.
Method
22 participants underwent single-session 82Rb dynamic PET imaging during rest and dipyridamole stress using one of 2 test–retest infusion protocols: CA–CA (n = 12) or CA–CF (n = 10). MBF was quantified using a single-tissue-compartment model (1TCM) and a simplified retention model (SRM). Non-parametric test–retest repeatability coefficients (RPCnp) were compared between groups. Myocardium-to-blood contrast and signal-to-noise ratios of the late uptake images (2 to 6 minutes) were also compared to evaluate standard myocardial perfusion image (MPI) quality.
Results
MBF values in the CA–CA group were more repeatable (smaller RPCnp) than the CA–CF group using the 1TCM at rest alone, rest and stress combined, and stress/rest reserve (21% vs 36%, 16% vs 19%, and 20% vs 27%, P < 0.05, respectively), and using the SRM at Rest and Stress alone, Rest and Stress combined, and stress/rest reserve (21% vs 38%, 15% vs 25%, 22% vs 38%, and 23% vs 49%, P < 0.05, respectively). In terms of image quality, myocardium-to-blood contrast and signal-to-noise ratios were not significantly different between groups.
Conclusions
Constant-activity-rate ‘square-wave’ infusion of 82Rb produces more repeatable tracer injection profiles and decreases the test–retest variability of MBF measurements, when compared to a constant-flow-rate ‘bolus’ administration of 82Rb, especially with SRM, and without compromising standard MPI quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Absolute myocardial blood flow (MBF) quantification addresses the limitation of relative myocardial perfusion imaging (MPI) by measuring blood flow in absolute units of mL/min per gram of tissue (mL/min/g), with proven precision and accuracy1,2 and incremental prognostic value.3,4 MBF quantification with rubidium-82-chloride (82Rb) positron emission tomography (PET) requires minimal changes to the conventional image acquisition protocol, with no additional risk or discomfort to patients. A series of dynamic PET images are acquired starting at the time of tracer administration to measure the time course of tracer clearance from arterial blood and uptake into the myocardium. Tracer kinetic modeling analysis is then used to estimate MBF based on the rate of tracer uptake measured in the dynamic image sequence.
To enable high diagnostic confidence in the MBF measurements, the test–retest repeatability should have low variance, which is dependent on several factors during image acquisition and analysis. We have previously shown that automation of processing helps to minimize operator-induced variability,5 and standardized analysis protocols are reproducible between laboratories.6 In theory, tracer kinetic analysis should be robust against variations in the shape of tracer injection profiles; however, in practice, the estimated kinetic parameters may be biased due to limitations in instrumentation and modeling assumptions.7 There are limited studies investigating the influence of tracer infusion profiles on MBF quantification.8,9
82Rb is the PET tracer used most commonly for clinical MPI, and its use is growing for MBF quantification. Test-retest repeatability of MBF imaging has been reported in the range of 25% to 40% using dipyridamole10–12 and 25% to 30% using regadenoson stress.13 Due to the short radioactive half-life (76 second) of the generator-produced 82Rb isotope, the tracer must be eluted directly to the patient through an intravenous (IV) catheter. The shape of the eluted activity profile vs time can vary dramatically over the life of the generator, as the parent 82Sr isotope decays. We previously reported the performance of a custom elution system to allow accurate administration of 82Rb activity using variable flow rates within a preset time interval, e.g., 30 second ‘slow-bolus’ infusions.14 Subsequent developments have aimed to improve the repeatability of tracer infusion profiles that would otherwise change with the age of the 82Sr/82Rb generator. This infusion system now employs a generator bypass-line and a feedback-control system to achieve two key features: (1) administration of 82Rb activity at a constant activity rate (MBq/s) to avoid saturating the PET detectors during dynamic image acquisition, and (2) repeatable ‘square-wave’ 82Rb activity profiles with progressive generator aging.
The primary goal of this work was to characterize the influence of variable tracer infusion profiles on the test–retest repeatability of MBF quantification, using two common tracer kinetic models: (1) the single-tissue-compartment model (1TCM)15 and (2) a simplified retention model (SRM).16,17 A secondary goal was to evaluate the effect of tracer infusion profiles on standard MPI signal-to-noise and contrast as measures of image quality. We hypothesized that MBF estimates may be dependent on the shape of the tracer infusion profiles, and that the test–retest variability of MBF quantification may be reduced by infusing 82Rb at a constant activity rate (CA) vs a constant flow rate (CF), without a significant impact on standard perfusion image quality.
Materials and Methods
Study Population
This study consisted initially of 24 participants: 15 clinical patients with known or suspected coronary artery disease (CAD) and 9 healthy subjects with low risk of CAD.18 Patients with acute coronary syndrome or unstable angina, heart failure, pulmonary edema, severe valve disease, or contraindication to dipyridamole such as hypotension, heart block, or asthma were excluded. One healthy subject was excluded due to interstitial tracer injections resulting from poor IV cannulation, and one clinical patient was excluded due to paced rhythm that was discovered following enrolment. Subjects were instructed to abstain from caffeine intake for 12 hours, fast for 4 hours (except for water intake), and withhold cardiac medications prior to the study, according to our clinical protocol and society guidelines.19 All participants provided written informed consent to participate under a research protocol approved by the University of Ottawa Heart Institute Human Research Ethics Board.
Image Acquisition
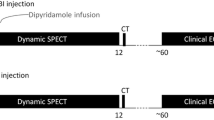
Our standard clinical imaging protocol20 was modified to acquire two rest and two stress (test–retest) scans in a single imaging session, to maintain consistent patient positioning and hemodynamic conditions. Patients were positioned in a Discovery 690 PET/VCT-64 scanner (GE Healthcare, Waukesha, WI) with ECG leads placed for patient monitoring and cardiac gating. A scout scan was performed for patient positioning, followed by a low-dose [0.14 to 0.37 mSv] (median 0.26 mSv) CT scan for attenuation correction. Four list-mode PET scans were acquired in 3D-mode; two scans at rest and two during dipyridamole-induced hyperemic stress. 82Rb was administered over a 30-second interval as a standard ‘square-wave’ infusion using the CA elution mode for the first (test) rest and stress scans, according to our routine clinical practice (Ruby-Fill® generator and prototype Rb-82 elution system [v2], Jubilant DraxImage, Kirkland, QC).20 For the second (retest) rest and stress scans, either the same CA infusion or a 30 mL/min CF ‘bolus’ infusion mode was used.
For all scans, the injected activity was adjusted for patient weight (10 MBq/kg) to limit the scanner coincidence dead-time to <35% and the corresponding dead-time correction factors (DTF < 1.54) to ensure accurate measurement of the bolus first-pass activity.21 82Rb PET images were aligned with the CT images for accurate attenuation correction prior to dynamic image reconstruction. List-mode scans (6 minute) were rebinned into 14 time frames (9 × 10, 3 × 30, 1 × 60, 1 × 120 seconds) and reconstructed using the vendor iterative algorithm (OSEM 24 subsets, 4 iterations) and 8 mm Hann post-filter.
Tracer Infusion
Participants were randomly assigned to one of two groups to avoid selection bias. In the CA–CA group, all four scans (test and retest at rest and stress) were performed using the CA infusion mode. In the CA–CF group, imaging was performed using the CA infusion mode for the first (test) rest and stress scans, and the CF infusion mode for the second (retest) rest and stress scans. CA infusions were always performed first to conform to our standard clinical imaging protocol. CF infusions were performed at the maximum flow rate of 30 mL/min, to achieve the shortest possible bolus infusion. All scans were initiated manually after 82Rb infusion was started, and the scanner-reported coincidence (prompt) count rates exceeded 10 kcps. The first rest scan was followed immediately by a second rest scan. The stress agent, dipyridamole (0.14 mg/kg/min), was infused for 5, and 3 minutes later, the two stress scans were performed in rapid succession, as illustrated in Figure 1.
Image Quality
Since MPI is currently the standard for clinical interpretation, the effect of CA vs CF infusion profiles on image quality was also evaluated. Several metrics were utilized: the total coincidence (prompt) counts recorded during the 2 to 6 minutes retention phase, the left ventricle (LV) activity polar map myocardium-to-blood ratio (MBR), contrast-to-noise ratio (CNR), and the myocardium signal-to-noise ratio (SNR). LV activity polar maps were sampled from the 2 to 6 minutes retention-phase images using FlowQuant® V2.4 (UOHI, Ottawa, ON). MBR was defined as the mean tracer activity in the LV polar map divided by the arterial blood value during the same time frames (2 to 6 minutes). A higher MBR value indicates a higher retention of radioactive tracer in the myocardium with respect to the residual activity in the blood. A narrower peak of the bolus first-pass in the blood input using the CF elution mode might improve the MBR due to longer blood clearance time, but may also increase the PET detector dead-time losses; therefore, the peak dead-time correction factor (DTF) was also recorded. SNR was computed as the mean/standard deviation (SD) of the myocardium activity polar map. CNR was defined as the mean (myocardium—blood) contrast divided by the SD of the myocardium value in the LV activity polar map. Higher CNR and SNR are indicative of lower image noise and higher uniformity of tracer retention in the myocardium.
Myocardial Blood Flow
Reconstructed dynamic PET image sequences were analyzed with FlowQuant® to quantify MBF in the 3 vascular territories using the 1TCM with dual-spillover correction and tracer extraction correction, previously shown to have good test–retest repeatability at rest.10 This method includes automatically derived left ventricle orientation and segmentation with optional operator adjustments. The arterial blood volume of interest was automatically derived by thresholding segmentation of the peak-activity blood pool image in a restricted region including the left atrium and aortic outflow tract as described previously.10 A second kinetic model, the SRM was also used to quantify MBF using the same regions described above, as reported previously using a fixed recovery coefficient (RC = 0.76), a blood integration interval from time zero to the blood peak-time + 1.4 minutes, and an extraction correction consistent with the 1TCM values of MBF.17
Statistical Analysis
Continuous and discrete data are presented as mean ± standard deviation (SD) and range [minimum, maximum] or median and inter-quartile range (IQR) for non-Gaussian-distributed data. Demographic and hemodynamic variables were compared using unpaired or paired Student’s t-tests, with Bonferroni corrections as appropriate. test–retest MBF values were compared using Spearman’s rank correlation (R). Differences in repeated measurements were calculated both in absolute units (retest–test) mL/min/g, and relative to the test–retest mean values [(retest − test)/(retest + test)/2 × 100%]. The measured test–retest MBF differences did not follow a Gaussian distribution so non-parametric repeatability coefficients (RPCnp=1.45×IQR) were used as a more robust measure to characterize the repeatability. For Gaussian-distributed data, the conventional RPC=1.96×SD and RPCnp are equivalent.22 To account for small measured differences in the test–retest values measured separately at Rest and Stress, repeatability of the combined Stress & Rest data was assessed using values adjusted for the median differences. Changes in the heart rate × systolic blood pressure = rate × pressure product (RPP) between test and retest were compared to differences in MBF using Spearman’s rank correlation. Wilcoxon and Levene’s non-parametric tests were used to assess the statistical significance of differences in medians and variances, respectively. P values less than 0.05 were considered statistically significant. All analyses were performed using Matlab R2013b (Mathworks, Natick, MA).
Results
Study Population
Demographics and cardiac risk factors for the patients and normal volunteers are summarized in Table 1. The CA–CF cohort had a higher number of healthy normals who tended to be younger with lower BMI than the CA–CA subjects. Hemodynamic measurements are summarized in Table 2. As expected during pharmacologic stress, there were significant increases in HR, BP, and RPP vs the resting state values. There were no changes in resting hemodynamics between test and retest; however, at stress, there was a small decrease observed in all the hemodynamic values during the retest scans compared to the initial stress test scans (P < 0.05).
Retest vs test RPP values were highly correlated (R ≥ 0.90; P < 0.001), showing a small but significant decrease both at rest and stress (Figure 2A). The test–retest changes in MBF (Delta) were not significantly correlated with changes in RPP (R < 0.30; P = NS) as shown in Figure 2B using 1TCM, nor with SRM (data not shown), therefore no RPP-adjustments of rest or stress MBF values were performed.
Tracer Infusion
Depending on the amount of 82Rb activity available from the 82Sr/82Rb generator and the amount of requested activity, the CF-mode of elution resulted in variable time intervals and peak amplitudes of tracer activity, as illustrated in Figure 3. In contrast, the CA-mode elution profiles were much less variable in shape, with very consistent weight-adjusted amplitude over the time course of infusion.
82Rb infusion profiles for the CA–CF test–retest cohort. The CA-mode elution profiles follow an approximate ‘square-wave’ infusion profile with a constant activity rate of 0.33 MBq/s/kg, resulting in a consistent total injected activity of 10 MBq/kg (shaded blue area) administered over a standard time interval of 30 seconds. The CF-mode profiles result in the same total activity, but injected over variable time intervals from 10 to 30 s and with variable peak amplitudes from 0.4 to 2.2 MBq/s/kg
Image Quality
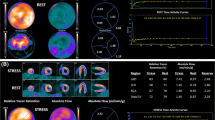
Comparison of the image quality metrics between the CA vs CF infusions in the CA–CF cohort (n = 10) is summarized in Figure 4. The CA ‘square-wave’ infusions had significantly lower peak DTF values compared to the CF ‘bolus’ infusions (1.43 ± 0.10 vs 1.51 ± 0.12; P < 0.001). A larger proportion of CF scans was above the target dead-time factor (1.54) and correction inaccuracy recommended on the particular PET scanner used in this study (8/20 vs 2/20, P = 0.01) as shown in Figure 5A; likely due to the higher injected activity rates as shown in Figure 3. The total coincidence (prompt) counts recorded in the uptake phase were >10% higher (69 vs 62 M; P < 0.001) using the CA vs CF infusion mode. Despite this small increase in recorded counts, there was no significant difference observed in the uptake image quality metrics including MBR, CNR, and SNR between CA and CF infusion modes (Figure 4).
Myocardial perfusion image quality in the CA–CF cohort (mean + SD). There were no significant differences in myocardium-to-blood ratio (MBR), contrast-to-noise ratio (CNR), and myocardial signal-to-noise ratio (SNR) between CA vs CF infusion modes at rest or stress. There was a significant improvement in MBR and CNR at stress vs rest, as expected, using both infusion modes. *P < 0.05 increased vs rest
System dead-time factors (DTF) effect on myocardial blood flow (MBF). (A) Correlation of DTF values for paired rest (blue) and stress (red) scans using constant activity (CA) and constant flow (CF) infusions. Dotted lines are shown at the DTF value of 1.54, corresponding to the maximum recommended dead-time limit of 35%. Differences in DTF between infusion types did not correlate with changes in MBF, using either the 1TCM (B) or SRM (C) analysis method
Myocardial Blood Flow
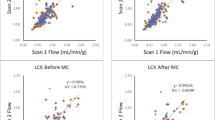
Test–retest MBF scatter plots are shown in Figure 6 using consistent (CA–CA) and variable (CA–CF) infusion profiles, as analyzed using the 1TCM and SRM tracer kinetic methods. The CA–CA cohort (Figure 6A, B) generally displayed test–retest values closer to the line of identity (ideal) compared to the CA–CF values (Figure 6C, D), using both the 1TCM and SRM methods. The measured range of MBF values was wider at rest and stress in the CA–CF vs CA–CA group, due to several outliers apparent in the CA–CF scatter plots, using both the 1TCM and SRM methods.
The corresponding Bland–Altman plots of the relative test–retest Delta/Mean [%] values are shown in Figure 7, together with the 95% limits of agreement (median ± RPCnp). The subjects with consistent tracer infusion profiles (CA–CA cohort) generally had a smaller range of mean MBF values at rest and stress, with narrower limits of agreement (Figure 7A, B), indicating improved test–retest repeatability.
Bland–Altman plots of MBF repeatability at rest and stress. Retest–test delta/mean [%] values are plotted at rest (blue) and stress (red) vs the respective Mean MBF [mL/min/g]. Median values are plotted as thick solid lines within the shaded regions illustrating the limits of agreement of the median ± 1.45 × IQR (inter-quartile range). Values are indicated as median and [lower, upper] limits of agreement
The full list of test–retest median and RPCnp values at Stress and Rest, as well as the combined Stress & Rest, stress/rest, and stress–rest reserve values are shown in Table 3; expressed both as absolute (delta) and relative (delta/mean [%]) differences in MBF. Significantly better test–retest variability (lower RPCnp) was measured in the CA–CA vs CA–CF cohort at rest, stress & rest combined, stress/rest, and stress–rest reserve, using both the 1TCM and SRM analysis methods (P < 0.05). The test–retest variability was also significantly lower at stress in the CA–CA vs CA–CF cohort using the SRM analysis method.
Box-plots (median and IQR) and repeatability coefficients (RPC) of the relative Delta/Mean [%] values are illustrated in Figures 8A, B, respectively, for stress, rest, stress & rest combined, stress/rest, and stress–rest reserve. It is apparent that the CA–CA cohort analyzed using the 1TCM method generally displayed the smallest median delta and RPCnp values, as well as the fewest outliers, whereas the CA–CF cohort analyzed using the SRM method had the largest RPCnp values at Stress, Rest, Stress & Rest combined, Stress/Rest, and stress–rest reserve, as well as the largest outliers. Similar patterns were observed in the RPCnp values using the absolute scale differences, as shown in the Supplementary Figure S1.
Test–retest RPCnp values of delta MBF [%] relative to the mean. (A) Box-plots of the median and inter-quartile range in the CA–CA and CA–CF cohorts, measured using the 1TCM and SRM methods; possible outliers shown with red ‘+’ symbols are beyond the median ± 1.5 × IQR. (B) Repeatability coefficients (RPCnp) in the CA–CA and CA–CF cohorts, measured using the 1TCM and SRM methods. *P < 0.05 decreased variance in CA–CA vs CA–CF cohort
While the peak DTF values were approximately 5% higher using CF compared to the corresponding CA-mode values (Figure 5A), there were no significant differences in MBF observed as a result of changing DTF values using either the 1TCM or SRM (Figure 5B, C, respectively). The single outlier at stress observed using the SRM (Figures 5C, 6D) did not appear as an outlier using the 1TCM (Figures 5B, 6C), and therefore cannot be attributed to changes in dead-time alone.
Discussion
To the best of our knowledge, this is the first study to evaluate the impact of tracer administration profiles on the test–retest repeatability of MBF quantification using dynamic 82Rb PET imaging. This work demonstrates that the 82Rb tracer infusion profile can be a significant source of variability in measured MBF values, and that precision can be improved using the more consistent CA infusion mode. Variability in the combined Stress&Rest MBF and Reserve measurements was substantially lower on average (20% vs 27% RPCnp) when using consistent infusions (CA–CA) compared to variable infusion profiles (CA–CF), with the 1TCM analysis method (Table 3). This improvement in repeatability was even more pronounced when using the SRM kinetic analysis method (25% vs 52% RPCnp), which appears to be more sensitive to changes in the shape of the infusion profile than the 1TCM approach. The widely used 1TCM appears to be more robust to variations in the infusion profile than the simple retention model, but neither model fully corrected for unexpected changes in the shape of the arterial blood input function. In theory, the quantitative MBF results should not depend on the shape of the input function, because the compartment model is formulated to predict the myocardial tissue response curve for any arbitrary shape of input. However, our results show that in practice, there is still some residual bias which can be reduced by standardizing the shape of the blood input function. Conversely, the simplified retention model does not predict the myocardial tissue response curve explicitly, and is therefore more reliant on a consistent shape of input function to provide repeatable measurements of MBF. While the repeatability of MBF quantification improved with consistent infusion profiles, it is equally important to note that the quality of the late-phase uptake images was not adversely affected using longer infusions.
Using the Rb-82 elution system that delivers reproducible ‘square-wave’ infusion profiles over the life of the generator, our results suggest that the CA infusion mode is preferable for optimal repeatability, in particular when using the SRM for MBF quantification with 82Rb PET. This finding is of clinical significance since non-invasive MBF and flow reserve imaging is being used increasingly as part of the routine clinical evaluation of stress myocardial perfusion. The results of this study indicate that the repeatability of MBF measurements with 82Rb PET is improved using an elution system capable of maintaining reproducible infusion profiles irrespective of generator age.
MBF Tracer Kinetic Models
We evaluated the repeatability of MBF quantification using two commonly employed methods: the single-tissue-compartment kinetic model (1TCM) and a simplified retention model (SRM). The 1TCM is widely accepted in the community to be both accurate and robust.1,2,6 Previous studies have shown good reproducibility of the 1TCM for 82Rb MBF measurement, as implemented in several research and commercial software packages.6,23 Simplified retention models may be attractive due to the lower computational complexity but suffer from relatively poor reproducibility among results reported by various laboratories.17 For example, despite the widespread acceptance of 82Rb and 13N-ammonia PET imaging for the quantification of MBF, quite a wide range of tracer retention fractions have been reported (20 to 30% for 82Rb and 30 to 60% for 13N-ammonia at peak stress) using several variations of a simple retention model, each with slightly different underlying assumptions and numerical implementations.17
To date, there has been no study comparing the 1TCM vs SRM methods, in terms of MBF test–retest repeatability. Although it was not the primary objective of the present study, we observed substantially lower test–retest variability using the 1TCM compared to SRM in the CA–CF cohort, for the combined Stress&Rest MBF and Reserve data (27% vs 52% RPCnp). However, when using consistent infusion profiles (CA–CA), the differences in repeatability between the 1TCM vs SRM were smaller (20% vs 25% RPCnp) and did not reach statistical significance. These findings support our hypothesis that changes in the tracer infusion profile shapes can adversely impact MBF variability and that a reproducible infusion profile can help to reduce test–retest variability, especially when assuming SRM kinetics.
Outlier Values
The regression and Bland–Altman plots in Figures 6 and 7 suggest that one of the patients in the CA–CF cohort appears to be a clear outlier. This is particularly evident using the SRM method which resulted in abnormally high mean MBF values of 3 to 4 mL/min/g at rest, and up to 6 mL/min/g at stress. Several quality assurance metrics were investigated to identify a potential cause; there was no significant patient body motion or hemodynamic changes between test and retest scans. The tracer elution profiles, had substantially different shapes between the CA ‘square-wave’ vs CF ‘bolus’ infusion modes, but were highly repeatable between rest and stress as shown in Supplementary Figure S2. The corresponding blood input and myocardium TACs for this particular patient are presented in Supplementary Figure S3. The initial rest scan had a delayed rise in blood activity following the initial bolus, which may be associated with an interstitial infusion or partially blocked IV catheter. The second rest and both stress scans had a similar delayed but smaller-amplitude blood peak at approximately 1.5 min following the initial bolus. The shape of the blood input curves was reflected in abnormally long mean-transit-times, as derived using a gamma-variate curve fit to the first-pass blood peak activity. Consequently, tracer delivery to the myocardium proceeded well beyond the standard blood integration time of the SRM, leading to substantial overestimation of the MBF values (>4 mL/min/g) during the initial test scan at rest. In contrast, the 1TCM was better able to account for the complete shape of the blood and myocardium TACs, resulting in more repeatable MBF estimates despite the dramatic changes in shape. These results suggest an improved ability of the 1TCM to accommodate a wider range of arterial blood and myocardium TAC shapes that may be encountered in clinical practice.
Comparison to Previous Studies
Table 4 summarizes previously published repeatability values for stress flow, rest flow, and flow reserve using PET, compared with the results of the current study. The values reported in this study are among the lowest, which may benefit from our previous work on minimizing several sources of variability including: kinetic model parameters, image-derived blood input ROI,10 and operator variability.5 In the current study, we used these previously determined optimal parameters and investigated the infusion profile shape as an additional source of variability. The present results demonstrate that 82Rb PET MBF quantification using the CA elution mode to deliver consistently shaped infusion profiles can improve test–retest precision.
This work was performed using a high-count-rate LYSO-based PET system. High-count-rate capabilities are essential for MBF quantification with 82Rb due to the wide range of count rates encountered over the course of dynamic image acquisition, associated with rapid tracer distribution and short radioactive half-life (76 s). This is especially true in a clinical setting where high-quality MPI (and ECG-gated) images are desired in addition to dynamic imaging for MBF quantification using a single tracer injection. CA infusions may prove to be even more advantageous on lower count-rate systems (e.g., using BGO detectors) in which a tradeoff exists between higher 82Rb activity required for diagnostic quality MPI images, vs lower 82Rb activity to avoid detector dead-time saturation in early time frames for accurate MBF quantification.
Potential Limitations
This study enrolled a relatively small number of subjects (n = 22). Nevertheless, we were able to demonstrate a significant improvement in MBF repeatability using a consistent CA-mode infusion of 82Rb compared to variable CF-mode infusions, which demonstrated changes in amplitude and duration as a function of generator age and injected activity.
The present study did not investigate the test–retest repeatability of the CF-mode infusions alone, with variable shape profiles at the start and end of the generator shelf-life, as may be encountered in clinical practice. Our results suggest that the CA ‘square-wave’ infusion mode might be expected to improve MBF repeatability compared to CF-mode studies performed on different days, by removing the variations between infusion profiles that typically occur as the generator ages. This could be confirmed in a future study using CA–CA vs CF–CF test–retest studies acquired on different days within the normal generator shelf-life. In the present study, we were bound by the need to comply with our clinical-standard procedure for cardiac imaging and MBF quantification using the established CA-mode infusion, and selected a rapid test–retest protocol to achieve stable hemodynamics, which could otherwise affect the test–retest repeatability. Furthermore, the protocol was limited to 4 scans per subject in order to reduce participant discomfort and radiation exposure (estimated to be an additional 1.5 mSv above the clinical routine).30
Small differences in patient demographics between the CA–CA and CA–CF cohorts (Table 1) were an unintended consequence of our randomization strategy. Nevertheless, we believe that the conclusions remain valid since we evaluated the test–retest differences relative to the mean for each subject, and using paired comparisons in which each patient served as their own control.
Conclusions
Myocardial blood flow quantification with 82Rb PET can be influenced by the shape of the time-activity infusion profile of the tracer. Constant-activity-rate ‘square-wave’ infusion of 82Rb produces more consistent activity profiles and improves the test–retest variability of MBF measurements, when compared to constant-flow-rate ‘bolus’ administration of 82Rb, especially using the simplified retention model. Standard MPI uptake-phase image quality was not influenced by the variations in tracer infusion profiles.
New Knowledge Gained
The reproducibility of myocardial blood flow quantification from 82Rb dynamic PET may be influenced by inconsistent tracer infusion profiles. The one-tissue kinetic model is more robust to variations in tracer infusion than the simplified retention model. Therefore, a reproducible infusion profile over the life of a 82Sr/82Rb generator such as constant activity rate (square-wave) is preferable, especially when using a simplified retention model. Conventional MPI quality is not degraded by CA infusions.
Abbreviations
- MBF:
-
Myocardial blood flow
- CA:
-
Constant-activity-rate
- CF:
-
Constant-flow-rate
- 1TCM:
-
1-Tissue compartment model
- SRM:
-
Simplified retention model
- DTF:
-
Dead-time factor
- RPCnp :
-
Reproducibility coefficient (non-parametric-based estimate)
- MBR:
-
Myocardium-to-blood ratio
- CNR:
-
Contrast-to-noise ratio
- SNR:
-
Signal-to-noise ratio
References
El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, et al Reproducibility and accuracy of quantitative myocardial blood flow assessment with 82Rb PET: Comparison with 13N-ammonia PET. J Nucl Med 2009;50:1062–71.
Nesterov SV, Deshayes E, Sciagrà R, Settimo L, Declerck JM, Pan XB, et al Quantification of myocardial blood flow in absolute terms using (82)Rb PET imaging: The RUBY-10 Study. JACC Cardiovasc Imaging 2014;7:1119–27.
Ziadi MC, deKemp RA, Williams KA, Guo A, Chow BJW, Renaud JM, et al Impaired myocardial flow reserve on Rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740–8.
Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, et al Improved cardiac risk assessment with non-invasive measures of coronary flow reserve. Circulation. 2011;124:2215–24.
Klein R, Renaud JM, Ziadi MC, Thorn SL, Adler A, Beanlands RS, et al Intra- and inter-operator repeatability of myocardial blood flow and myocardial flow reserve measurements using rubidium-82 pet and a highly automated analysis program. J Nucl Cardiol. 2010;17:600–16.
deKemp RA, Declerck J, Klein R, Pan X-B, Nakazato R, Tonge C, et al Multisoftware reproducibility study of stress and rest myocardial blood flow assessed with 3D dynamic PET/CT and a 1-tissue-compartment model of 82Rb kinetics. J Nucl Med 2013;54:571–7.
Kolthammer JA, Muzic RF. Optimized dynamic framing for PET-based myocardial blood flow estimation. Phys Med Biol 2013;58:5783–801.
Klein R, Beanlands RS, Wassenaar RW, Thorn SL, Lamoureux M, DaSilva JN, et al Kinetic model-based factor analysis of dynamic sequences for 82-rubidium cardiac positron emission tomography. Med Phys 2010;37:3995–4010.
Raylman RR, Caraher JM, Hutchins GD. Sampling requirements for dynamic cardiac PET studies using image-derived input functions. J Nucl Med 1993;34:440–7.
Efseaff M, Klein R, Ziadi MC, Beanlands RS, deKemp RA. Short-term repeatability of resting myocardial blood flow measurements using rubidium-82 PET imaging. J Nucl Cardiol 2012;19:997–1006.
Sdringola S, Johnson NP, Kirkeeide RL, Cid E, Gould KL. Impact of unexpected factors on quantitative myocardial perfusion and coronary flow reserve in young, asymptomatic volunteers. JACC Cardiovasc Imaging. 2011;4:402–12.
Johnson NP, Gould KL. Regadenoson versus dipyridamole hyperemia for cardiac PET imaging. J Am Coll Cardiol Imaging 2015;8:438–47.
Moody J, Murthy V, Lee B, Corbett J, Ficaro E. Variance estimation for myocardial blood flow by dynamic PET. IEEE Trans Med Imaging 2015;34:2343–53.
Klein R, Adler A, Beanlands RS, deKemp RA. Precision-controlled elution of a 82Sr/82Rb generator for cardiac perfusion imaging with positron emission tomography. Phys Med Biol 2007;52:659–73.
Lortie M, Beanlands RSB, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging 2007;34:1765–74.
Yoshida K, Mullani N, Gould KL. Coronary flow and flow reserve by PET simplified for clinical applications using rubidium-82 or nitrogen-13-ammonia. J Nucl Med 1996;37:1701–12.
Renaud JM, DaSilva JN, Beanlands RS, deKemp RA. Characterizing the normal range of myocardial blood flow with 82rubidium and 13N-ammonia PET imaging. J Nucl Cardiol 2013;20(4):578–91.
Gibbons RJ, Balady GJ, Beasley JW, Faafp JW, Bricker JT, Duvernoy WFC, et al ACC/AHA guidelines for exercise testing: Executive summary: A report of the american college of cardiology/ american heart association task force on practice guidelines (committee on exercise testing). Circulation 1997;96:345–54.
Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, et al ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. PET myocardial perfusion and metabolism clinical imaging. J Nucl Cardiol 2016;23:1187–226.
Kaster T, Mylonas I, Renaud JM, Wells GA, Beanlands RS, deKemp RA. Accuracy of low-dose rubidium-82 myocardial perfusion imaging for detection of coronary artery disease using 3D PET and normal database interpretation. J Nucl Cardiol 2012;19:1135–45.
Renaud JM, Yip K, Guimond J, Trottier M, Pibarot P, Turcotte E, et al Characterization of 3D PET systems for accurate quantification of myocardial blood flow. J Nucl Med. 2016. doi:10.2967/jnumed.116.174565.
Peck R, Olsen C, Devore J. Introduction to statistics and data analysis. Toronto: Nelson Education; 2011.
Tahari AK, Lee A, Rajaram M, Fukushima K, Lodge MA, Lee BC, et al Absolute myocardial flow quantification with (82)Rb PET/CT: Comparison of different software packages and methods. Eur J Nucl Med Mol Imaging 2014;41:126–35.
Kaufmann PA, Gnecchi-Ruscone T, Yap JT, Rimoldi O, Camici PG. Assessment of the reproducibility of baseline and hyperemic myocardial blood flow measurements with 15O-labeled water and PET. J Nucl Med 1999;40:1848–56.
Wyss CA, Koepfli P, Mikolajczyk K, Burger C, von Schulthess GK, Kaufmann PA. Bicycle exercise stress in PET for assessment of coronary flow reserve: Repeatability and comparison with adenosine stress. J Nucl Med. 2003;44:146–54.
Siegrist PT, Gaemperli O, Koepfli P, Schepis T, Namdar M, Valenta I, et al Repeatability of cold pressor test-induced flow increase assessed with H2[15O] and PET. J Nucl Med. 2006;47:1420–6.
Schindler TH, Zhang X-L, Prior JO, Cadenas J, Dahlbom M, Sayre J, Schelbert HR. Assessment of intra- and interobserver reproducibility of rest and cold pressor test-stimulated myocardial blood flow with (13)N-ammonia and PET. Eur J Nucl Med Mol Imaging. 2007;34:1178–88.
Nagamachi S, Czernin J, Kim AS, Sun KT, Böttcher M, Phelps ME, et al Reproducibility of measurements of regional resting and hyperemic myocardial blood flow assessed with PET. J Nucl Med. 1996;37:1626–31.
Manabe O, Yoshinaga K, Katoh C, Naya M, deKemp RA, et al Repeatability of Rest and Hyperemic Myocardial Blood Flow Measurements with 82Rb Dynamic PET. J Nucl Med. 2009;50:68–71.
Hunter CRRN, Hill J, Ziadi MC, Beanlands RSB, deKemp RA. Biodistribution and radiation dosimetry of 82Rb at rest and during peak pharmacological stress in patients referred for myocardial perfusion imaging. Eur J Nucl Med Mol Imaging. 2015;42:1032–42.
Disclosure
Ran Klein and Robert deKemp receive royalties from Jubilant DraxImage for the sale of Ruby-Fill generators. Ran Klein, Robert deKemp, and Rob Beanlands are consultants to Jubilant DraxImage. Ran Klein, Robert deKemp, and Jennifer Renaud receive revenue shares from the sales of FlowQuant.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Funding
Institute for Cardiovascular Therapeutics grant from the Ontario Research Fund (ORF-RE02038) and Jubilant DraxImage. Natural Sciences and Engineering Research Council of Canada (436149). Rob Beanlands is a Career Investigator supported by the HSFO; Tier 1 uOttawa Chair in Cardiac Imaging Research; Vered Chair in Cardiology.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Ran Klein and Adrian Ocneanu contributed equally to this study.
See related editorial, doi:10.1007/s12350-016-0690-1.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Klein, R., Ocneanu, A., Renaud, J.M. et al. Consistent tracer administration profile improves test–retest repeatability of myocardial blood flow quantification with 82Rb dynamic PET imaging. J. Nucl. Cardiol. 25, 929–941 (2018). https://doi.org/10.1007/s12350-016-0698-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-016-0698-6