Abstract
Introduction
Cardiovascular-kidney-metabolic (CKM) syndrome is highly prevalent in the US Medicare population and is projected to increase further. Sodium-glucose co-transporter 2 inhibitors have indications in chronic kidney disease (CKD), heart failure (HF), and type 2 diabetes (T2D), providing protective efficacy across conditions within CKM syndrome. The objective of this study was to develop a model to extrapolate key outcomes observed in pivotal clinical trials to the US Medicare population, and to assess the potential direct cost offsets associated with dapagliflozin therapy.
Methods
All US 2022 Medicare beneficiaries (≥ 65 years of age) eligible to receive dapagliflozin were estimated according to drug label indication and Medicare enrollment and claims data. Incidence of key outcomes from the dapagliflozin clinical program were modelled over a 4-year time horizon based on patient-level data with CKD, HF, and T2D. Published cost data of relevant clinical outcomes were used to calculate direct medical care cost-offset associated with treatment with dapagliflozin.
Results
In a population of 13.1 million patients with CKM syndrome, treatment with dapagliflozin in addition to historical standard of care (hSoC) versus hSoC alone led to fewer incidents of HF-related events (hospitalization for HF, 613,545; urgent HF visit, 98,896), renal events (kidney failure, 285,041; ≥ 50% sustained decline in kidney function, 375,137), and 450,355 fewer deaths (of which 225,346 and 13,206 incidences of cardiovascular and renal death were avoided). In total this led to medical care cost offsets of $99.3 billion versus treatment with hSoC only (dapagliflozin plus hSoC, $310.3 billion; hSoC, $211.0 billion).
Conclusion
By extrapolating data from trials across multiple indications within CKM syndrome, this broader perspective shows that considerable medical care cost offsets may result through attenuated incidence of clinical events in CKD, T2D, and HF populations if treated with dapagliflozin in addition to hSoC over a 4-year time horizon.
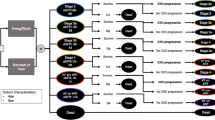
Graphical abstract available for this article.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cardiovascular-kidney-metabolic (CKM) syndrome is highly prevalent in the US Medicare population and is projected to increase further, with healthcare costs disproportionately higher in this population. |
Sodium-glucose co-transporter 2 inhibitors, such as dapagliflozin, are indicated for multiple conditions within CKM syndrome—chronic kidney disease (CKD), heart failure (HF), and type 2 diabetes (T2D). |
Our objective was to extrapolate clinical outcomes observed in CKM syndrome populations from key dapagliflozin trials to the US Medicare population and calculate costs associated with averted outcomes from the perspective of the Centers for Medicare and Medicaid Services (CMS). |
Over 4 years, the avoidance of key clinical outcomes in those treated with dapagliflozin plus historical standard of care, such as hospitalization for HF (613,545), onset of kidney failure (285,041), and death (450,355), resulted in an estimated total medical care cost offset of $99.3 billion versus historical standard of care alone. |
By considering a multidisciplinary approach in the Medicare population, our analysis suggests that patients with comorbidities may experience considerable positive effects through dapagliflozin treatment which may confer considerable offsets. |
Digital Features
This article is published with digital features, including a graphical abstract to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.25959445.
Introduction
The interconnectivity between metabolic risk factors, chronic kidney disease (CKD), and cardiovascular health plays a pivotal role in shaping overall morbidity and mortality. This complex interplay, known as cardiovascular-kidney-metabolic (CKM) syndrome, has far-reaching multisystem consequences, with the most significant clinical impact on cardiovascular disease (CVD) and mortality [1]. Poor CKM health is prevalent in the US population, with a disproportionate burden seen among those with adverse social determinants of health and in those over the age of 65 years [2]. Among older adults with Medicare fee-for-service (FFS), a third of the population had CKD, heart failure (HF), and/or type 2 diabetes (T2D) but these patients accounted for over 50% of total healthcare spending (including Part D, an optional prescription drug coverage) [2]. The presence of one or more CKM comorbidities increases a patient’s risk of hospitalization, kidney failure, and mortality [3,4,5,6], substantially increasing healthcare costs [7, 8]. Prevalence of these diseases and associated healthcare resource use are projected to increase, imposing a considerable strain on the US healthcare system [9].
Dapagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, has demonstrated clear efficacy across individual disease indications and comorbid subpopulations in a series of T2D [10], HF [11, 12], and CKD trials [13]. In the context of the US healthcare system, dapagliflozin treatment in those with one or more of these diseases has the potential to decrease the prevalence of new comorbid CKM syndrome conditions and reduce total healthcare delivery costs [9]. The closely interconnected nature of CKM syndrome contributes to high morbidity and mortality in this patient population and SGLT2 inhibitors can contribute to value-based approaches for interdisciplinary care that can address the urgent need for effective management across all aspects of CKM syndrome [1, 14].
Our study objective was to extrapolate clinical outcomes observed in key dapagliflozin trials in CKM syndrome populations to the US Medicare population and calculate short-term costs associated with averted outcomes from the perspective of the Centers for Medicare and Medicaid Services (CMS) over a 4-year period.
Methods
The analysis considered Medicare subpopulations with CKD, HF, or T2D, with separate subpopulations derived that accounted for patients with two or three of these conditions (Fig. 1). We developed a cohort-level partitioned survival model to quantify the short-term predicted clinical and health economic outcomes in these populations when treated with dapagliflozin, specifically in relation to key primary and secondary outcomes from four placebo-controlled, phase 3 randomized clinical trials. Incidence of clinical events was calculated for the total population of US adults ≥ 65 years with Medicare coverage, with published cost estimates applied to calculate direct medical care cost-offset associated with the avoidance of such events through treatment with dapagliflozin.
Two scenarios were compared, where all patients were assumed to receive either (1) dapagliflozin in addition to historical standard of care (hSoC), or (2) hSoC alone. Definitions of what treatments were received as part of hSoC in each trial are detailed in the supplementary materials (Table S1).
Model Structure
We modeled health states aligned to the definitions of key cardiovascular and renal events across the four pivotal dapagliflozin clinical trials (DECLARE-TIMI 58 [NCT01730534], DAPA-HF [NCT03036124], DAPA-CKD [NCT03036150], and DELIVER [NCT03619213]; Fig. 2). Outcomes were estimated over a 4-year period, which was broadly comparable to the typical length of trial follow-up. Given the relatively short time horizon, event rates within a cohort were assumed to remain constant over the modeled time horizon across all considered endpoints, in effect, that increased hazard of disease progression is adequately captured by event data derived from the clinical trials. Clinical outcomes were therefore estimated via exponential survival curves.
Specifically, cardiovascular-related health states were:
-
Hospitalization for HF (stratified by initial and recurrent hospitalizations)
-
Urgent HF visits defined as an urgent, unscheduled office/practice or emergency department visit for a primary diagnosis of HF
-
Death from cardiovascular causes
Modeled renal-related health states were:
-
≥ 50% sustained decline in estimated glomerular filtration rate (eGFR) from baseline
-
Kidney failure defined as a composite of either eGFR < 15 ml/min per 1.73 m2 for ≥ 28 days, maintenance dialysis for ≥ 28 days or kidney transplantation
-
Death from renal causes
The model also estimated the rate of all-cause mortality across the Medicare population, thereby considering deaths due to causes other than cardiovascular and renal causes. Treatment-related adverse events were not incorporated, in line with similar published analyses [15,16,17]. Furthermore, dapagliflozin is considered to have a favorable safety profile, with no increases in overall rates of serious adverse events. The rate of serious adverse events and a summary of adverse events considered of special interest reported across the trials is provided in Table S2.
Derivation of US Medicare Population with CKM Syndrome Eligible to Receive Dapagliflozin
A population of 54.2 million adults aged ≥ 65 years were estimated to be covered by Medicare, according to the US 2022 census data [18]. The prevalence of CKM syndrome was first estimated across this population based on 2021 Medicare FFS claims data and diagnosis codes from International Classification of Diseases, Tenth Revision (ICD-10), provided in the supplementary materials (Table S3), according to the approach outlined in a recent database analysis [19]. Label indications for dapagliflozin were used to refine the populations that are eligible to receive dapagliflozin treatment. The final population includes patients diagnosed with T2D with cardiovascular risk factors, HF, and CKD stage 2–4, with a total estimated prevalence rate of 24.2% (Table 1). The total modeled population comprised 13.1 million US adults ≥ 65 years with Medicare coverage.
Application of Clinical Efficacy Data to CKM Syndrome Subpopulations
Event rates for each subpopulation were derived from the trial deemed most representative of the population as shown in Fig. 1, as outlined in the supplementary materials (Table S4).
The DECLARE TIMI 58 trial, which assessed dapagliflozin in patients with T2D with established CVD or multiple risk factors for CVD, informed the event incidence in patients with T2D without prior HF or CKD (those with eGFR ≥ 60 ml/min/1.73 m2 at baseline) [10]. It was assumed that patients with T2D only did not experience renal events due to the relatively low incidence of kidney failure in the DECLARE-TIMI 58, and that the secondary endpoint component for CKD progression was ≥ 40% decline in eGFR from baseline, while the corresponding endpoint for CKD progression was a ≥ 50% sustained decline in eGFR across the other trials.
The DAPA-CKD trial informed renal outcomes in patients with CKD and was the first trial to assess an SGLT2 inhibitor in patients with CKD regardless of T2D status [13]. The DAPA-CKD trial informed outcomes in all subpopulations with CKD, including those with comorbid HF and/or T2D at baseline.
The DAPA-HF and DELIVER trials enrolled patients with HF with reduced ejection fraction and preserved ejection fraction, respectively [11, 12], and were pooled to inform event rates in the subpopulations with HF with or without T2D at baseline.
Event rates per 1000 patient years sourced from respective clinical study reports and published post hoc trial analyses were used to derive event rates specific to CKM syndrome subpopulations dependent on their comorbidity status and the treatment received. Event rates for individual endpoints stratified by comorbidity status at baseline were extracted where possible; where these were unavailable, composite endpoints were employed as proxy measures for rate of occurrence. Event rates applied in the model are provided in the supplementary materials (Table S5).
Cost and Healthcare Resource Use
The analysis estimated economic outcomes from the perspective of the CMS, considering only direct costs specific to the US setting. Event costs were applied at the incidence of an event and maintenance costs were applied following incidence of an event, until death, or the end of the modeled time horizon (Table 2). We assumed that the costs associated with a ≥ 50% sustained decline in kidney function were equated to the marginal cost between a patient’s CKD stage at baseline and the destination CKD stage following a 50% decline in eGFR from baseline. Deaths from other causes were assumed to occur in an inpatient setting only [20]. All costs were expressed as 2022 US dollars, adjusted for inflation if necessary according to the US Medical Care consumer price index [21]. All future costs were discounted at 3% per annum in line with the Institute for Clinical and Economic Review (ICER) reference case [22].
Model Outcomes
Incidence of clinical events was calculated for the total Medicare population who have T2D, HF, and/or CKD over the 4-year model horizon. Numbers needed to treat (NNT), defined as the average number of patients who need to receive treatment with the intervention to avoid one clinical event in the time specified, were calculated for each clinical event. Medical care cost offsets were the measure of economic benefit associated with each clinical event.
Scenario and Sensitivity Analyses
A series of scenario analyses were conducted to test key model parameters to identify which parameters were the largest cost drivers. In the first scenario, we increased event rates by 5% intervals from 5% to 20% in the dapagliflozin arm only. In a second scenario, prevalence rates for each subpopulation were varied by ± 5% of the base-case value. The final scenario analysis explored the influence of the costs of end-of-life care on model outcomes, considering deaths in outpatient settings in addition to inpatient settings, as in the base-case [20]. We also conducted deterministic sensitivity analysis on the event and maintenance cost parameters, adjusting by ± 30% of the base-case values.
Compliance with Ethics Guidelines
This study was conducted in line with ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines. This study did not require informed consent or institutional/ethical review board approval since the analysis does not report any new studies with human participants or animals performed by any of the authors and was based on previously conducted clinical trials. Any clinical data were anonymized for the purpose of the study.
Results
Overall, the ≥ 65 years US Medicare population with CKM syndrome were predicted to experience fewer adverse clinical events with dapagliflozin plus hSoC versus hSoC alone over 4 years. The total costs associated with clinical events if treated with dapagliflozin plus hSoC were estimated to be $211.0 billion compared with $310.3 billion when treated with hSoC over the 4-year time horizon; therefore, an estimated $99.3 billion in event-associated costs were avoided in the dapagliflozin plus hSoC arm (Fig. 3).
CKM outcomes in the US Medicare populations: a stacked bar chart of modelled clinical events experienced when treated with hSoC (orange) and the proportion that could be prevented when treated with dapagliflozin in addition to hSoC (blue); b total event-related costs by treatment received and incremental cost offsets associated with preventable events. CKM cardiovascular-kidney-metabolic, eGFR estimated glomerular filtration rate, HF heart failure, hSoC historical standard of care
Cardiovascular Events
In patients treated with dapagliflozin plus hSoC, fewer events occurred across all cardiovascular outcomes compared with patients treated with hSoC (Table 3; Fig. 3a), which was associated with a cost avoidance of $25.9 billion (dapagliflozin plus hSoC, $66.0 billion; hSoC, $92.0 billion). Treatment with dapagliflozin was estimated to prevent the incidence of initial hospitalization for HF in 513,751 people (dapagliflozin plus hSoC, 1,215,262; hSoC, 1,729,013; NNT, 26; Table 3)—a reduced incidence rate of 29.7% over 4 years. This was associated with a cost avoidance of $21.3 billion (dapagliflozin plus hSoC, $54.0 billion; hSoC, $75.3 billion; Fig. 3b). In addition, dapagliflozin was expected to prevent 99,794 subsequent hospitalizations for HF (dapagliflozin plus hSoC, 257,034; hSoC, 356,828; NNT, 43; Table 3)—a 28.0% lower incidence rate over 4 years and medical care cost offset of $4.5 billion (dapagliflozin plus hSoC, $11.9 billion; hSoC, $16.4 billion; Fig. 3b).
Renal Events
Similarly, fewer events occurred across all renal outcomes with dapagliflozin plus hSoC treatment as shown in Table 3 and Fig. 3a. Prevention of renal outcomes was associated with a cost avoidance of $63.0 billion (dapagliflozin plus hSoC, $108.1 billion; hSoC, $171.1 billion). The largest cost avoidance when considering renal outcomes of $53.3 billion is associated with kidney failure (dapagliflozin plus hSoC, $93.8 billion; hSoC, $147.1 billion; Fig. 3a) specifically the prevention of 285,041 patients reaching kidney failure with dapagliflozin treatment (dapagliflozin plus hSoC, 442,972; hSoC, 728,013; NNT, 35; Table 3)—a 39.2% lower rate of progression over 4 years. A notable medical care cost offset was also seen with a ≥ 50% sustained eGFR decline where 375,137 fewer patients experienced a sustained eGFR decline with dapagliflozin treatment (dapagliflozin plus hSoC, 505,330; hSoC, 880,467; NNT, 27; Table 3)—a 42.6% lower incidence rate over 4 years associated with an avoided cost of $9.7 billion (dapagliflozin plus hSoC, $14.3 billion; hSoC, $24.0 billion; Fig. 3b).
Mortality
Across the ≥ 65 years US Medicare population with CKM syndrome, 450,355 deaths from any cause were avoided if treated with dapagliflozin plus hSoC versus hSoC alone (dapagliflozin plus hSoC, 1,810,452; hSoC, 2,260,806; NNT, 29; Table 3)—an estimated 19.9% reduction in the number of deaths over a 4-year period from treatment initiation. Of these, 225,346 deaths prevented would be of cardiovascular causes (dapagliflozin plus hSoC, 794,712; hSoC, 1,020,057; NNT, 58) and 13,206 deaths prevented would be of renal causes (dapagliflozin plus hSoC, 8740; hSoC, 21,946; NNT, 763).
Costs avoided by the prevention of deaths due to cardiovascular and renal causes were estimated to be $6.5 billion (dapagliflozin plus hSoC, $23.0 billion; hSoC, $29.5 billion; Fig. 3b) and $1.0 billion (dapagliflozin plus hSoC, $0.6 billion; hSoC, $1.6 billion), respectively. Deaths avoided that would be of other causes equate to a cost offset of $2.8 billion (dapagliflozin plus hSoC, $13.2 billion; hSoC, $16.0 billion; Fig. 3b) due to the avoidance of death over the modelled period.
Costs Avoided per Patient by Subpopulation
When considering total medical care cost offsets over 4 years per patient treated with dapagliflozin plus hSoC for each subpopulation, the greatest cost avoidance per patient ($21,248) was observed in the “HF + T2D + CKD” population. This was followed by the “HF + CKD” and “T2D + CKD” populations where the estimated costs avoided were $13,396 and $13,340, respectively (Table 4). This indicates that the largest medical care cost offsets are seen when using dapagliflozin to treat patients with comorbidities.
Scenario Analyses
Additional analyses whereby event rates for dapagliflozin plus hSoC were increased in 5% intervals up to 20% over base-case are presented in the supplementary materials (Table S6). The total medical care cost offsets in the dapagliflozin arm reduced from $99.3 billion in the base case to $65.0 billion when event rates were increased by 20%. Upon varying the prevalence rates of each subpopulation by ± 5%, minimal changes in medical care cost offset were estimated in the “CKD + T2D” and “HF + T2D + CKD” populations ($100.5 billion and $100.3 billion, respectively, Table S7). Model outcomes were also not sensitive to the change when accounting for the cost of end-of-life care in either an inpatient or outpatient setting (Table S8).
Deterministic Sensitivity Analysis
Deterministic sensitivity analyses of event and maintenance costs where estimates were adjusted by ± 30% were conducted. When considering event costs, results demonstrated that the modelled estimated cost offset outcomes were most sensitive to initial hospitalization for HF, ranging from $96.6 billion to $101.9 billion (Fig. 4a). Kidney failure was the biggest driver of maintenance cost, ranging from $83.3 billion to $115.3 billion (Fig. 4b). When considering both event and maintenance costs, kidney failure was the most influential followed by initial hospitalization for HF. The estimated costs offsets ranged from $83.3 billion to $115.3 billion and $92.9 billion to $105.6 billion, respectively (Fig. 4c).
Discussion
The analysis presented here suggests that a substantial proportion of major cardiorenal outcomes could be avoided or reduced across the Medicare population with CKM syndrome if treated with dapagliflozin. Over 4 years, major clinical events prevented through treatment with dapagliflozin plus hSoC compared with hSoC alone led to medical care cost offsets of $99.3 billion to the CMS. Capturing clinical and health economic outcomes across linked disease subpopulations simultaneously can characterize the potential for reduced incidence of comorbidities and attenuated progression. For example, medical care cost offsets per patient treated with dapagliflozin were approximately 60% greater in the subpopulation with CKD, HF, and T2D versus those with two comorbid CKM syndrome conditions, and considerably greater in comparison to those with one condition. When considering individual outcomes, prevention of kidney failure was the most influential, with a medical care cost offset of $53.3 billion due to the considerable efficacy of dapagliflozin in delaying CKD progression. This highlights the importance of timely diagnosis and treatment of CKD to delay or prevent progression to kidney failure and the need for dialysis, which has a high associated cost burden [23]. Conditions within CKM syndrome are notably more common among African-American adults and other minority groups compared to non-Hispanic white adults [24,25,26]. African Americans and Hispanics or Latinos have an approximately fourfold and twofold greater risk of progressing from early-stage CKD to end-stage kidney disease, respectively, than non-Hispanic white persons [25]. This disparity underscores the importance of prioritizing early detection and treatment to mitigate the progression and impact of CKD in diverse populations.
It is well documented that SGLT2 inhibitors provide distinct, additive treatment benefits to traditional therapies which encompasses the cardiovascular, renal, and glucose control aspects of CKM syndrome; however, siloed approaches to care may be a significant factor affecting therapeutic inertia with limited uptake of newer pharmacotherapies [27]. Furthermore, mismanagement of comorbidities through unnecessarily complex treatment regimens is estimated to cost the US healthcare system $2 billion per year [28, 29] and, given the high rate of comorbid diseases within the Medicare population, it is important to consider the broader cost and benefit for each therapy.
The burden of CKM syndrome is predicted to escalate over the next 10 years [9], in large part due to an ageing population increasing the relative burden associated with the US Medicare healthcare system. Cost-effectiveness analyses in the US setting have demonstrated SGLT2 inhibitors to be cost-effective treatments in certain CKM syndrome subpopulations [30,31,32,33,34,35,36]. Additionally, previous short-term economic analyses have also considered SGLT2 inhibitors only in the context of a single indication [15,16,17, 37, 38]. However, these analyses have not considered the broader treatment effect of SGLT2 inhibitors across the full spectrum of CKM syndrome.
Treatments such as SGLT2 inhibitors, with effects across multiple indications, encompass the shift to value-based care approaches to healthcare in the USA. The implementation of such a framework encourages healthcare professionals to consider the wider health determinants of their patients to improve health outcomes considering multiple factors in combination [14], detaching from a siloed treatment approach. As the Inflation Reduction Act aims to improve access to valuable prescription drugs for those with Medicare coverage [39], applying a multidisciplinary approach to CKM syndrome is important to accurately value the cost of treatment and provide efficient healthcare delivery [1, 40]. Many patients with CKM syndrome have overlapping comorbidities leading to high costs and associated healthcare resource use driven by accelerated disease progression. The demonstrated efficacy of dapagliflozin and other SGLT2 inhibitors across CKM syndrome subpopulations can lead to a reduction in the complexity of care and disease progression that drive the need for further care.
This modelled analysis, as with any extrapolation, is subject to certain limitations. The scope of this analysis was to estimate the short-term economic offsets associated with dapagliflozin treatment and not to account for all components of medical cost, such as treatment-related costs, and therefore the value of dapagliflozin treatment was not assessed. Alongside acquisition costs, treatment-related adverse event management costs were not incorporated in the analyses. However, the safety profile of dapagliflozin is well documented [41], and the drug is generally well tolerated in patients across the pivotal trials. Nevertheless, certain serious adverse events can lead to hospitalization, and considerable associated cost, such as diabetic ketoacidosis and Fournier’s gangrene. However, the net incidence of such serious adverse events was similar across treatment groups in the trials, as observed in wider literature [42]. Therefore, when considering the net effect of hospitalization and other costs pertaining to these events, the total incremental cost offsets would not be substantively affected.
The population is a closed cohort aligned to prevalence estimates derived from relevant ICD-10 diagnosis codes that broadly align with the trial populations and eligible to receive dapagliflozin per US FDA label indications. Nevertheless, these populations are not fully represented by the trial populations (aged ≥ 65 years) from which efficacy data were derived. In addition, the relative size of the modeled estimates will invariably be sensitive to the epidemiological inputs. Further, differential dosing was not accounted for as the clinical trials only considered a dose of 10 mg and therefore no data was available to assess the outcomes independently of the 10 mg dose. It should also be noted that the treatment received in the clinical studies will not necessarily reflect the typically lower degree of adherence to treatment guidelines observed in real-world practice.
The model considered outcomes over a relatively short time horizon, in line with previous analyses [15,16,17], and as a result may not fully capture the benefit of dapagliflozin in terms of mortality outcomes. Furthermore, our analysis was unable to fully capture the burden of kidney failure within the Medicare population, as patients initiating dialysis who are under 65 years of age are enrolled on Medicare after 30 months of dialysis treatment. Younger patients initiating dialysis have greater life expectancy and therefore are expected to spend a higher proportion of their lifetime on dialysis. For example, the 5-year survival rate in patients aged 45–64 years with kidney failure on dialysis is 49% from onset, versus 35% and 21% for those aged 65–74 and 75 years of age, respectively [2].
The model approach assumes health states are independent, possibly limiting the extrapolation of events that are structurally dependent (e.g., progressed CKD increases the risk of death). As the time horizon is broadly aligned to the four pivotal clinical trials and uses patient-level data this may eliminate an element of uncertainty. As incidence rate ratios for multiple comorbid diseases were not readily available, a degree of risk exists in relation to double counting of effects. Furthermore, while composite endpoints were considered as suitable proxy outcomes for component event rates, it was necessary to assume equal distribution across component outcomes. In addition, costs for death by causes other than cardiovascular or renal causes were sourced from an analysis of Medicare beneficiaries [20]. The cost of end-of-life care considered death from any cause in an inpatient setting, and therefore may partially comprise healthcare costs associated with cardiovascular or renal death, potentially overestimating economic effects. No discontinuation was assumed beyond that observed in the intention-to-treat trial populations; therefore, the costs presented here provide an upper limit of what could be achieved, as any discontinuation of dapagliflozin would lead to a smaller reduction in incremental events.
Conclusions
By considering a holistic, multidisciplinary approach aligned to the principles of value-based care in the Medicare population, our analysis suggests that patients with comorbidities across the CKM syndrome can experience considerable treatment benefits and reduced incidence of mortality if treated with dapagliflozin in addition to established care strategies. This broader perspective shows that considerable medical care cost offsets may result from treatment with dapagliflozin through attenuated disease progression across CKD, T2D, and HF populations, particular through reduced frequency of hospitalization in patients with HF and onset of kidney failure [1].
Data Availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
References
Ndumele CE, Rangaswami J, Chow SL, et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation. 2023;148(20):1606–35.
United States Renal Data System. USRDS Annual Data Report: Epidemiology of kidney disease in the United States. 2023. https://adr.usrds.org/2023. Accessed 28 Nov 2023.
Zareini B, Blanche P, D'Souza M, et al. Type 2 diabetes mellitus and impact of heart failure on prognosis compared to other cardiovascular diseases: a nationwide study. Circ Cardiovasc Qual Outcomes. 2020;e006260.
Lawson CA, Seidu S, Zaccardi F, et al. Outcome trends in people with heart failure, type 2 diabetes mellitus and chronic kidney disease in the UK over 20 years. eClinicalMedicine. 2021;32:100739.
Sud M, Tangri N, Pintilie M, et al. Risk of end-stage renal disease and death after cardiovascular events in chronic kidney disease. Circulation. 2014;130(6):458–65.
Kamalesh M, Cleophas TJ. Heart failure due to systolic dysfunction and mortality in diabetes: pooled analysis of 39,505 subjects. J Card Fail. 2009;15(4):305–9.
Ferdinand KC, Norris KC, Rodbard HW, et al. Humanistic and economic burden of patients with cardiorenal metabolic conditions: a systematic review. Diabetes Ther. 2023;14(12):1979–96.
Nichols GA, Amitay EL, Chatterjee S, et al. Health care costs associated with the development and combination of cardio-renal-metabolic diseases. Kidney360. 2023;4(10):1382–8.
McEwan P, Morgan AR, Boyce R, et al. Cardiorenal disease in the United States: future health care burden and potential impact of novel therapies. J Manag Care Spec Pharm. 2022;28(4):415–24.
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–98.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46.
Teisberg E, Wallace S, O’Hara S. Defining and implementing value-based health care: a strategic framework. Acad Med. 2020;95(5):682–5.
Kamstra R, Durkin M, Cai J, et al. Economic modelling of costs associated with outcomes reported for type 2 diabetes mellitus (T2DM) patients in the CANVAS and EMPA-REG cardiovascular outcomes trials. J Med Econ. 2019;22(3):280–7.
Manceur AM, Durkin M, Kharat A, et al. Costs associated with renal and cardiovascular events among patients with type 2 diabetes mellitus and nephropathy: a cost model based on the CREDENCE clinical trial. Curr Med Res Opin. 2020;36(4):563–70.
McEwan P, Hafner M, Jha V, et al. Translating the efficacy of dapagliflozin in chronic kidney disease to lower healthcare resource utilization and costs: a medical care cost offset analysis. J Med Econ. 2023;26(1):1407–16.
United States Census Bureau. Health insurance coverage in the United States: 2022. Current population reports. 2023. https://www.census.gov/content/dam/Census/library/publications/2023/demo/p60-281.pdf. Accessed 14 Dec 2023.
Wittbrodt ET, Eudicone JM, Bell KF, et al. Eligibility varies among the 4 sodium-glucose cotransporter-2 inhibitor cardiovascular outcomes trials: implications for the general type 2 diabetes US population. Am J Manag Care. 2018;24(8 Suppl):S138–45.
Duncan I, Ahmed T, Dove H, et al. Medicare cost at end of life. Am J Hospice Palliat Med. 2019;36(8):705–10.
United States Department of Labor—Bureau of Labor Statistics. Medical care in U.S. city average, all urban consumers, not seasonally adjusted. 2022. https://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths. Accessed 12 Jan 2023.
Institute for Clinical and Economic Review. ICER’s reference case for economic evaluations: principles and rationale. 2018. https://icer-review.org/wp-content/uploads/2018/07/ICER_Reference_Case_July-2018.pdf. Accessed 29 Jul 2020.
Golestaneh L, Alvarez PJ, Reaven NL, et al. All-cause costs increase exponentially with increased chronic kidney disease stage. Am J Manag Care. 2017;23(10 Suppl):S163–72.
Bozkurt B, Ahmad T, Alexander KM, et al. Heart failure epidemiology and outcomes statistics: a report of the Heart Failure Society of America. J Cardiac Fail. 2023;29(10):1412–51.
Centers for Disease Control and Prevention. Chronic kidney disease in the United States, 2023. 2023. https://www.cdc.gov/kidneydisease/pdf/CKD-Factsheet-H.pdf. Accessed 15 Mar 2023.
Centers for Medicare & Medicaid Services. Diabetes prevalence. 2023. https://www.cms.gov/files/document/cms-diabetes-infographic-6-2023.pdf. Accessed 27 Jul 2023.
Cromer SJ, Lauffenburger JC, Levin R, et al. Deficits and disparities in early uptake of glucagon-like peptide 1 receptor agonists and SGLT2i among medicare-insured adults following a new diagnosis of cardiovascular disease or heart failure. Diabetes Care. 2022;46(1):65–74.
Aitken M, Valkova S. Avoidable costs in U.S. healthcare: the $200 billion opportunity from using medicines more responsibly. IMS Institute for Healthcare Informatics. 2013. http://offers.premierinc.com/rs/381-NBB-525/images/Avoidable_Costs_in%20_US_Healthcare-IHII_AvoidableCosts_2013%5B1%5D.pdf. Accessed 28 Nov 2023.
Almodóvar AS, Nahata MC. Associations between chronic disease, polypharmacy, and medication-related problems among medicare beneficiaries. J Manag Care Spec Pharm. 2019;25(5):573–7.
Abegaz TM, Diaby V, Sherbeny F, et al. Cost effectiveness of dapagliflozin added to standard of care for the management of diabetic nephropathy in the USA. Clin Drug Investig. 2022;42(6):501–11.
Tisdale RL, Cusick MM, Aluri KZ, et al. Cost-effectiveness of dapagliflozin for non-diabetic chronic kidney disease. J Gen Intern Med. 2022;37(13):3380–7.
Bhatt AS, Vaduganathan M, Claggett BL, et al. Cost Effectiveness of dapagliflozin for heart failure across the spectrum of ejection fraction: an economic evaluation based on pooled, individual participant data from the DELIVER and DAPA-HF trials. J Am Heart Assoc. 2024;13(5):e032279.
Nguyen E, Coleman CI, Nair S, et al. Cost-utility of empagliflozin in patients with type 2 diabetes at high cardiovascular risk. J Diab Comp. 2018;32(2):210–5.
Reifsnider OS, Kansal AR, Wanner C, et al. Cost-effectiveness of empagliflozin in patients with diabetic kidney disease in the united states: findings based on the EMPA-REG OUTCOME trial. Am J Kidney Dis. 2022;79(6):796–806.
Reifsnider OS, Tafazzoli A, Linden S, et al. Cost-effectiveness analysis of empagliflozin for treatment of patients with heart failure with reduced ejection fraction in the US. J Am Heart Assoc. 2024;13(4):e029042.
Zheng J, Parizo JT, Spertus JA, et al. Cost-effectiveness of empagliflozin in patients with heart failure with preserved ejection fraction. JAMA Intern Med. 2022;182(12):1278–88.
Chakravarty A, Rastogi M, Dhankhar P, et al. Comparison of costs and outcomes of dapagliflozin with other glucose-lowering therapy classes added to metformin using a short-term cost-effectiveness model in the US setting. J Med Econ. 2018;21(5):497–509.
Dwyer JP, Agiro A, Desai P, et al. Short-term costs in patients with chronic kidney disease treated with dapagliflozin: a retrospective cohort study. Expert Rev Pharmacoecon Outcomes Res. 2023;23(9):1057–66.
Centers for Medicare & Medicaid Services. Inflation Reduction Act and Medicare. 2023. https://www.cms.gov/inflation-reduction-act-and-medicare. Accessed 19 Dec 2023.
Rangaswami J, Tuttle K, Vaduganathan M. Cardio-renal-metabolic care models. Circ: Cardiovasc Qual Outcomes. 2020;13(11):e007264.
Mascolo A, Di Napoli R, Balzano N, et al. Safety profile of sodium glucose co-transporter 2 (SGLT2) inhibitors: a brief summary. Front Cardiovasc Med. 2022;9:1010693.
Jabbour S, Seufert J, Scheen A, et al. Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diab Obes Metab. 2018;20(3):620–8.
United States Census Bureau. Health insurance coverage in the United States: 2022. 2023. https://www.census.gov/content/dam/Census/library/publications/2023/demo/p60-281.pdf. Accessed 27 Jul 2023.
Childers CP, Dworsky JQ, Kominski G, et al. A comparison of payments to a for-profit dialysis firm from government and commercial insurers. JAMA Intern Med. 2019;179(8):1136–8.
Kilgore M, Patel HK, Kielhorn A, et al. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63–70.
Nicholson G, Gandra SR, Halbert RJ, et al. Patient-level costs of major cardiovascular conditions: a review of the international literature. Clinic Outcomes Res. 2016;8:495–506.
Voigt J, Sasha John M, Taylor A, et al. A reevaluation of the costs of heart failure and its implications for allocation of health resources in the US. Clin Cardiol. 2014;37(5):312–21.
Axelrod DA, Schnitzler MA, Xiao H, et al. An economic assessment of contemporary kidney transplant practice. Am J Transp. 2018;18(5):1168–76.
Naccarelli GV, Johnston SS, Lin J, et al. Cost burden of cardiovascular hospitalization and mortality in ATHENA-like patients with atrial fibrillation/atrial flutter in the United States. Clin Cardiol. 2010;33(5):270–9.
Acknowledgements
Medical Writing/Editorial Assistance
The authors thank Peter Gabb and Chloe Hembury of Health Economics and Outcomes Research Ltd. for providing medical writing support and Jani Silvanto of Health Economics and Outcomes Research Ltd. for providing analytical support, all of which was funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Funding
This work was supported by AstraZeneca who provided support for model development, analysis, and medical writing for this study, and funded the journal’s Rapid Service and Open Access Fees.
Author information
Authors and Affiliations
Contributions
Ryan L Miller, Raymond C Chang and Joanna C Huang conceptualized and designed the study. Ryan L Miller and Raymond C Chang were responsible for data analysis. Katherine W Kwon provided expert guidance. All authors contributed to interpretation of the results, preparation and review of the manuscript, and approval of the final manuscript for publication.
Corresponding author
Ethics declarations
Conflict of Interest
Raymond C Chang and Joanna C Huang are employees of AstraZeneca. Ryan L Miller was an employee of Health Economics and Outcomes Research Ltd. Health Economics and Outcomes Research Ltd. received fees from AstraZeneca in relation to this study. Katherine W Kwon is a speaker with AstraZeneca.
Ethical Approval
This study was conducted in line with ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines. This study did not require informed consent or institutional/ethical review board approval since the analysis does not report any new studies with human participants or animals performed by any of the authors and was based on previously conducted clinical trials. Any clinical data were anonymized for the purpose of the study.
Additional information
Prior Presentation: Part of this work was presented in poster format at NKF Spring Clinical Meetings 2024 and published in the associated issue of the American Journal of Kidney Diseases (https://doi.org/10.1053/j.ajkd.2024.01.505).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chang, R.C., Miller, R.L., Kwon, K.W. et al. Cost Offset of Dapagliflozin in the US Medicare Population with Cardio-Kidney Metabolic Syndrome. Adv Ther 41, 3247–3263 (2024). https://doi.org/10.1007/s12325-024-02919-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02919-5