Abstract
Introduction
Trofinetide is the first drug to be approved for the treatment of Rett syndrome. Hepatic impairment is not expected to affect the pharmacokinetic (PK) profile of trofinetide because of predominant renal excretion. This study was conducted to help understand the potential impact of any hepatic impairment on trofinetide PK.
Methods
This study used physiologically based PK modeling to estimate trofinetide exposure (maximum drug concentration and area under the concentration-time curve from time zero to infinity) in virtual patients with mild, moderate, and severe hepatic impairment (per Child-Pugh classification) compared with virtual healthy subjects following a 12 g oral trofinetide dose.
Results
In individual deterministic simulations for matched individuals and stochastic simulations at the population level (100 virtual individuals simulated per population), as anticipated, predicted plasma exposures were similar for healthy subjects and for patients with mild, moderate, and severe hepatic impairment. However, predicted blood concentration exposures slightly increased with increasing severity of hepatic impairment because of change in hematocrit levels.
Conclusion
This study indicates that hepatic impairment is not expected to have a clinically relevant effect on exposure to trofinetide.
Graphical Abstract

Plain Language Summary
Trofinetide is the first approved treatment for Rett syndrome, a rare genetic condition that affects brain development. When a person takes trofinetide, most is removed from the body via the urine in its unchanged form (no chemical alteration). Regulatory requirements mean researchers must confirm the safety of any pharmaceutical drug and evaluate whether changes in liver function lead to harmful levels of drug exposure. Researchers used a computer model to predict how much trofinetide would be present in the blood and plasma (the liquid portion of blood) over time in virtual healthy subjects and virtual patients with varying degrees of liver disease (mild, moderate, or severe). Computer simulations showed that predicted trofinetide levels in plasma were similar in virtual healthy subjects and each virtual patient group with liver disease. Predicted levels of trofinetide in blood were slightly elevated with increasing severity of liver disease. This is because people with liver disease have fewer red blood cells, so the cell portion of blood becomes smaller relative to the liquid portion (plasma), which leads to higher trofinetide concentrations in whole blood (trofinetide minimally enters the red blood cell). The small increase in trofinetide levels in blood and the absence of any change in trofinetide levels in plasma means that people with Rett syndrome and liver disease are unlikely to be exposed to harmful levels of trofinetide after a 12 g oral dose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Trofinetide, the first drug to be approved for the treatment of Rett syndrome, mainly undergoes renal elimination, but as with any drug, it is still important to confirm whether systemic exposure is affected by varying degrees of hepatic impairment |
This study used physiologically based pharmacokinetic modeling to estimate trofinetide exposure in virtual patients with mild, moderate, and severe hepatic impairment compared with virtual healthy subjects following a 12 g oral trofinetide dose |
What was learned from the study? |
Predicted plasma exposures were similar for healthy subjects and for patients with mild, moderate, and severe hepatic impairment. Predicted blood concentration exposures slightly increased with increasing severity of hepatic impairment due to change in hematocrit levels |
This study indicates that hepatic impairment is not expected to have a clinically relevant effect on exposure to trofinetide; however, when renal comorbidity is also present, patients may need closer monitoring and/or trofinetide dose adjustment |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.26015668.
Introduction
Rett syndrome (RTT) is a rare, X-linked neurodevelopmental disorder, primarily caused by loss-of-function mutations in the MECP2 gene which encodes methyl-CpG-binding protein 2 (MeCP2), a DNA-binding protein involved in the epigenetic regulation of gene expression [1] whose deficiency leads to abnormal neuronal maturation and plasticity [2,3,4]. RTT affects mainly females, with an incidence of approximately 1 in 10,000–15,000 female births worldwide [5,6,7,8] but also affects a smaller number of males [9]. The life expectancy of people with RTT has improved over time, with many individuals now surviving into adulthood; however, those affected require lifelong support and medical care from caregivers and healthcare systems [8, 10, 11].
In 2009, Tropea et al. found that an active tripeptide fragment (glycine-proline-glutamate [GPE]) of insulin-like growth factor 1, which occurs naturally in the brain, partially reversed RTT-like symptoms when administered to Mecp2-deficient mice [12]. Trofinetide (DAYBUE™, Acadia Pharmaceuticals Inc., CA, USA), the first and only US Food and Drug Administration-approved treatment for RTT, is a novel synthetic analog of GPE that is preserved for longer in the blood/plasma versus GPE because of modifications that make it more resistant to degradation by proteases [13, 14]. In clinical studies, twice daily oral administration of trofinetide solution significantly improved clinician- and caregiver-assessed efficacy measures, including in a phase II study of 82 children and adolescent females with RTT [15] and the phase III LAVENDER study in 187 children, adolescents, and adult females with RTT [16]. In the LAVENDER study, the coprimary efficacy endpoints (Rett Syndrome Behaviour Questionnaire and Clinical Global Impression-Improvement scale) were significantly improved after 12 weeks of treatment with trofinetide compared with placebo. A post hoc CGI-I responder analysis (responders defined as score ≤ 3 at week 12) showed a greater proportion of responders in the trofinetide group (37.7%) compared with placebo (15.2%). Trofinetide had an acceptable safety and tolerability profile in the LAVENDER study with diarrhea and vomiting reported as the most frequent treatment emergent adverse events in the trofinetide (80.6% and 26.9%, respectively) and placebo groups (19.1% and 9.6%, respectively).
Despite evidence of perturbations in cholesterol metabolism and fatty liver disease in Mecp2-deficient mice [17], hepatic disease is rarely reported as a comorbidity in individuals with RTT. Nevertheless it is feasible that individuals with RTT may have coexisting but rare hepatic morbidities that can lead to drug accumulation [18, 19]; therefore, understanding the pharmacokinetics (PK) of trofinetide in individuals with hepatic impairment is of clinical interest to manage dosing in these rare cases. Clinical PK data in healthy adult subjects indicate that trofinetide predominantly undergoes renal elimination [20], with < 5% metabolic elimination [21]. Therefore, it is expected that patients with hepatic impairment and no underlying renal impairment would not have higher trofinetide exposures. Despite the lack of hepatic metabolism with trofinetide and the rarity of liver failure in RTT, for regulatory purposes it is necessary to confirm whether dose modifications are required where there is the potential for exposure to be significantly altered. As there are no previously published studies investigating the PK of trofinetide in individuals with hepatic impairment, the present study employed a physiologically based PK (PBPK) modeling approach to estimate trofinetide exposure in virtual patients with mild, moderate, and severe hepatic impairment and normal renal function compared with virtual healthy subjects.
Methods
Overview of Trofinetide PBPK Model
Modeling of trofinetide exposure was carried out using a validated PBPK model [22]. GastroPlus® version 9.7 (Simulation Plus, Lancaster, CA) and PBPKPlus™ were used to perform the PBPK simulations. Microsoft Excel and R software version 3.4.3 (Foundation for Statistical Computing, Vienna, Austria) were used for the compilation and post-processing of GastroPlus-simulated output. Other analyses and generation of graphs were performed using a family of Tidyverse packages in R.
The clinical studies used to validate the PBPK model were conducted in accordance with the ethical standards of the local Institutional Review Boards for each site and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained by the parent or legal guardian on behalf of the study participants.
To evaluate the possible impact of hepatic impairment on the PK of trofinetide, various trofinetide dosing scenarios were simulated using validated healthy adult subject physiologies and physiologies representing the stages of hepatic impairment using Child-Pugh classification [23] (A = mild impairment, B = moderate impairment, C = severe impairment). Due to general renal impairment comorbidity with hepatic impairment physiologies, an evaluation of the impact of hepatic impairment alone required some modifications to the hepatic impairment physiologies to resemble the renal function in the matching healthy control subjects.
The model incorporated experimental and predicted physicochemical properties of trofinetide alongside information on absorption, dissolution, and elimination that were determined experimentally or optimized during model development and were described previously [22]. Intestinal dissolution, absorption, and metabolism of trofinetide were described using the Advanced Compartmental Absorption and Transit (ACAT™) model. Physiological model parameters (volumes, blood flow, organ weights) were generated using the Population Estimates for Age-Related Physiology (PEAR Physiology™) module within PBPKPlus™. Systemic tissue distribution of trofinetide was modeled using a permeability-limited model for all tissues, and tissue to plasma partition coefficients were predicted by the Poulin and Theil extracellular method [24]. Elimination via renal excretion was defined by glomerular filtration rate (GFR) multiplied by the fraction of unbound drug in the plasma.
The following assumptions were applied to the final trofinetide PBPK model: (1) trofinetide is absorbed from the gut via a passive diffusion process; (2) elimination in urine occurs via passive renal filtration with no measurable amount of metabolism; (3) a trofinetide blood-to-plasma concentration ratio (Rbp) value of 0.525 was used for healthy adult subjects, which was based on nonclinical findings and was confirmed in the clinical setting in a PK radiolabeled study [21]. The model-predicted PK profile for trofinetide was validated using available PK data from clinical studies after oral trofinetide solution or intravenous trofinetide administration [22].
Hepatic Impairment Assessment
Deterministic Simulations
Using the standard physiology (except for GFR, as explained at the end of this section) for each of the three defined stages of hepatic impairment (Child-Pugh Class A, Class B, and Class C) and the PBPK model settings developed for trofinetide, single-dose deterministic simulations were run using an oral dose of 12 g trofinetide (the highest intended clinical dose) administered to 65-year-old individuals with and without hepatic impairment. A 65-year-old individual typifies the average age associated with an increased risk of hepatic impairment [25].
Initial deterministic simulations included four age-, body weight-, height-, sex-, and GFR-matched individuals representing a healthy subject and each class of hepatic impairment (Child-Pugh Class A, B, and C). Predicted exposure measures, including the maximum drug concentration (Cmax) and area under the concentration-time curve from time zero to infinity (AUCinf), were determined. The results were presented as both plasma and blood concentrations with the aid of a conversion formula utilizing adjusted hematocrit levels based on the physiologies. Changes in the trofinetide Rbp due to reduced hematocrit values for each hepatic impairment physiology were considered in the predictions of blood concentrations and PK parameters using the following equation:
where: Rbpadult is the blood-to-plasma concentration ratio in healthy adults (0.525 for trofinetide); Rbpadj is the adjusted blood-to-plasma concentration ratio for the assessed population; Hctadj is the hematocrit (expressed as a fraction) in the assessed population; 0.45 represents hematocrit (expressed as fraction) in healthy adults.
Patients with hepatic impairment often present with renal impairment comorbidity [26], which is included in the GastroPlus-defined physiologies for hepatic impairment. The extent of contribution of this renal impairment comorbidity to the trofinetide exposure after oral dosing was attenuated to determine the expected impact due primarily to hepatic impairment. Specifically, for deterministic simulations, the GFR for the hepatic impairment physiologies was set to the defined value for the matched healthy subject physiology.
Stochastic Simulations
The expected population variability in the model-simulated trofinetide PK in patients with hepatic impairment was assessed through population (stochastic) simulations. Virtual patient population characteristics were created within each defined stage of hepatic impairment.
The population simulation module in GastroPlus runs a series of simulations, for a number of virtual individuals, created by random sampling of physiological input parameters to generate a predicted population outcome; this can help evaluate the combined effects of variations in population physiology and formulation variables that are not precise values but for which distributions of values can be estimated. The default variability (coefficient of variation expressed as a percent [%CV]) within GastroPlus was used for all parameters to simulate the potential interindividual variability expected in trofinetide PK.
The GFR for each virtual hepatic impairment population was proportionally adjusted to match the mean GFR for the healthy population. The simulations were run using an oral dose of 12 g for each population.
Bioequivalence Assessment
Based on the stochastic simulations, a parallel design bioequivalence assessment was completed for each hepatic impairment population compared with the matching healthy control population for trofinetide Cmax and AUCinf predictions. Bioequivalence was assessed using an analysis of variance on natural log-transformed Cmax and AUCinf values. Geometric mean ratios and 90% confidence intervals (CIs) were calculated for the natural log-transformed AUCinf and Cmax values. The criterion for passing the bioequivalence test was the 90% CI falling within the limits of 0.80 and 1.25.
Results
Deterministic Simulations of Hepatic Impairment
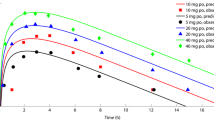
Single deterministic simulations were completed for the virtual healthy subject and each patient representing one of the three classes of hepatic impairment using the default physiologies in GastroPlus, with matched age, body weight, sex, and GFR, and are presented in Fig. 1. Predicted exposures for blood and plasma after trofinetide administration to the virtual subject and the patients were determined and are presented in Table 1. Plasma Cmax was similar for all four groups: 257.5 μg/ml for the patient with mild hepatic impairment, 252.7 μg/ml for the patient with moderate hepatic impairment, 250.9 μg/ml for the patient with severe hepatic impairment, and 262.4 μg/ml for the healthy subject. Both the shape of the plasma exposure profile and predicted AUCinf were similar among the four groups (Fig. 1 and Table 1).
Deterministic simulation of trofinetide exposure in a healthy subject and in patients with hepatic impairment administered a 12 g oral dose of trofinetide. Class A, Class B, and Class C refer to the Child-Pugh classifications of varying degrees of hepatic impairment (mild, moderate, and severe, respectively). Normal represents the age- and body weight-matched healthy subject
As hepatic impairment physiologies have lower hematocrit levels than healthy physiologies, higher trofinetide Rbp was predicted with increasing severity of hepatic impairment (predicted Rbp increased from 0.525 [Class A] to 0.630 [Class C]). In line with this, with conversion of the plasma concentration data to the corresponding blood units, both Cmax and AUCinf increased with increasing severity of hepatic impairment, with the highest blood exposures in the severe hepatic impairment group (Table 1).
Stochastic Simulations of Hepatic Impairment
The demographics of the virtual individuals used in the stochastic simulations of hepatic impairment are presented in Table 2. Populations were well matched by age (range = 55–75 years), body weight (range = 72.5–102.2 kg), height (range = 151–187.9 cm), body surface area (range = 1.7–2.3 m2), and GFR (range = 59.9–147.6 ml/min). Overall reduced hematocrit and increased trofinetide Rbp were predicted with increasing severity of hepatic impairment across the virtual populations.
Stochastic simulations were carried out for each of the four evaluated populations with a 12 g oral administration of trofinetide, with 100 virtual individuals simulated in each population. Results from the population simulations are presented for plasma and blood concentration data in Fig. 2, and predicted PK parameters are presented in Table 3. Consistent with the deterministic simulations, the plasma concentration data for all groups were similar in median, range, and mean outputs. With conversion of the plasma data to the corresponding blood units, both Cmax and AUCinf were increasingly elevated across the three classes of hepatic impairment compared with the healthy subject group. The mild hepatic impairment group showed the least difference in the mean output compared with the healthy subject group.
Box plots showing the predicted blood and plasma trofinetide exposures in healthy subjects and in patients with hepatic impairment administered a 12 g oral dose of trofinetide. Boxes represent the 25th and 75th percentiles, and the lines represent the median. Whiskers extend to the most extreme values within the 1.5 interquartile range. Values outside the range are marked with open circles. Class A, Class B, and Class C refer to the Child-Pugh classifications of varying degrees of hepatic impairment (mild, moderate, and severe, respectively). AUCinf area under the concentration-time curve from time zero to infinity, Cmax maximum drug concentration
Bioequivalence Assessments
For the plasma concentration data (Table 4), all three hepatic impairment populations were bioequivalent to the healthy population based on Cmax and AUCinf. For the blood concentration data, only the mild hepatic impairment group was bioequivalent to the healthy population based on Cmax and AUCinf. Both the moderate and severe hepatic impairment populations were bioequivalent to the healthy group for AUCinf but not Cmax: the upper CI bounds for Cmax were 1.254 and 1.251 for the moderate and severe hepatic impairment populations, respectively. Although these upper bounds did not meet the upper acceptance criterion of 1.250, they were within 0.004 units (moderate impairment) and 0.001 units (severe impairment) of meeting the criterion for bioequivalence.
Discussion
This in silico PBPK modeling study is the first to investigate the effect of hepatic impairment on the PK of trofinetide. PBPK modeling was used to predict the effects of hepatic impairment by accounting for known trofinetide clinical PK properties and the physiologic alterations due to hepatic impairment.
As renal elimination accounts for most of the systemic clearance of trofinetide, with minimal metabolic elimination, hepatic impairment was not expected to impact the PK of trofinetide. There is minimal to no accumulation of trofinetide following multiple-dose administration in healthy subjects and individuals with RTT [14, 15], and, as such, the single-dose PK profile is considered representative of the steady-state profile.
When 12 g oral trofinetide was administered to a virtual healthy subject and virtual patients with hepatic impairment, deterministic simulations predicted trofinetide plasma exposure in healthy subjects to be equivalent to exposure in patients with mild, moderate, or severe hepatic impairment. These findings suggest that hepatic impairment did not impact single-dose trofinetide plasma exposures after oral administration, which indicates there is no accumulation of trofinetide in hepatically impaired individuals given that single dosing is representative of the steady-state profile.
Bioequivalence in whole blood concentrations was lower compared with plasma concentrations, which was attributed to the substantial decrease in hematocrit levels concurrent with worsening hepatic impairment, which consequently increased the Rbp value used for converting trofinetide plasma concentrations to blood concentrations. The estimated low Rbp of 0.525 used for simulations in healthy subjects (based on the experimentally determined Rbp in rat blood) suggests that minimal red blood cell penetration or binding occurs for trofinetide. This drug characteristic, along with the decreased hematocrit in patients with hepatic impairment, resulted in predictions of elevated trofinetide whole blood concentrations in patients with hepatic impairment.
Stochastic simulations demonstrated that the hepatic impairment populations were bioequivalent to the healthy subject population regarding plasma exposures, but only the mild impairment population was bioequivalent based on whole blood exposures. The moderate and severe hepatic impairment groups had exposures bioequivalent to the healthy group for AUCinf but not Cmax. However, the differences in exposures based on Cmax were not clinically relevant given that the moderate and severe hepatic impairment populations had only a small excursion from bioequivalence for Cmax, with the upper CI bounds being within 0.004 (moderate impairment) and 0.001 (severe impairment) units of the acceptance criterion for bioequivalence.
Generally, plasma concentrations are used to determine PK parameters. For a limited number of drugs where there is a better relationship between blood concentration response and dose response, measurement of blood concentration is a valuable surrogate index of drug exposure [27]. As such, the predicted trofinetide plasma concentrations in this hepatic impairment assessment provide a better indicator of the clinically relevant impact of hepatic impairment on trofinetide PK than whole blood concentrations. Despite predicted minor increases in trofinetide exposure in blood in patients with increasing degrees of hepatic impairment, the impact of hepatic impairment on the clinically relevant exposure to trofinetide is expected to be minimal.
The limited impact of hepatic impairment on trofinetide exposure is consistent with the findings of a PBPK model investigating potential drug-drug interactions between trofinetide and cytochrome P450 3A4-metabolized drugs in which it was shown that trofinetide did not affect CYP3A4 enzyme activity in the liver [22]. Nonclinical enzyme inhibition studies (unpublished data) have also previously indicated that trofinetide demonstrates either poor or no inhibition of the following human cytochrome P450 enzymes: CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4.
The limitations of the study should be considered. To evaluate the impact of hepatic impairment alone, the modeling was modified so that renal function reflected that in healthy subjects, as would be accomplished through exclusion criteria in a typical hepatic impairment clinical trial. However, in clinical practice, patients with hepatic impairment could present with a renal impairment comorbidity [28]; given that trofinetide is predominantly eliminated renally, there is the potential that the exposures to trofinetide reported in plasma and whole blood in this study may be underestimated in patients with both hepatic and renal impairment. The potential impact of renal impairment on the PK of trofinetide has been investigated in a phase I, open-label, single-dose study and using a PBPK modeling approach and will be published separately. Other limitations include the absence of virtual physiologies that are more reflective of a typical RTT population characterized by younger age, other comorbidities, and the need for polypharmacy, as these factors could influence drug metabolism.
Conclusion
Using PBPK modeling, the predicted trofinetide plasma exposure in healthy subjects is bioequivalent to the trofinetide plasma exposure in patients with mild, moderate, or severe stages of hepatic impairment. Although there are potential confounding factors to consider in a typical RTT population such as younger age, other comorbidities and polypharmacy that could affect drug metabolism, hepatic impairment is not anticipated to have any clinically relevant effect on trofinetide exposure particularly as trofinetide is known to undergo minimal hepatic metabolism.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to data confidentiality but are available from the corresponding author on reasonable request providing a confidentiality agreement is signed.
References
Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–8.
Baj G, Patrizio A, Montalbano A, Sciancalepore M, Tongiorgi E. Developmental and maintenance defects in Rett syndrome neurons identified by a new mouse staging system in vitro. Front Cell Neurosci. 2014;8:18.
Bedogni F, Cobolli Gigli C, Pozzi D, et al. Defects during Mecp2 null embryonic cortex development precede the onset of overt neurological symptoms. Cereb Cortex. 2016;26:2517–29.
Belichenko PV, Wright EE, Belichenko NP, et al. Widespread changes in dendritic and axonal morphology in Mecp2-mutant mouse models of Rett syndrome: evidence for disruption of neuronal networks. J Comp Neurol. 2009;514:240–58.
Fehr S, Bebbington A, Nassar N, et al. Trends in the diagnosis of Rett syndrome in Australia. Pediatr Res. 2011;70:313–9.
Hagberg B. Rett’s syndrome: prevalence and impact on progressive severe mental retardation in girls. Acta Paediatr Scand. 1985;74:405–8.
Leonard H, Bower C, English D. The prevalence and incidence of Rett syndrome in Australia. Eur Child Adolesc Psychiatry. 1997;6(suppl 1):8–10.
Kirby RS, Lane JB, Childers J, et al. Longevity in Rett syndrome: analysis of the North American Database. J Pediatr. 2010;156:135–138.e1.
Neul JL, Benke TA, Marsh ED, et al. The array of clinical phenotypes of males with mutations in Methyl-CpG binding protein 2. Am J Med Genet B Neuropsychiatr Genet. 2019;180:55–67.
Lane JB, Salter AR, Jones NE, et al. Assessment of caregiver inventory for Rett syndrome. J Autism Dev Disord. 2017;47:1102–12.
Tarquinio DC, Hou W, Neul JL, et al. The changing face of survival in Rett syndrome and MECP2-related disorders. Pediatr Neurol. 2015;53:402–11.
Tropea D, Giacometti E, Wilson NR, et al. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci U S A. 2009;106:2029–34.
Batchelor DC, Lin H, Wen JY, et al. Pharmacokinetics of glycine–proline–glutamate, the N-terminal tripeptide of insulin-like growth factor-1, in rats. Anal Biochem. 2003;323:156–63.
Oosterholt SP, Horrigan J, Jones N, Glass L, Della Pasqua O. Population pharmacokinetics of NNZ-2566 in healthy subjects. Eur J Pharm Sci. 2017;109:S98–107.
Glaze DG, Neul JL, Kaufmann WE, et al. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology. 2019;92:e1912–1925.
Neul JL, Percy AK, Benke TA, et al. Trofinetide for the treatment of Rett syndrome: a randomized phase 3 study. Nat Med. 2023;29:1468–75.
Kyle SM, Vashi N, Justice MJ. Rett syndrome: a neurological disorder with metabolic components. Open Biol. 2018;8: 170216.
Committee for Medicinal Products for Human Use (CHMP). Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with impaired hepatic function. CPMP/EWP/2339/02. 2005. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-pharmacokinetics-medicinal-products-patients-impaired-hepatic-function_en.pdf. Accessed 20 Sep 2022.
Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. Guidance for industry 2003. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pharmacokinetics-patients-impaired-hepatic-function-study-design-data-analysis-and-impact-dosing-and. Accessed 20 Sep 2022.
Darwish M, Youakim JM, Harlick J, DeKarske D, Stankovic S. A phase 1, open-label study to evaluate the effects of food and evening dosing on the pharmacokinetics of oral trofinetide in healthy adult subjects. Clin Drug Investig. 2022;42:513–24.
Darwish M, Nunez R, Youakim JM, Robertson P Jr. Characterization of the pharmacokinetics and mass balance of a single oral dose of trofinetide in healthy male subjects. Clin Drug Investig. 2024;44:21–33.
Darwish M, Youakim JM, Darling I, Lukacova V, Owen JS, Bradley H. A physiologically based pharmacokinetic modeling approach to assess the potential for drug interactions between trofinetide and CYP3A4-metabolized drugs. Clin Ther. 2024;46:194–200.
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9.
Poulin P, Theil F-P. A priori prediction of tissue:plasma partition coefficients of drugs to facilitate the use of physiologically-based pharmacokinetic models in drug discovery. J Pharm Sci. 2000;89:16–35.
Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol. 2015;31:184–91.
Johnson TN, Boussery K, Rowland-Yeo K, Tucker GT, Rostami-Hodjegan A. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet. 2010;49:189–206.
Gross AS. Best practice in therapeutic drug monitoring. Br J Clin Pharmacol. 2001;52(suppl 1):5–9.
Betrosian AP, Agarwal B, Douzinas EE. Acute renal dysfunction in liver diseases. World J Gastroenterol. 2007;13:5552–9.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial support were provided by Lesley Taylor, PhD, and Stuart Murray, MSc, of Evidence Scientific Solutions, Inc. (Philadelphia, PA) and funded by Acadia Pharmaceuticals Inc.
Funding
This trial was sponsored by Acadia Pharmaceuticals Inc. The journal’s Rapid Service and Open Access fees were also funded by Acadia Pharmaceuticals Inc.
Author information
Authors and Affiliations
Contributions
Conceptualization: Mona Darwish. Methodology: Mona Darwish, Obinna N. Obianom, Inger Darling, Viera Lukacova. Data curation, formal analysis, investigation, visualization: Mona Darwish, Obinna N. Obianom, Inger Darling, Viera Lukacova. Writing (original draft/previous versions/approval): Mona Darwish, Obinna N. Obianom, James M. Youakim, Inger Darling, Viera Lukacova, Heather Bradley.
Corresponding author
Ethics declarations
Conflict of Interest
Mona Darwish, James M. Youakim, and Heather Bradley are employees and hold stock of Acadia Pharmaceuticals Inc. Obinna N. Obianom, Inger Darling, and Viera Lukacova are employees and hold stock of Simulations Plus, Inc.
Ethical Approval
The clinical studies that were used to validate the PBPK model were conducted in accordance with the ethical standards of the local Institutional Review Boards for each site and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained by the parent or legal guardian on behalf of the study participants.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Darwish, M., Obianom, O.N., Youakim, J.M. et al. Effect of Hepatic Impairment on Trofinetide Exposures Using an In Silico Physiologically Based Pharmacokinetic Model. Adv Ther 41, 3328–3341 (2024). https://doi.org/10.1007/s12325-024-02926-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02926-6