Abstract
Von Hippel-Lindau disease (VHL) is frequently associated with pancreatic neuroendocrine tumors (PNETs). Here, we report a case of tumor-to-tumor metastasis in a VHL patient in whom colon cancer metastasized to the interior of a PNET. A 65-year-old man had undergone bilateral adrenalectomy for pheochromocytomas in both adrenal glands in his 50 s. Genetic screening was performed considering his family history of pheochromocytoma, and he was diagnosed with VHL. PNET was detected, for which the patient was regularly monitored by follow-up imaging. One year ago, the patient underwent right hemicolectomy to remove a tumor in the ascending colon (pT3N0M0, pStage IIA). He was admitted to our department for detailed examination because the pancreatic tumor had grown, and thus, pancreaticoduodenectomy was performed. Diagnostic imaging and histological findings indicated tumor-to-tumor metastasis, in which the patient’s previous colon cancer had metastasized to and proliferated within the PNET. Colon cancer metastasizing to a PNET is extraordinarily rare and has never been reported in the literature. Thus, practitioners should be vigilant for tumor-to-tumor metastasis when performing imaging surveillance of PNETs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Von Hippel-Lindau disease (VHL) is an autosomal dominant hereditary disease and is frequently associated with pancreatic neuroendocrine tumors (PNETs). PNETs associated with VHL metastasize at a lower rate than typical PNETs; thus, they are typically monitored by surveillance imaging. Tumor-to-tumor metastasis is a phenomenon in which one tumor type serves as the recipient for a second, biologically unrelated, primary donor tumor within the same patient [1]. Here, we report a very rare case of tumor-to-tumor metastasis in a VHL patient whose colon cancer metastasized to the interior of a PNET and was detected during surveillance imaging.
Case report
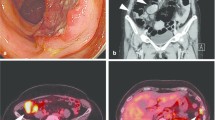
A 65-year-old man was referred to our department for a detailed examination of a pancreatic tumor. In his 30 s, he underwent distal gastrectomy (B-II reconstruction) for a perforated duodenal ulcer. In his 50 s, he underwent bilateral adrenalectomy to remove pheochromocytomas from both adrenal glands. At this time, genetic screening was performed considering his family history of pheochromocytoma, and he was diagnosed with VHL. A systemic search revealed a 12-mm-sized plethoric tumor in the uncus of the pancreas (Fig. 1a), in addition to spinal hemangioblastomas, which was to be followed up with surveillance imaging. One year before his first visit to our department, he underwent right hemicolectomy to remove the ascending colon cancer (UICC TNM Classification, 8th edition: T3(SS)N0M0, Stage IIA).
He had no symptoms. Blood test results revealed slight elevation in the levels of the tumor marker carcinoembryonic antigen (15.4 ng/mL) but no signs of carbohydrate antigen 19-9 elevation (12.6 U/ml) or abnormal glucose tolerance. Urinary total metanephrines (metanephrine + normetanephrine) were within normal limits. Contrast computed tomography revealed that the well-enhanced tumor in the uncus of the pancreas had grown to 22 mm, and a hypodense ischemic region had developed inside it, showing a ring-shaped enhancement effect (Fig. 1b). This core region was hypointense on T1 and T2-weighted magnetic resonance imaging (MRI); diffusion was restricted at the tumor margin on diffusion-weighted MRI. On dynamic MRI, the tumor interior was poorly enhanced, but ring-shaped enhancement appeared at the margin in early phase images and persisted until later phases (Fig. 2a–d). On endoscopic ultrasonography, the tumor appeared as a hypoechoic mass with a slightly hyperechoic central component (Fig. 3); however, we could not perform endoscopic ultrasonography-guided fine-needle aspiration cytology because the access route could not be secured due to his surgically altered gastrointestinal anatomy. Therefore, we diagnosed the plethoric tumor as a PNET and attributed the ischemic region in its core to intratumoral hemorrhage. Pancreaticoduodenectomy was planned given the tumor’s large maximum diameter (22 mm) with rapid doubling time (415 days). Histological specimens prepared from the resected tissue were examined to establish a pathological diagnosis. While classic NET cells (G1) circumscribed the tumor margin, moderately differentiated adenocarcinoma and signs of necrosis were observed in the interior (Fig. 4a–c). This adenocarcinomatous region had histopathological features similar to specimens prepared from the resected colon tumor. On immunohistochemical examination, both specimens were negative for CK7 and positive for CK20 and CDX2 (Fig. 5a–f). Genetic testing revealed the same KRAS mutation in both tumors (G12V). Based on these findings, the adenocarcinomatous region inside the excised tumor was diagnosed as metastatic colon cancer, representing a rare case of tumor-to-tumor metastasis. After surgery, carcinoembryonic antigen normalized and no recurrence has been detected for more than 21 months (Fig. 6).
Magnetic resonance imaging (MRI). a: T2-weighted MRI showed a hypointense core region (arrow). b–d: Dynamic contrast-enhanced MRI showed a ring-shaped enhancement that appeared at the margin in early phase images and persisted until delayed phases (arrow) (b: plain phase, c: early phase, d: delayed phase)
Histopathological findings. a: Gross photograph of a pancreatic tumor (arrow heads). Microscopic area is indicated with a square. b, c: Pathological images of Hematoxylin and Eosin staining showed classic NET cells (G1) of resected pancreas region circumscribed the tumor margin, moderately differentiated adenocarcinoma and signs of necrosis were observed in the interior (magnification. b: × 40, c × 100)
Pathological images of immunohistochemical examination showed that both the adenocarcinomatous region of the pancreas a–c and the resected colon tumor specimen d–f- were negative for CK7 and positive for CK20 and CDX2 (a, d: CK7 staining, b, e: CK20 staining, c, f: CDX2 staining) (magnification. a-f: × 100)
Discussion
VHL is an autosomal dominant hereditary disease characterized by multiple and recurrent neoplastic lesions in a variety of organs. In addition to PNETs, which are estimated to affect 10–17% of patients with VHL, CNS hemangioblastomas, retinal hemangioblastomas, endolymphatic sac tumors, pheochromocytomas, renal cell carcinomas, and pancreatic cysts have been described in VHL patients [2,3,4].
PNETs associated with VHL metastasize at a lower rate than typical PNETs. In addition, VHL patients often require multiple operations to manage non-neuroendocrine tumors that develop in other organs. For these reasons, practitioners should be cautious when deciding whether surgery is suitable for VHL-associated PNETs [5, 6]. Blansfield, Ganeshan, and others proposed the following criteria as surgical indications for PNETs in VHL patients: (1) tumor size > 3 cm (or > 2 cm for lesions in the head of the pancreas), (2) tumor doubling time < 500 days, (3) mutation in exon 3, and (4) suspicion of regional nodal metastases [4, 7]. After fully explaining our rationale to the patient, we decided to perform pancreaticoduodenectomy because of the tumor’s diameter and doubling rate. Based on diagnostic histopathological examination findings, we identified an adenocarcinomatous region in the inner core of the PNET; based on confirmatory evidence from immunohistochemical and genetic testing, we finally diagnosed it as tumor-to-tumor metastatic colon cancer.
Tumor-to-tumor metastasis has become more widely recognized in recent years. The following four criteria have been proposed as a definition of tumor-to-tumor metastasis: (1) more than one primary tumor must exist within the same patient; (2) the recipient tumor must be a true neoplasm; (3) the metastatic neoplasm should be a true metastasis with established growth within the host tumor and not the result of contiguous growth or embolization of tumor cells; and (4) tumors that have metastasized to the lymphatic system where lymphoreticular malignant tumors already exist are excluded [1, 8]. All these criteria were met in this case.
Cancers that are richly vascularized and have slow growth rates are theorized to be most receptive to tumor-to-tumor metastasis, and renal cell carcinoma, sarcoma, and meningioma are common recipient tumors. Conversely, lung and breast cancers are regarded as the most common donors (i.e., sites of origin) in tumor-to-tumor metastasis [9]. Despite being well-vascularized, PNETs are unlikely to host a secondary tumor because they require immediate surgery. In this case, the patient was scheduled for routine surveillance to monitor his PNET because of his status as a VHL patient; metastasis likely occurred during follow-up.
Only two cases of tumor-to-tumor metastasis to a PNET have been reported; the donor was renal cell carcinoma in both instances [10, 11]. Since renal cell carcinoma is the most common origin of metastatic tumors of the pancreas, it is also considered a likely donor of intratumor metastasis to the PNETs [12,13,14].
In our case, the donor was the colon cancer, which is unusual. Practitioners engaged in follow-up surveillance of PNET patients should exercise special caution when examining patients who have concurrent breast, lung, or colon cancers or any other malignancies known to frequently metastasize to the pancreas, not just renal cell carcinoma, and should continually monitor their course.
In conclusion, this report describes a very rare case of tumor-to-tumor metastasis in a VHL patient whose colon cancer metastasized to the interior of a PNET. Colon cancer metastasizing to a PNET is extraordinarily rare and has never been reported in the literature. Practitioners should be vigilant for tumor-to-tumor metastasis when performing imaging surveillance of PNETs.
References
Widdel L, Kleinschmidt-DeMasters BK, Kindt G. Tumor-to-tumor metastasis from hematopoietic neoplasms to meningiomas: report of two patients with significant cerebral edema. World Neurosurg. 2010;74:165–71.
Hammel PR, Vilgrain V, Terris B, et al. Pancreatic involvement in von-Hippel-Lindau disease. The groupe francophone d’Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119:1087–95.
Lonser RR, Glenn GM, Walther M, et al. Von Hippel-Lindau disease. Lancet. 2003;361:2059–67.
Blansfield JA, Choyke L, Morita SY, et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs). Surgery. 2007;142:814–8.
Yamasaki I, Nishimori I, Ashida S, et al. Clinical characteristics of pancreatic neuroendocrine tumors in Japanese patients with von Hippel-Lindau disease. Pancreas. 2006;33:382–5.
Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: nationwide survey analysis. J Gastroenterol. 2015;50:58–64.
Ganeshan D, Menias CO, Pickhardt PJ, et al. Tumors in von Hippel-Lindau syndrome: From head to toe-comprehensive state-of-art review. Radiographics. 2018;38:849–66.
Campbell LV Jr, Gilbert E, Chamberlain CR Jr, et al. Metastases of cancer to cancer. Cancer. 1968;22:635–43.
Erdogan H, Aydin MV, Tasdemiroglu E. Tumor-to-tumor metastasis of the central nervous system. Turk Neurosurg. 2014;24:151–62.
Cenkowski M, Gibson IW, Lategan B, et al. Tumor-to-tumor metastasis: report of a case of renal cell carcinoma metastasizing to a pancreatic endocrine neoplasm. J Clin Oncol. 2011;29:e303–4.
Bednarek-Rajewska K, Zalewski P, Bręborowicz D, et al. Renal cell carcinoma metastasizing to pancreatic neuroendocrine neoplasm-the second case described in the world literature. Pol J Pathol. 2017;68:82–5.
Minni F, Casadei R, Perenze B, et al. Pancreatic metastases: observations of three cases and review of the literature. Pancreatology. 2004;4:509–20.
Crippa S, Angelini C, Mussi C, et al. Surgical treatment of metastatic tumors to the pancreas: a single center experience and review of the literature. World J Surg. 2006;30:1536–42.
Hung JH, Wang SE, Shyr YM, et al. Resection for secondary malignancy of the pancreas. Pancreas. 2012;41:121–9.
Acknowledgements
The authors thank Editage for the critical reading of the manuscript and English language review.
Author information
Authors and Affiliations
Contributions
NH, KJ, and TS wrote the manuscript; YS, TM, YR, MM, and TT treated the patient, UH performed histological analysis; all authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Human/animal rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Written informed consent was obtained from the patients for publication of these case reports and accompanying images.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Natsui, H., Kohisa, J., Yoshikawa, S. et al. Tumor-to-tumor metastasis of colon cancer metastasizing to a pancreatic neuroendocrine tumor associated with von Hippel-Lindau disease: a case report. Clin J Gastroenterol 15, 1173–1178 (2022). https://doi.org/10.1007/s12328-022-01684-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-022-01684-8