Abstract
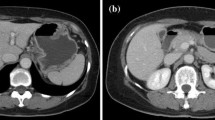
We describe a case of autoimmune hepatitis (AIH) that may have occurred following drug-induced liver injury with camostat mesilate and/or benzbromarone in an elderly patient. The patient’s liver biopsy showed chronic active hepatitis and autoimmune hepatitis. Stopping the use of these drugs did not lead to complete remission, but the use of a low dose of corticosteroids completely cured his liver dysfunction. In the present case, liver dysfunction was caused by an autoimmune mechanism. Special attention should be paid to idiopathic AIH and drug-induced AIH in elderly patients.


Similar content being viewed by others
References
Suzuki A, Brunt EM, Kleiner DE, et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011;54:931–9. doi:10.1002/hep.24481.
Scully LJ, Clarke D, Barr RJ. Diclofenac induced hepatitis. 3 cases with features of autoimmune chronic active hepatitis. Dig Dis Sci. 1993;38:744–51.
Kanda T, Yokosuka O, Tada M, et al. N-nitroso-fenfluramine hepatotoxicity resembling chronic hepatitis. J Gastroenterol Hepatol. 2003;18:999–1000.
Naito A, Terada J, Tanabe N, et al. Autoimmune hepatitis in a patient with pulmonary arterial hypertension treated with endothelin receptor antagonists. Intern Med. 2014;53:771–5.
Hochman JA, Woodard SA, Cohen MB. Exacerbation of autoimmune hepatitis: another hepatotoxic effect of pemoline therapy. Pediatrics. 1998;101:106–8.
Hisamochi A, Kage M, Ide T, et al. An analysis of drug-induced liver injury, which showed histological findings similar to autoimmune hepatitis. J Gastroenterol. 2015 [Epub ahead of print]
van der Klauw MM, Houtman PM, Stricker BH, Spoelstra P. Hepatic injury caused by benzbromarone. J Hepatol. 1994;20:376–9.
Wagayama H, Shiraki K, Sugimoto K, et al. Fatal fulminant hepatic failure associated with benzbromarone. J Hepatol. 2000;32:874.
Arai M, Yokosuka O, Fujiwara K, et al. Fulminant hepatic failure associated with benzbromarone treatment: a case report. J Gastroenterol Hepatol. 2002;17:625–6.
Babany G, Larrey D, Pessayre D, et al. Chronic active hepatitis caused by benzarone. J Hepatol. 1987;5:332–5.
Kaufmann P, Török M, Hänni A, et al. Mechanisms of benzarone and benzbromarone-induced hepatic toxicity. Hepatology. 2005;41:925–35.
CAMOSTAT MESILATE (in Japanese). http://database.japic.or.jp/pdf/newPINS/00060620.pdf#search=Camostat+mesilate. Accessed 29 Feb 2016.
Kanda T, Yokosuka O, Fujiwara K, et al. Fulminant hepatic failure associated with triazolam. Dig Dis Sci. 2002;47:1111–4.
Ichida F, Tsuji T, Omata M, et al. New Inuyama classification; new criteria for histological assessment of chronic hepatitis. Int Hepatol Commun. 1996;6:112–9.
Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–38.
Hennes EM, Zeniya M, Czaja AJ, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–76.
Takikawa H, Onji M, Takamori Y, et al. Proposal of diagnostic criteria of drug induced hepatic injury in DDW-J2004 workshop. Kanzo (in Japanese). 2005;46:85–90.
Kuo CF, Yu KH, Luo SF, et al. Gout and risk of non-alcoholic fatty liver disease. Scand J Rheumatol. 2010;39:466–71.
Rabinowich L, Shibolet O. Drug induced steatohepatitis: an uncommon culprit of a common disease. Biomed Res Int. 2015;2015:168905.
Tandra S, Yeh MM, Brunt EM, et al. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J Hepatol. 2011;55:654–9.
Czaja AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci. 2011;56:958–76.
Björnsson E, Talwalkar J, Treeprasertsuk S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51:2040–8.
Abe M, Mashiba T, Zeniya M, et al. Present status of autoimmune hepatitis in Japan: a nationwide survey. J Gastroenterol. 2011;46:1136–41.
Acknowledgments
The authors thank all the staff at Chiba University Hospital for the patient’s care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest:
Tatsuo Kanda received a research grant from Chugai Pharmaceutical and MSD; Osamu Yokosuka received a research grant from Chugai Pharmaceutical, Bayer, MSD, Daiichi-Sankyo, Tanabe-Mitsubishi, Bristol-Myers Squibb, Taiho Pharmaceutical, and Gilead Sciences; Junichiro Kumagai, Shin Yasui, Yuki Haga, Reina Sasaki, Masato Nakamura, Shuang Wu, Shingo Nakamoto, Makoto Arai and Yotaro Iino declare that they have no conflict of interest.
Human/Animal Rights:
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments.
Informed Consent:
Informed consent was obtained from the patient in the report.
Rights and permissions
About this article
Cite this article
Kumagai, J., Kanda, T., Yasui, S. et al. Autoimmune hepatitis following drug-induced liver injury in an elderly patient . Clin J Gastroenterol 9, 156–159 (2016). https://doi.org/10.1007/s12328-016-0648-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-016-0648-5