Abstract
Bleeding is a fatal complication after pancreatectomy. Although coil embolization is a widely accepted treatment option, ischemia of the remaining organs should be prevented. This study reports the successful treatment of intra-abdominal hemorrhage following distal pancreatectomy with en bloc celiac axis resection (DP-CAR) using balloon-assisted coil embolization (BACE). A 59-year-old man was diagnosed with locally advanced pancreatic cancer. The tumor involves the common hepatic artery, splenic artery, and celiac artery. After four cycles of treatment with gemcitabine/nab-paclitaxel, the soft-density masses, surrounding the artery, shrunk. DP-CAR and R0 resections were performed. A minor postoperative pancreatic fistula occurred. Six months postoperatively, the computed tomography showed delayed asymptomatic bleeding from an anterior superior pancreaticoduodenal artery (ASPDA) pseudoaneurysm located near the gastroduodenal artery confluence. BACE was performed by placing a microballoon catheter in the region of confluence of the ASPDA and posterior superior pancreaticoduodenal artery (PSPDA) to prevent coil migration. After inserting the microballoon catheter, coil embolization was performed in the ASPDA. Hepatic blood flow was maintained from the PSPDA. BACE is a useful technique to preserve blood flow to the remnant organs when performing coil embolization for bleeding following a distal pancreatectomy, especially following a DP-CAR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mortality rate among patients, undergoing pancreatectomy, has decreased to less than 5% due to the advances in surgical procedures and postoperative management. The complications of a pancreatectomy include postoperative pancreatic fistula (POPF) formation, delayed gastric emptying, and post-pancreatectomy hemorrhage (PPH). PPH is a fatal complication that often requires immediate treatment [1,2,3]. PPH occurs in 7.5–10% of patients who undergo surgery, and the mortality rate for patients with PPH was reportedly 16–50% [4, 5]. Bleeding within 24 h after surgery is considered early bleeding, and bleeding after 24 h postoperatively is considered late bleeding. Early bleeding is often caused by the technical failure of appropriate hemostasis during the operation. In contrast, late bleeding is mainly caused by POPF, intra-abdominal abscess, and bile leak [6, 7].
Laparotomy is the treatment of choice for early bleeding, while coil embolization is used to address late bleeding [8, 9]. When performing coil embolization in patients, who underwent distal pancreatectomy with en bloc celiac axis resection (DP-CAR), the hepatic arterial flow from the superior mesenteric artery (SMA) must be maintained. The disruption of the hepatic arterial flow causes liver failure and liver abscess formation [10, 11].
Balloon-assisted coil embolization (BACE) utilizes a balloon stent when performing coil embolization [12, 13]. BACE is effective for selective embolization of the anterior (ASPDA) or the posterior superior pancreaticoduodenal artery (PSPDA). It prevents the migration of the coil to another artery. This study presents a case of late-onset intra-abdominal hemorrhage, following DP-CAR, that was successfully treated with BACE.
Case report
The patient was a 59-year-old diabetic male with gradually increasing blood sugar levels. Computed tomography (CT) revealed a pancreatic body tumor, measuring 24 mm in diameter. It involved the common hepatic artery (CHA), splenic artery (SA), and celiac artery (CA) (Fig. 1a, b). The patient was referred to our department for further treatment. We performed endoscopic ultrasound guided fine needle aspiration (EUS-FNA) for diagnosis. The biopsy identified an adenocarcinoma, and the patient was diagnosed with locally advanced pancreatic cancer. We decided to perform induction chemotherapy initially and switch to surgery if the tumor shrinkage was obtained. The patient received induction chemotherapy with gemcitabine/nab-paclitaxel (GnP). After four cycles of chemotherapy, a CT scan revealed that the soft-density mass, surrounding the CHA, SA, and CA, partially shrunk (Fig. 1c). The elevated serum tumor markers also decreased after chemotherapy as follows: CEA level was 4.6–3.5 ng/mL, CA19-9 was 86–36 U/mL, DUPAN-2 was 57 U/mL to less than 25 U/mL, and Span-1 was 42–19 U/mL. The tumor was considered resectable via DP-CAR. Blood flow to the proper hepatic artery via the gastroduodenal artery (GDA) was secured under CHA clamping. The CA was ligated twice and dissected approximately 2 cm from the root. The operation time was 379 min, and the intraoperative bleeding volume was 1,292 mL. We started administration of somatostatin analogue on the day of surgery. Three days after surgery, the patient had an elevated inflammatory reaction and CT recognized fluid collection around the pancreatic stump. We diagnosed pancreatic fistula [International Study Group of Pancreas Fistula (ISGPF)] grade BL and changed the antibiotic from cefotiam to meropenem. The drain culture was negative and the tip of the drain was away from fluid collection, so we removed the drain five days after surgery. The somatostatin analog was stopped seven days after surgery and meropenem was stopped ten days after surgery because the inflammatory reaction improved. The length of postoperative hospital stay was 19 days. The pathological diagnosis was a moderately to poorly differentiated adenocarcinoma, T1cN1aM0 Stage IIB according to the 7th edition of classification of pancreatic carcinoma from Japan Pancreas Society. The effect of chemotherapy was grade 2 [14].
Enhanced computed tomography findings of the primary tumor. a: Enhanced CT showed the tumor involving the CHA (black arrowhead), SA (white arrow), and CA (white arrowhead) at the first visit. b: Magnified enhanced CT at the first visit showed that the tumor (black dotted circle), 24 mm in diameter. c: After four cycles of chemotherapy, enhanced CT showed that the tumor size had decreased 16 mm in diameter, and the soft-density mass (black dotted circle) around the CHA, SA, and CA had shrunk. CA celiac artery, CHA common hepatic artery, CT computed tomography, SA splenic artery
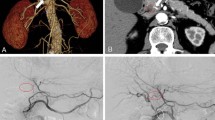
Two months postoperatively, the patient received adjuvant chemotherapy with GnP. Since we confirmed that GnP was effective in this patient during induction chemotherapy, we selected GnP as postoperative adjuvant chemotherapy. Three months postoperatively, CT showed a 3 mm pseudoaneurysm, arising from the ASPDA. The pseudoaneurysm was proximal to the area of confluence of the ASPDA and GDA. Fluid collection was also observed proximal to the pancreatic stump (Fig. 2). Although the patient had received two courses of GnP, chemotherapy was discontinued at this time. Trans-gastric drainage of the fluid and angiography was initially planned. However, CT showed spontaneous reduction of the fluid collection and partial shrinkage of the pseudoaneurysm when the patient admitted to our hospital. Therefore, the patient underwent close follow-up without treatment. Three months later, enhanced CT revealed asymptomatic bleeding, arising from the ASPDA pseudoaneurysm (Fig. 3a). The blood flow of the portal vein (PV) was maintained (Fig. 3b). Since the patient had stable vital signs, coil embolization was scheduled. Migration of the coil to the GDA results in hepatic infarction and liver failure. Therefore, BACE was performed by inserting a microballoon catheter in the area of confluence of the ASPDA and PSPDA to prevent coil migration. First, both femoral arteries were punctured, and 5-Fr long sheaths were placed. The SMA was visualized using a catheter, inserted through the left sheath, to confirm the bifurcation of the pancreatic duodenal arcade and the location of the pseudoaneurysm (Fig. 4a). A microballoon catheter (Attendant SP®, Terumo, Tokyo, Japan) was inserted through the right sheath to the region of confluence of the ASPDA and PSPDA (Fig. 4b, c). After microballoon placement, coil embolization was performed in the ASPDA from the microcatheter (Progreat β®, Terumo, Tokyo, Japan), inserted through the left sheath (Fig. 4d, e). Adequate blood flow to the liver was maintained after coil embolization (Fig. 5a). Before finishing the procedure, we confirmed the absence of bleeding (Fig. 5b). Magnetic resonance imaging showed no bleeding or coil migration one month after coil embolization.
Enhanced CT findings three months after surgery. a: Enhanced CT image revealed a pseudoaneurysm (black arrowhead), 3 mm in diameter, arising from ASPDA (black arrow). Fluid collection was also observed near the pancreatic stump. b: Three-dimensional reconstruction image of the blood vessels revealed an aneurysm in the ASPDA (white arrow). ASPDA anterior superior pancreaticoduodenal artery, CT computed tomography
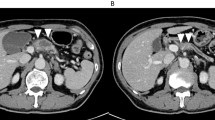
Enhanced CT findings six months after surgery. a: Enhanced CT showed a pseudoaneurysm arising from the ASPDA (black arrow) and asymptomatic active bleeding arising from the ASPDA pseudoaneurysm (black arrowhead). b: Enhanced CT showed that the blood flow of the PV was maintained (black arrow). ASPDA anterior superior pancreaticoduodenal artery, CT computed tomography, PV portal vein
Angiography and schema during coil embolization. a: Angiography revealed active bleeding (white arrowhead) arising from the ASPDA pseudoaneurysm. The pseudoaneurysm was located near the root of the GDA (black arrowhead). b, c: A balloon catheter was placed via the PSPDA (white arrowhead). The confluence of the ASPDA and GDA was occluded using a balloon. d, e: The coil was placed in the ASPDA with an occluding balloon placed in the PSPDA to the GDA. ASPDA anterior superior pancreaticoduodenal artery, GDA gastroduodenal artery, PSPDA posterior superior pancreaticoduodenal artery
Angiography after the coil embolization. a: Angiography after the coil embolization through the PSPDA confirmed hepatic arterial flow. b: Angiography after the coil embolization of the ASPDA confirmed no active bleeding. ASPDA anterior superior pancreaticoduodenal artery, PSPDA posterior superior pancreaticoduodenal artery
Discussion
PPH is a fatal complication of distal pancreatectomy. Occurring in 7.5–10% of patients, it has a mortality rate of 16–50% [5, 11, 15]. The severity of bleeding following a pancreatectomy was defined by the ISGPF. It is categorized based on the time of bleeding, bleeding volume, general condition of the patient, and necessity of treatment. Bleeding within 24 h postoperatively is defined as early bleeding [8, 16, 17]. In terms of bleeding volume, a decrease in hemoglobin of less than 3 mg/dl is defined as mild, while a decrease in Hb of 3 mg/dl or more is defined as severe. Grade A refers to early and mild bleeding, that is treated conservatively. Meanwhile, Grade B refers to early but severe bleeding, or mild but late bleeding. Lastly, Grade C refers to late and severe bleeding. Early treatment is important because hemorrhagic shock and coagulopathy have a poor prognosis [4, 10, 18]. Grades B and C require invasive treatments, including endovascular treatment and laparotomy [16, 17, 19]. In the present case, an ASPDA pseudoaneurysm was observed three months postoperatively. At that time, there was no bleeding from the pseudoaneurysm and no complaints of abdominal symptoms from the patient. We planned to hospitalize the patient for angiography and drainage of fluid collection in the pancreatic stump. However, CT at the time of admission showed spontaneous reduction of the fluid collection and partial shrinkage of the pseudoaneurysm, so we decided to follow-up in a short period of time after multi-professional team conference. Six months postoperatively, an enhanced CT showed asymptomatic bleeding from the ASPDA pseudoaneurysm, which required treatment.
Due to the advances in endovascular treatment, several cases have been treated without laparotomy [2, 20, 21]. In a study comparing endovascular treatment and laparotomy, the complication and mortality rates of endovascular treatment were lower than those of laparotomy [9, 19, 22]. Endovascular treatment is particularly useful for managing late bleeding.
Maintaining hepatic arterial flow during endovascular treatment is associated with a reduced incidence of complications, such as liver failure and liver abscess formation [18, 20, 21]. Therefore, limited embolization of the GDA stump has been performed to address intra-abdominal bleeding following pancreaticoduodenectomy. However, the rebleeding rates remained high. Therefore, extended embolization of the hepatic arteries is recommended for patients with severe bleeding because it immediately stops the bleeding [22]. Stent grafts are also effective in patients with stable vital signs because they maintain blood flow to the peripheral organs. However, there is a specified distance, thickness, and angle of blood vessels for stent-graft placement [18, 22]. In the present case, the pseudoaneurysm was proximal to the region of confluence of the ASPDA and GDA, so the distance for stent-graft placement was inappropriate. Furthermore, the diameter of the ASPDA was too small for stent-graft placement.
In patients who underwent DP-CAR, the blood supply of the liver comes from the pancreaticoduodenal arcade through the SMA. When a pseudoaneurysm occurs in the ASPDA or PSPDA, blood flow through another vessel should be maintained to prevent reduced hepatic arterial flow. BACE prevents coil migration by temporarily occluding the artery with a balloon stent [12, 13]. Selective coil embolization of the target area reduces the incidence of complications.
BACE is suitable for patients with blood vessels that need to preserve blood flow. BACE is often used for cerebral aneurysms [23]. When the severe bleeding with unstable vital signs occurred, there is little time. Therefore, it is often necessary to widely perform coil embolization. However, skilled radiologists can perform BACE when the patient condition is stable. BACE is a safe and reliable method of coil embolization for patients that are difficult to treat via simple endovascular therapy due to anatomical variations or postoperative complications. Although this is a first reported case in which BACE was performed on pseudoaneurysm after DP-CAR, we consider that BACE is reliable and effective treatment technique. If the patient condition is stable or the procedure can be quickly completed, BACE should be considered because it can maintain blood flow to the remnant organs.
In conclusion, bleeding following a pancreatectomy is a fatal complication, often requiring immediate treatment. When performing coil embolization for bleeding from a pseudoaneurysm, it is important to maintain the blood flow to the remnant organs. BACE is a useful technique for performing selective coil embolization at the intended site while maintaining blood flow.
Abbreviations
- ASPDA:
-
Anterior superior pancreaticoduodenal artery
- BACE:
-
Balloon-assisted coil embolization
- CA:
-
Celiac artery
- CHA:
-
Common hepatic artery
- CT:
-
Computed tomography
- DP-CAR:
-
Distal pancreatectomy with en bloc celiac axis resection
- EUS-FNA:
-
Endoscopic ultrasound guided fine needle aspiration
- GnP:
-
Gemcitabine/nab-paclitaxel
- GDA:
-
Gastroduodenal artery
- ISGPF:
-
International Study Group of Pancreatic Fistula
- POPF:
-
Postoperative pancreatic fistula
- PPH:
-
Post-pancreatectomy hemorrhage
- PSPDA:
-
Posterior superior pancreaticoduodenal artery
- PV:
-
Portal vein
- SA:
-
Splenic artery
- SMA:
-
Superior mesenteric artery
References
Boufi M, Belmir H, Hartung O, et al. Emergency stent graft implantation for ruptured visceral artery pseudoaneurysm. J Vasc Surg. 2011;53:1625–31.
Lee HG, Heo JS, Choi SH, et al. Management of bleeding from pseudoaneurysms following pancreaticoduodenectomy. World J Gastroenterol. 2010;16:1239–44.
de Castro SM, Kuhlmann KF, Busch OR, et al. Delayed massive hemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg. 2005;241:85–91.
Blanc T, Cortes A, Goere D, et al. Hemorrhage after pancreaticoduodenectomy: when is surgery still indicated? Am J Surg. 2007;194:3–9.
Stampfl U, Sommer CM, Bellemann N, et al. The use of balloon-expandable stent grafts for the management of acute arterial bleeding. J Vasc Interv Radiol. 2012;23:331–7.
Hankins D, Chao S, Dolmatch BL, et al. Covered stents for late postoperative arterial hemorrhage after pancreaticoduodenectomy. J Vasc Interv Radiol. 2009;20:407–9.
Modestino F, Cappelli A, Mosconi C, et al. Balloon-assisted coil embolization (BACE) of a wide-necked aneurysm of the inferior pancreaticoduodenal artery. CVIR Endovasc. 2020;3:62.
Schäfer M, Heinrich S, Pfammatter T, et al. Management of delayed major visceral arterial bleeding after pancreatic surgery. HPB (Oxford). 2011;13:132–8.
Yekebas EF, Wolfram L, Cataldegirmen G, et al. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007;246:269–80.
Hasegawa T, Ota H, Matsuura T, et al. Endovascular treatment of hepatic artery pseudoaneurysm after pancreaticoduodenectomy: risk factors associated with mortality and complications. J Vasc Interv Radiol. 2017;28:50-59.e5.
Sato A, Yamada T, Takase K, et al. The fatal risk in hepatic artery embolization for hemostasis after pancreatic and hepatic surgery: importance of collateral arterial pathways. J Vasc Interv Radiol. 2011;22:287–93.
Yoshida R, Yoshizako T, Maruyama M, et al. Hemosuccus pancreaticus successful treatment by double balloon-assisted coil embolization for active bleeding from the main trunk of the superior mesenteric artery. Radiol Case Rep. 2018;13:644–7.
Ueda T, Murata S, Saito H, et al. Balloon-assisted transcatheter arterial embolization using N-butyl cyanoacrylate for iatrogenic arterial bleeding by groin puncture: a new technology. CVIR Endovasc. 2020;3:42.
Japan Pancreas Society. Classification of pancreatic carcinoma. 7th ed. Tokyo: Kanehara & Co; 2016.
Ellison EC. Evidence-based management of hemorrhage after pancreaticoduodenectomy. Am J Surg. 2007;194:10–2.
Grützmann R, Rückert F, Hippe-Davies N, et al. Evaluation of the international study group of pancreatic surgery definition of post-pancreatectomy hemorrhage in a high-volume center. Surgery. 2012;151:612–20.
Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an international study group of pancreatic surgery (ISGPS) definition. Surgery. 2007;142:20–5.
Gwon DI, Ko GY, Sung KB, et al. Endovascular management of extrahepatic artery hemorrhage after pancreatobiliary surgery: clinical features and outcomes of transcatheter arterial embolization and stent-graft placement. AJR Am J Roentgenol. 2011;196:W627–34.
Gaudon C, Soussan J, Louis G, et al. Late postpancreatectomy hemorrhage: predictive factors of morbidity and mortality after percutaneous endovascular treatment. Diagn Interv Imaging. 2016;97:1071–7.
Miura F, Asano T, Amano H, et al. Management of postoperative arterial hemorrhage after pancreato-biliary surgery according to the site of bleeding: re-laparotomy or interventional radiology. J Hepatobiliary Pancreat Surg. 2009;16:56–63.
Tajima Y, Kuroki T, Tsutsumi R, et al. Extrahepatic collaterals and liver damage in embolotherapy for ruptured hepatic artery pseudoaneurysm following hepatobiliary pancreatic surgery. World J Gastroenterol. 2007;13:408–13.
Hur S, Yoon CJ, Kang SG, et al. Transcatheter arterial embolization of gastroduodenal artery stump pseudoaneurysms after pancreaticoduodenectomy: safety and efficacy of two embolization techniques. J Vasc Interv Radiol. 2011;22:294–301.
Taqi MA, Quadri SA, Puri AS, et al. A prospective multicenter trial of the TransForm occlusion balloon catheter: trial design and results. Interv Neurol. 2018;7:53–64.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by SI, YM, RM, SF, MT, HK, YH, YT, SG, and HT. The first draft of the manuscript was written by SI and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human rights
Not applicable.
Informed consent
Informed consent was obtained from the patient to publish this case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ida, S., Morita, Y., Muraki, R. et al. Anterior superior pancreaticoduodenal artery pseudoaneurysm after distal pancreatectomy with en bloc celiac axis resection successfully treated with balloon-assisted coil embolization. Clin J Gastroenterol 15, 1198–1203 (2022). https://doi.org/10.1007/s12328-022-01710-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-022-01710-9