Abstract
A 42-year-old Japanese woman complained of upper abdominal pain. Endoscopic examination demonstrated an elevated lesion in the body of the stomach, and a biopsy specimen demonstrated proliferation of atypical spindle cells. She underwent partial gastrectomy; the resected tumor measured 3.5 × 2.8 × 1.2 cm in size. Histological examination disclosed the haphazard proliferation of spindle cells in the mucosa mixed with less prominent epithelioglandular component. The spindle cells were positive for cytokeratin, vimentin, EMA and CD99, but not for KIT, DOG1, desmin or S100. Reverse transcription-polymerase chain reaction using paraffin sections amplified a SYT-SSX1 chimera transcript. A diagnosis of synovial sarcoma was made. There has been no sign of recurrence or metastasis for 6 years after the operation. Synovial sarcoma in the stomach is very rare. Since differential diagnosis of synovial sarcoma from carcinosarcoma and mesenchymal tumors is critical for the treatment and prediction of prognosis, accurate diagnosis with molecular analysis is essential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Synovial sarcoma is a mesenchymal tumor of unknown origin [1]. The tumor is characterized by a variable degree of epithelial differentiation and by translocation t (X;18) (p11;q11) forming a characteristic SYT-SSX chimera gene. The tumor develops mainly in the extremities of young adults, but exceptionally in the gastrointestinal (GI) tract. Correct diagnosis is crucial for the appropriate treatment and prediction of prognosis. Here, we report a case of primary synovial sarcoma arising in the stomach.
Case report
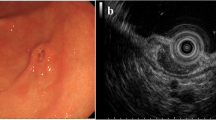
A 42-year-old Japanese woman complained of upper abdominal pain. She had no remarkable past medical or family history. Her laboratory data showed anemia, and the serum levels of CEA and CA19-9 were within normal limits. Endoscopic examination revealed an elevated lesion in the body of the stomach (Fig. 1a). The surface of the tumor was covered with normal mucosa, on the top of which ulcer was noted (Fig. 1b). Abdominal computed tomography (CT) showed an elevated lesion protruding into the luminal side of the stomach (Fig. 1c). The tumor was confined to the mucosa, and no tumor was detected in the serosa. There was no focus of metastasis to lymph nodes or to the liver. Endoscopic biopsy revealed the proliferation of spindle cells in the propria, suggesting poorly differentiated adenocarcinoma and carcinosarcoma of the stomach. The patient underwent partial gastrectomy. Through the pathological examination and molecular analysis, a diagnosis of synovial sarcoma was made. The patient received no adjuvant therapy, and there has been no evidence of local recurrence (Fig. 1d) or distant metastasis for 6 years after the operation.
Pathological findings
The resected tumor was 3.5 × 2.8 × 1.2 cm in size (Fig. 2a). Histological examination showed that the tumor was confined from the propria of the mucosa to the submucosal layer of the gastric wall. The surface of the tumor was ulcerated (Fig. 2b). The tumor was composed of bundles of spindle cells with slightly swollen nuclei, and the spindle cells infiltrated into the propria (Fig. 2c). In most areas of the tumor, the spindle cells proliferated monotonously (Fig. 2d), and glandular structures composed of epithelioid cells with atypical nuclei were sparsely mixed (Fig. 2e). There was no apparent chondroid, osteoid or muscular component. Vascular and perineurial invasion was not found. Mitotic count was 2 in 10 high power fields (HPFs). Margins of the resected tumor were negative.
Pathological features of the gastric tumor. a The loupe image of the resected tumor. b The surface of the tumor was ulcerated. c The tumor cells infiltrated into the propria. d Sheet-like proliferation of spindle cells. e Glandular structure with atypical cells was also observed. f, g Positive immunostaining for cytokeratin (AE1/AE3) in spindle cells (f) and epithelioid cells with glandular structure (g). h Spindle cells showed strong positive reaction for CD99. i Ki-67 index was ~20 %. (c ×40, d ×100, d–i ×200)
Immunostaining was done on the 4-μm-thick paraffin sections by Ventana GX System (Roche Diagnostics KK, Tokyo, Japan). The antibodies used in this study and the results are summarized in Table 1. The spindle cells showed focal positive reaction for cytokeratin (Fig. 2f), and epithelioid cells with glandular structure also showed positive reaction (Fig. 2g). Spindle cells showed positive reactions for EMA, Bcl2 and CD99 (Fig. 2h). No positive reaction was found with other antibodies (Table 1). The Ki67 index was calculated as ~20 % (Fig. 2i).
Molecular analysis
The SYT-SSX chimera transcript was analyzed by reverse transcription (RT)-polymerase chain reaction (PCR) using total RNA extracted from paraffin sections. As a control case, gastric adenocarcinoma was used. Total RNA was extracted using RNeasy FFPE Kit (Qiagen, KK, Tokyo, Japan). Briefly, two paraffin sections of the tumors were deparaffinized and transferred to 1.5 ml tubes. The tissues were digested with proteinase K solution, and total RNA was extracted with a spin column according to the protocol recommended by the manufacturer. Complementary DNA (cDNA) was synthesized from 1 μg total RNA by RT using the SuperScript III First Strand cDNA Synthesis System (Invitrogen Corp., Tokyo, Japan). A non-RT sample was prepared in the same way except that the reaction was done without reverse transcriptase. The SYT-SSX chimera transcript was amplified by PCR using primers SYT: 5′-CAG CAG AGG CCT TAT GGA TAT GA-3′ and SSX: 5′-TCA TTT TGT GGG CCA GAT GC-3′, which were reported by Guillou [2]. cDNA transcribed from 100 ng total RNA and non-RT sample was used as a template. The amplified product was electrophoresed in Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Yokohama, Japan). The PCR product was subcloned into pCRII vector (Invitrogen Corp.) and sequenced using a BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Inc., Tokyo, Japan).
A single amplified product was obtained in the RT sample of the current case (Fig. 3a). No amplification was observed in the non-RT sample of the current case, and in RT and non-RT samples of gastric adenocarcinoma. Sequencing of the subcloned fragment revealed that the product was 101 base pairs in length, and sequencing of the product determined that the product is a chimera transcript of SYT and SSX1 genes (Fig. 3b).
Discussion
The current case presented a localized elevated lesion of the gastric mucosa, resembling a submucosal tumor (SMT). The tumor was mainly located in the mucosa and submucosa, and invasion beyond the muscular layer was not found. Histologically, the tumor was composed of spindle cells, which showed positive reactions for cytokeratin, EMA and CD99. The final diagnosis of synovial sarcoma was obtained by the demonstration of a chimera transcript of SYT-SSX1 by RT-PCR using paraffin sections.
Primary synovial sarcoma is rare in the stomach. To date, only 13 cases have been reported in the English-language literature (Table 2); [3–5]. This is the first report of a Japanese case of primary gastric synovial sarcoma. Among the 14 reported cases, there were 5 males and 9 females. The ages ranged from 29 to 68 years, and the mean is 50 years. The cases followed a favorable clinical course. Most of the cases were monophasic subtype, and foci of poorly differentiated component were noted in two cases [3, 4]. Definitive diagnosis was obtained by intensive immunohistochemical studies and molecular analyses using fluorescence in-situ hybridization (FISH) and RT-PCR [3–5].
The spindle cell growth in the gastric wall in our case resembled those of other SMTs arising in the stomach, such as gastrointestinal stromal tumor (GIST), leiomyoma, leiomyosarcoma and Schwannoma. A careful differential diagnosis was thus required. In the current case, the spindle cells were negative for the markers of other mesenchymal tumors (Table 1). In contrast, they showed positive for cytokeratin and EMA, as did epithelioid cells with glandular structure, reminiscent of the pathological features of carcinosarcoma.
Carcinosarcoma comprises two subtypes: true carcinosarcoma and “so-called” carcinosarcoma. True carcinosarcoma refers a tumor mixed with adenocarcinoma and sarcomatous components with chondroid, osteoid and muscular differentiations. So-called carcinosarcoma is a tumor composed of a bland growth of spindle cells. It should be noted that the most of the reported cases of synovial sarcoma in the stomach exhibited a monophasic pattern (Table 2); [3–5]. The differential diagnosis of synovial sarcoma from so-called carcinosarcoma is thus essential. Cytokeratin and EMA can be positive in either tumor. Immunostaining for CD99 may be useful. The positive reaction for CD99 is a characteristic feature of synovial sarcoma in the soft tissues, as documented in the previous two cases of gastric synovial sarcoma [4, 5] and in the current case.
Molecular analysis is indispensable for confirming a diagnosis of synovial sarcoma. Translocation of chromosomes t(X;18) gives rise to a SYT-SSX chimera transcript, which is detected exclusively in synovial sarcoma [6]. For the detection of this gene, FISH and RT-PCR are frequently applied to paraffin sections. In the current case, we were able to identify the genetic alteration by RT-PCR, and the fusion of SYT and SSX1 was confirmed by sequencing. In the previous studies, 4 cases had SYT-SSX1 and 4 cases had SYT-SSX2 chimera transcript [4, 5]. It was reported that the epithelial differentiation is more prominent and metastasis is more frequent in the synovial sarcomas of the soft tissue with SYT-SSX1 [7]. The clinical significance of the genetic alterations of the primary gastric synovial sarcoma needs to be elucidated.
Compared to gastric carcinosarcoma [8, 9], synovial sarcoma affects slightly younger people. While male predominance was reported in carcinosarcoma, female predominance is noted in gastric synovial sarcoma (Table 2); [3–5]. Primary gastric synovial sarcoma follows a favorable course. On the other hand, prognosis of carcinosarcoma is poor, and approximately half of the patients died within 6 months [9]. Among these, there are a couple of cases with a relatively favorable prognosis [8, 10]. Although early surgical treatment is essential for better prognosis, it is speculated that some primary gastric synovial sarcoma might have been diagnosed as carcinosarcoma. Accurate distinction between synovial sarcoma and carcinosarcoma is necessary for the determination of postoperative treatment and prediction of prognosis.
The histological prognostic factors of synovial sarcoma of the stomach are not yet known. Although the number of cases of primary gastric synovial sarcoma is limited, their histology is not different from those in the soft tissue. It was reported that patients with tumor <5 cm in size, <10 mitoses in 1.7 mm2 and without necrosis follow a favorable course in cases of synovial sarcoma of soft tissue [11]. Similarly, in primary gastric synovial sarcoma, four out of six patients with tumor >5 cm in diameter recurred or died of the disease (Table 2). Mitotic count varied widely from case to case [4, 5]. Two patients with poorly differentiated component died of metastatic disease [3, 4]. It was also reported that vascular invasion and perineurial invasion could be a risk factor for recurrence and metastasis of synovial sarcoma of soft tissue. Hence, pathological diagnosis of primary gastric synovial sarcoma should include the size, mitotic count, the presence or absence of necrosis and poorly differentiated component, vascular and perineurial invasions and the presence or absence of tumors cells at the margin. The risk factors should be validated by accumulating cases.
References
Suurmeijer AJH, de Bruijn D, van Geurts Kessel A, Miettinen MM. Synovial sarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. “WHO classification of tumours of soft tissue and bone”. Lyon: International Agency for Research on Cancer; 2013. p. 213–5.
Guillou L, Coindre J, Gallagher G, Terrier P, Gebhard S, de Saint Aubain Somerhausen N, et al. Detection of the synovial sarcoma translocation t(X;18) (SYT;SSX) in paraffin-embedded tissues using reverse transcriptase-polymerase chain reaction: a reliable and powerful diagnostic tool for pathologists. A molecular analysis of 221 mesenchymal tumors fixed in different fixatives. Hum Pathol. 2001;32:105–12.
Billings SD, Meisner LF, Cummings OW, Tejada E. Synovial sarcoma of the upper digestive tract: a report of two cases with demonstration of the X;18 translocation by fluorescence in situ hybridization. Mod Pathol. 2000;13:68–76.
Makhlouf HR, Ahrens W, Agarwal B, Dow N, Marshalleck JJ, Lee EL, et al. Synovial sarcoma of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 10 cases. Am J Surg Pathol. 2008;32:275–81.
Wang CC, Wu MC, Lin MT, Lee JC. Primary gastric synovial sarcoma. J Formos Med Assoc. 2012;111:516–20.
Ladanyi M, Antonescu CR, Leung DH, Woodruff JM, Kawai A, Healey JH, et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res. 2002;62:135–40.
Ladanyi M. Fusions of the SYT and SSX genes in synovial sarcoma. Oncogene. 2001;20:5755–62.
Ikeda Y, Kosugi S, Nishikura K, Ohashi M, Kanda T, Kobayashi T, Hatakeyama K. Gastric carcinosarcoma presenting as a huge epigastric mass. Gastric Cancer. 2007;10:63–8.
Yoshida H, Tanaka N, Tochigi N, Suzuki Y. Rapidly deforming gastric carcinosarcoma with osteoblastic component: an autopsy case report. World J Gastroenterol. 2012;18:4064–8.
Ashida K, Wamata T, Sugesawa A, Miyano Y, Iwai N, Tani H. A case of so-called carcinosarcoma of the stomach. J Jpn Surg Assoc. 1998;59:702–6.
Bergh P, Meis-Kindblom JM, Gherlinzoni F, Berlin O, Bacchini P, Bertoni F, et al. Synovial sarcoma: identification of low and high risk groups. Cancer. 1999;85:2596–607.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kamata, K., Wada, R., Yajima, N. et al. Primary gastric synovial sarcoma: molecular diagnosis and prediction of prognosis. Clin J Gastroenterol 6, 303–308 (2013). https://doi.org/10.1007/s12328-013-0403-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-013-0403-0