Abstract
Adalimumab (ADL, Humira®, reference product), an anti-TNF-α biologic, has transformed the treatment of chronic, immune-mediated inflammatory diseases. However, the high cost of ADL therapy has driven the development of more affordable ADL biosimilars, agents with no clinically meaningful differences from the reference product. This review summarizes the product attributes of reference ADL and the nine ADL biosimilars approved and available in the USA in relation to patient experience of injection-site pain (ISP). Product formulation, delivery volume and device features (e.g., type and needle gauge size) influence patient experience of ISP with potential clinical consequences. Citrate-free formulations generally cause less ISP; injection volumes of > 1.5 ml may be associated with increased ISP. Reference ADL and all ADL biosimilars offer a citrate-free formulation, and reference ADL and four ADL biosimilars offer a high-concentration solution that allows a smaller injection volume. All available ADL products are injected subcutaneously using either a pre-filled pen (PFP) or pre-filled syringe (PFS). Patients prefer the PFP, but the PFS permits better control over the speed and duration of injection. Smaller (29-gauge) needle outer diameter is associated with less ISP; reference ADL and seven ADL biosimilars offer a device with a 29-gauge needle. In the USA, an approved biosimilar can be designated “interchangeable,” allowing pharmacy-level substitution, where state law permits. In the USA, two ADL biosimilars have received interchangeability designation; others are seeking interchangeability designation from the Food and Drug Administration (n = 2), are being evaluated in clinical studies to support interchangeability (n = 2), or do not have/are not seeking interchangeability designation (n = 3). Product-related attributes influence patient experience of ISP caused by subcutaneous ADL injection. Reference ADL and ADL biosimilar products differ in their attributes, so discussion with patients about treatment options is essential to optimize adherence and outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adalimumab (ADL), an anti-TNF-α biologic, is administered by subcutaneous injection using a pre-filled syringe or pre-filled pen. |
Subcutaneous administration of biologic drugs, such as ADL, can be associated with injection-site pain (ISP), and patient experience of ISP is influenced by various product-related factors (e.g., formulation, delivery device and needle gauge size, and injection volume). |
Biosimilar versions of reference ADL (Humira®) are approved and available on the US market; these agents are highly similar to and have no clinically meaningful differences in safety, purity, or potency from reference ADL. |
ADL products (reference and biosimilar) differ in their attributes, and this can impact the injection experience and, by extension, treatment adherence and patient outcomes. |
It is important for healthcare professionals to consider the various features of ADL products (reference and biosimilar) as well as the impact of interchangeable biosimilars and availability of patient assistance programs on patient access to these medications when discussing ADL treatment options with their patients. |
Digital features
This article is published with digital features, including a plain language summary as supplementary material, to facilitate understanding of the article. To view digital feature for this article, go to https://doi.org/10.6084/m9.figshare.2516446
Introduction
Adalimumab (ADL) is a recombinant, fully human IgG1 monoclonal antibody against tumor necrosis factor alpha (TNF-α) that reduces symptoms and improves physical function in patients with chronic immune-mediated inflammatory diseases [1]. ADL has been marketed as Humira® ([adalimumab], AbbVie Inc, North Chicago, IL, USA; AbbVie Deutschland GmbH Co. KG, Ludwigshafen, Germany) in the USA since 2002 [2].
ADL is used in the clinical practice of dermatologic, rheumatologic, and gastroenterologic diseases [3,4,5,6,7,8,9,10,11], and biosimilar versions of ADL are also available. A biosimilar is a biologic product that is highly similar to and has no clinically meaningful differences in safety, purity, or potency from the licensed reference or originator product [12]. The development and regulatory approval of a biosimilar is different from the process used for a reference biologic product or small-molecule drug and so is described with unique terminology (Table 1) [12,13,14,15,16,17,18]. The Biologics Price Competition and Innovation (BPCI) Act of 2009 created an abbreviated development pathway for biosimilars in the USA [19]. The BPCI Act established a framework to foster innovation and promote competition through the development of biosimilar products that have the potential to reduce treatment costs and expand access to safe and effective biologic therapies [19, 20].
Biologic drugs, such as ADL, cannot be administered orally; they can be administered by intravenous infusion or, most commonly, subcutaneous (SC) injection using a pre-filled syringe (PFS) or pre-filled pen (PFP). SC administration of biologic drugs can be associated with a subjective level of local pain and irritation [21]. Many product-related factors may influence the extent of injection-site pain (ISP) during SC injection, including formulation, delivery volume, and needle gauge size [21, 22]. In addition, patients may prefer one device type (PFS or PFP) because of aspects such as convenience and ease of use, perception of pain, or fear of injection [21, 23,24,25,26,27,28,29,30,31,32].
Many ADL products (reference and biosimilar) are available that differ in their formulations and delivery device features, which can impact the injection experience. Here, we review the attributes of reference ADL and the ADL biosimilars approved and available in the USA that may help to better inform patient choice of treatment.
Methods
Relevant peer-reviewed literature on the topics of SC injection with ADL and ADL biosimilars were identified based on the experience of the authors. Furthermore, regulatory documents (e.g., Food and Drug Administration [FDA] submissions/reviews) and product labels (e.g., US prescribing information) were searched for details of product attributes for ADL products.
This article is based on previously conducted studies and other sources; it does not contain any studies with human participants or animals performed by any of the authors.
ADL Biosimilars in the USA
As of October 2023, nine ADL biosimilars have been approved and are available on the market in the USA (Table 2) [33,34,35,36,37,38,39,40,41,42,43]. Additionally, a biologics license application for one proposed ADL biosimilar (AVT02, Alvotech, Reykjavik, Iceland) was accepted by the US FDA with anticipated approval in February 2024 [44].
Relevance of ADL Product Attributes to the Injection Experience
Formulation
Controlling pH is necessary for the solubility and stability of SC-injected biologic drugs. Slightly acidic formulations may be preferred for drug stability, but formulations with pH < 3.0 can cause pain and inflammation [21, 22]. Basic formulations with pH > 9.0 are associated with tissue necrosis [21, 22]. pH is often controlled by adding buffer salts to a product formulation [53].
Citrate and phosphate are two of the most used buffers and have a wide buffering range (pH 2.1–6.2 and 3.0–8.0, respectively) [22, 54]. Newer monoclonal antibody formulations use histidine or acetate as the preferred buffer; however, the buffering range (pH 5.0–6.5 and 3.8–5.8, respectively) is narrower versus phosphate or citrate [22, 55]. Citrate has been used in > 100 injectable product formulations [54]. However, stabilizing pH with citrate buffers has been associated with ISP [56, 57]. In addition, glutamate has been used as an excipient for product stability, and SC injection of glutamate has been associated with ISP and injection-site reactions [21, 58].
In a prospective single-center study of 54 adult healthy volunteers, a significantly greater number of participants (38/54; 70%) injected with a citrate-containing solution reported more or much more pain immediately after injection than participants injected with a histidine-containing solution (p = 0.002) [56]. In a prospective observational registry study in patients with inflammatory bowel disease, switching from a citrate-free reference ADL to a citrate-containing ADL biosimilar was correlated with ISP (p = 0.004) [57].
The consequences of ISP can be clinically meaningful and may contribute to non-adherence and the patient’s decision to discontinue treatment [59,60,61]. For example, in an online survey of patients with rheumatoid arthritis (RA; n = 250), the injection experience, including pain, burning, or discomfort during or after administration, was reported among the primary reasons for discontinuing a TNF inhibitor (TNFi) [59]. In a real-world study of rheumatology, dermatology, and gastroenterology patients receiving ADL (n = 744), the switch failure rate was higher for patients switched from citrate-free reference ADL to a citrate-containing ADL biosimilar than for patients switched to a citrate-free ADL biosimilar [61].
Formulating reference ADL to minimize ISP has been demonstrated to reduce the sensation of pain on administration [62, 63]. For example, in a pooled analysis of two identical, Phase 2, randomized, crossover studies in patients with RA, patients given citrate-free reference ADL reported less pain immediately after injection (p < 0.001) and were more likely to rate ISP severity as mild compared with patients given citrate-containing reference ADL (86.9% vs 42.6%) [62]. Citrate-free reference ADL was found to reduce ISP and was associated with greater treatment adherence, higher treatment persistence, and better experience of self-administration [64, 65].
Product formulations of ADL biosimilars available in the USA are summarized in Table 3 [2, 33,34,35,36,37,38,39,40,41]. High-concentration reference ADL and nearly all the ADL biosimilars offer versions that are citrate-, phosphate-, and glutamate-free [2, 33,34,35,36,37,38,39,40,41]. This is an important consideration when discussing ADL treatment options as patients and hospitals may not want to use products containing excipients that contribute to ISP.
Delivery Volume
Large SC injection volumes are generally associated with pain and adverse events at the injection site. In addition, the association between injection volume and ISP may be influenced by injection site location as injections into the thigh have been reported to be more painful than those into the abdomen [22, 77, 78]. Volumes of up to 0.8 ml are not expected to increase pain [22, 77, 78], but volumes of > 1.5 ml may be associated with increased ISP [21, 22, 79]. This observation is consistent with anecdotal reports from physicians and nurses that patients receiving 80 mg/0.8 ml loading doses of citrate-free ADL have not reported greater levels of ISP compared with those who received 40 mg/0.4 ml doses, though no formal assessments were conducted.
Reference ADL is available in a standard- (50 mg/ml) and high-concentration (100 mg/ml) solution for PFS or PFP injection [2]. Among currently available ADL biosimilars in the USA (Table 3), eight offer a standard-concentration solution (50 mg/ml) for injection by PFS or PFP [33,34,35,36,37,38,39, 41], and four offer a high-concentration solution (100 mg/ml) that allows a smaller volume for PFS or PFP injection: Amjevita™ (adalimumab-atto; Amgen Inc., Thousand Oaks, CA, USA), Hadlima™ (adalimumab-bwwd; Samsung Bioepis Co., Ltd., Incheon, Republic of Korea/Organon & Co., Jersey City, NJ, USA), Hyrimoz® (adalimumab-adaz; Sandoz Inc., Princeton, NJ, USA), and Yuflyma® (adalimumab-aaty; Celltrion, Inc., Incheon, Republic of Korea) [34, 36, 38, 40].
Device: Type, Needle Gauge Size, and Other Features
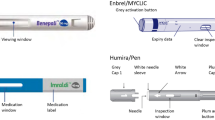
Reference ADL and all ADL biosimilars in the USA are available for injection as a PFS or PFP (Table 3) [2, 33,34,35,36,37,38,39,40,41]. Key features of ADL products available as a PFS or PFP are summarized in Figs. 1 and 2, respectively [2, 33,34,35,36,37,38,39,40,41, 66,67,68,69,70,71,72,73,74, 80,81,82,83,84,85,86]. The ADL PFS is composed of a needle and a syringe that is pre-filled with the appropriate drug dose, which reduces the time taken for injection and the chance of a dosing error and reduces the possibility of microbiologic contamination [87]. ADL PFPs have been designed so that the injection process is automated; their features make the injection process easier, which increases patient confidence [87, 88].
PFS features of reference ADL and ADL biosimilars approved and available on the US market.a aThis is a generic depiction of the device based on features of available PFSs. bLabeling for Humira® (adalimumab) indicates the needle cover of standard-concentration presentations may contain natural rubber latex, while the needle cover of high-concentration presentations is not made with natural rubber latex [2]. Labeling for Hulio® (adalimumab-fkjp), Idacio® (adalimumab-aacf), and Yusimry™ (adalimumab-aqvh) indicates a particular part of the presentation (plunger stopper, needle cover/cap, or both) is not made with natural rubber latex [39, 41, 85]. Labeling for Amjevita™ (adalimumab-atto), Hadlima™ (adalimumab-bwwd), Hyrimoz® (adalimumab-adaz), and Yuflyma® (adalimumab-aaty) includes the statement “not made with natural rubber latex” as it applies to the entire device [34, 38, 71, 72]. In addition, updated labeling for Abrilada™ (adalimumab-afzb) has received regulatory approval to include the statement “not made with natural rubber latex” as it applies to the entire device; this updated labeling is expected in April 2024 [33]. ADL, adalimumab; G, gauge; INN, international non-proprietary name; PFS, pre-filled syringe
PFP features of reference ADL and ADL biosimilars approved and available on the US market.a aThis is a generic depiction of the device based on features of available PFPs. bLabeling for Humira® (adalimumab) indicates the needle cover of standard-concentration presentations may contain natural rubber latex, while the needle cover of high-concentration presentations is not made with natural rubber latex [2]. Labeling for Hulio® (adalimumab-fkjp), Idacio® (adalimumab-aacf), and Yusimry™ (adalimumab-aqvh) indicates a particular part of the presentation (plunger stopper, needle cover/cap, or both) is not made with natural rubber latex [39, 41, 85]. Labeling for Amjevita™ (adalimumab-atto), Hadlima™ (adalimumab-bwwd), Hyrimoz® (adalimumab-adaz), and Yuflyma® (adalimumab-aaty) includes the statement “not made with natural rubber latex” as it applies to the entire device [34, 38, 71, 72]. In addition, updated labeling for Abrilada™ (adalimumab-afzb) has received regulatory approval to include the statement “not made with natural rubber latex” as it applies to the entire device; this updated labeling is expected in April 2024 [33]. ADL, adalimumab; G, gauge; INN, international non-proprietary name; PFP, pre-filled pen
It is also important to consider both the inner and outer diameters of needles to fully understand the potential impact of varying needle gauges (Fig. 3). Smaller needle outer diameter is directly correlated with less pain on injection [89]. This may be an important factor to consider when discussing ADL treatment options as some patients may be able to differentiate between standard 27-gauge and smaller 29-gauge needle sizes [90]. In addition, it is important to consider whether natural rubber latex or synthetic derivatives of natural rubber latex were used as materials in the manufacture of the ADL product or its container to avoid potential allergic reactions [91]. Reference ADL offers a PFS and PFP with a smaller, 29-gauge needle size, and the needle cover of high-concentration PFS and PFP presentations of reference ADL is not made with natural rubber latex [2, 66]. Among currently available ADL biosimilars in the USA, seven offer a PFS or PFP with a smaller 29-gauge needle size (Table 3; Figs. 1 and 2) [34, 37,38,39, 67, 71,72,73], and eight are not made with natural rubber latex (Figs. 1 and 2) [33, 34, 38, 39, 41, 71, 72, 85]. Specifically, labeling for three ADL biosimilars (Hulio® [adalimumab-fkjp], Idacio® [adalimumab-aacf], and Yusimry™ [adalimumab-aqvh]) indicates a particular part of the presentation (plunger stopper, needle cover/cap, or both) is not made with natural rubber latex [39, 41, 85], and labeling for four ADL biosimilars (Amjevita [adalimumab-atto], Hadlima [adalimumab-bwwd], Hyrimoz [adalimumab-adaz], and Yuflyma [adalimumab-aaty]) does not specify a particular part of the presentation as not made with natural rubber latex meaning that it applies to the entire device [34, 38, 71, 72]. In addition, updated labeling for one ADL biosimilar (Abrilada™ [adalimumab-afzb], Pfizer Inc., New York, NY, USA) has received regulatory approval to include the statement “not made with natural rubber latex” as it applies to the entire device; this updated labeling is expected in April 2024 [33].
Comparison of 29-G thin-walled and 27-G regular-walled needle diameters. aThe 29-G thin-walled and 27-G regular-walled needles have different outer diameters, but a comparable inner diameter. Therefore, patients can apply the same force to the injection device to administer medicine at the same speed because it passes through the same size area. bThe 29-G thin-walled needle measurements are based on the needle used in Abrilada™ (adalimumab-afzb). The outer diameter measurements relate to all 29-G and 27-G needles; however, the inner diameter measurement may not be representative of needles used with other ADL products. ADL adalimumab; G gauge
Currently available PFPs (Fig. 2) offer various features that support the safe and correct self-administration of ADL, including smaller needle outer diameter for less painful injections, a viewing window to inspect medication and ensure it is not cloudy or contaminated, a visual indicator that moves along the window to show injection progress, simple injection activation mechanisms to initiate drug delivery, audible clicks to signal the start and end of injection, and a needle guard to minimize needle phobia and protect from injury [2, 33,34,35,36,37,38,39,40,41, 66,67,68, 71,72,73, 80, 82, 84]. In addition, reference ADL and four ADL biosimilars offer single-dose pens that are currently licensed for the Arthritis Foundation’s Ease of Use Certification [69, 92, 93]. Products receiving the Arthritis Foundation’s Ease of Use Certification have been independently tested and proven as easier to use for people living with arthritis, chronic pain and limited functionality [92]. These device attributes should be considered when discussing ADL treatment options as patients switching from reference ADL to an ADL biosimilar may find it easier to transition to a PFP that is similar to the reference ADL PFP.
In general, most patients prefer a PFP over a PFS because they are convenient, quick, comfortable, and easy to use and because they have built-in safety features [24, 28, 30, 32]. However, some patients who experience ISP, which may be influenced by pain catastrophizing and can cause dread of injecting over time [21, 29], may prefer to use a PFS because the device allows better control over the speed and duration of injection as compared with a PFP [31, 94].
A preference for PFP devices has been demonstrated in patients administering reference ADL. In a Phase 2 trial (TOUCH study) of reference ADL in patients (N = 52) with RA, 88.5% preferred using a PFP compared with 5.8% who preferred using a PFS to administer reference ADL, citing less pain (76.9%), ease of use (94.2%), and convenience (92.3%) as advantages [28]. In a Phase 3 trial of the ADL biosimilar PF-06410293 (Abrilada [adalimumab-afzb]] in patients (N = 50) with RA, nearly all patients (95.9% [47/49]) elected to continue study treatment using PFP injections after completing the device usability sub-study [25]. In a Phase 2, open-label study of the ADL biosimilar SB5 (Imraldi® [adalimumab], Samsung Bioepis Co., Ltd., Incheon, Republic of Korea/Hadlima [adalimumab-bwwd]) in 49 patients with RA, 30.4% and 56.5% of patients (N = 46) reported an overall preference for the PFS and PFP, respectively, at week 6 [26].
Self-injection of a TNFi can improve the patient treatment experience, and availability of choice in self-injection device can address psychologic (e.g., injection anxiety, needle phobia) and physical (e.g., impaired hand dexterity, hand pain) barriers to safe self-injection leading to improved treatment adherence and patient outcomes [87, 88].
Out of Fridge Stability
Under normal circumstances, ADL must be refrigerated (2 °–8 °C [36 °–46 °F]); however, if needed (e.g., when traveling), ADL may be stored under alternative conditions (Table 3) [2, 33,34,35,36,37,38,39,40,41]. All ADL products can be stored at 25 °C (77 °F) with storage durations ranging from 14 to 31 days (Table 3), as determined from stability studies and specified in the product labelling instructions [2, 33,34,35,36,37,38,39,40,41, 95, 96]. One ADL biosimilar, Abrilada (adalimumab-afzb), may be stored at temperatures of up to 30 °C (86 °F) for 30 days [33]. Extended stability of ADL at room temperature conditions offers convenience and flexibility in storage, which could improve the injection experience and may lead to better treatment adherence and patient outcomes.
ADL Biosimilars: Relevance of Interchangeability Designation to Clinics
In the USA, an approved biosimilar can be designated as “interchangeable.” An interchangeable biosimilar “can be expected to produce the same clinical result as the reference product in any given patient” and demonstrates “for products that are administered more than once the risk in terms of safety or diminished efficacy of alternating or switching between use of the biologic product and the reference product is not greater than the risk of using the reference product without such alternation or switch” (Table 1) [16, 17, 97]. Where state law permits, this allows pharmacy-level substitution, meaning the interchangeable biosimilar may be substituted for the reference product without the need for a new prescription to be issued by the healthcare provider (HCP) who prescribed the reference product [17, 97]. A 2020 survey of physicians (N = 602) who prescribe biologics indicated that most (75%) were opposed to automatic substitution of biologics by pharmacists. The hesitancy of physicians toward automatic substitution could be due to a perceived lack of control over prescribing and patients’ treatment [98, 99].
To date, only two ADL biosimilars in the USA have received interchangeability designation: Cyltezo ([adalimumab-adbm], Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA), and Abrilada (adalimumab-afzb) (Table 2) [46, 51]. Two ADL biosimilars have applications for interchangeability either submitted to (Hadlima [adalimumab-bwwd]) or under review with (Yuflyma [adalimumab-aaty]) the FDA [48, 52], and two others are being evaluated in clinical studies designed to support an interchangeability designation (Amjevita [adalimumab-atto]; Hulio [adalimumab-fkjp], Mylan Pharmaceuticals Inc., Morgantown, WV, USA) (Table 2) [45, 49]. Lastly, an application for interchangeability included as part of the biologics license application for one additional proposed ADL biosimilar (AVT02) was accepted by the US FDA with anticipated approval in February 2024 [44].
The US FDA has issued draft guidance with recommendations on labeling for biosimilar and interchangeable biosimilar products [100]. Following this guidance, the agency recommends that labeling for both biosimilars and interchangeable biosimilars include a “biosimilarity statement” as all FDA-approved biosimilars must not have any clinically meaningful differences to their reference products, irrespective of their interchangeability designation [100]. The interchangeability designation is relevant at the point of pharmacy dispensing, i.e., an interchangeable biosimilar may be substituted for its reference product without the intervention of the prescribing HCP, where state law permits [17, 97]. Therefore, information about whether a biosimilar is licensed as an interchangeable biosimilar product is provided in the Purple Book, an online database of all licensed biologic products, including biosimilars and interchangeable biosimilars, that is accessible to pharmacists, HCPs, and the public [46, 100].
Reference ADL and both interchangeable ADL biosimilars are available for injection as a PFS or PFP. This would allow patients to continue using the same type of self-injection device after switching to an interchangeable ADL biosimilar. However, HCPs and advanced practice providers may need to consider how any differences between available devices could impact the injection experience for patients who are switched to an interchangeable ADL biosimilar with slightly different features than reference ADL (e.g., availability of smaller 29-gauge needle, whether it is manufactured with natural rubber latex, how it is supplied, whether the needle is attached, if the cap must be locked back on, etc.). Additionally, each time a patient switches from one device to another it would require a new technology visit and healthcare resources to educate the patient on how to use the device. Therefore, the feasibility of switching between different devices would also need to be considered.
Patient Support Programs
Attributes of the various ADL biosimilars will help to inform prescriber and patient choice of treatment. Resources and services provided through company patient assistance programs (PAPs) may also influence treatment choices. In the USA, companies that manufacture ADL products offer ADL-specific and/or non-ADL-specific PAPs to provide reimbursement or other financial assistance to patients for the cost of their medicine. In addition, some PAPs also provide resources to help patients understand the cost of their medicine; navigate insurance processes and provide information related to their insurance coverage; and help them understand their disease, treatment options, and prescribed medication. These programs have the potential to improve patient access to ADL products and will be an important factor for doctors to consider when discussing ADL treatment options with their patients.
Conclusions
ADL is an effective treatment for patients with chronic immune-mediated inflammatory diseases. Biologic drugs, such as ADL, are commonly administered via SC injection (by PFS or PFP), which can be associated with ISP. The extent of ISP during SC injection is influenced by various product-related factors, such as formulation, delivery volume, and device features (e.g., type and needle gauge size). Multiple ADL biosimilars that differ in delivery options, formulation, and dosing presentations are available to patients. As differences between product attributes can impact the injection experience and, by extension, treatment adherence and patient outcomes, various features of ADL biosimilars should be considered to better inform patient choice of treatment. Additionally, the impact of interchangeable biosimilars and availability of PAPs that ensure access to medications are important factors for HCPs to consider when discussing ADL treatment options with their patients.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Armuzzi A, Lionetti P, Blandizzi C, et al. Anti-TNF agents as therapeutic choice in immune-mediated inflammatory diseases: focus on adalimumab. Int J Immunopathol Pharmacol. 2014;27(1 Suppl):11–32.
AbbVie Inc. HUMIRA® (adalimumab) injection, for subcutaneous use [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125057s417lbl.pdf. Published 2021. Updated Feb 2021. Accessed 15 Nov 2023.
Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81(1):91–101.
Dick AD, Rosenbaum JT, Al-Dhibi HA, et al. Guidance on noncorticosteroid systemic immunomodulatory therapy in noninfectious uveitis: fundamentals of care for uveitis (FOCUS) initiative. Ophthalmology. 2018;125(5):757–73.
Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2021;73(7):924–39.
Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481–517.
Martora F, Megna M, Battista T, et al. Adalimumab, ustekinumab, and secukinumab in the management of hidradenitis suppurativa: a review of the real-life experience. Clin Cosmet Investig Dermatol. 2023;16:135–48.
Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–72.
Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384–413.
Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol. 2019;71(1):5–32.
Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019;71(10):1599–613.
US Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product: guidance for industry. 2015. https://www.fda.gov/media/82647/download. Accessed 12 Feb 23.
Declerck P, Danesi R, Petersel D, Jacobs I. The language of biosimilars: clarification, definitions, and regulatory aspects. Drugs. 2017;77(6):671–7.
GaBi Online. Small molecule versus biological drugs. https://www.gabionline.net/biosimilars/research/Small-molecule-versus-biological-drugs. Published 2012. Updated 29 June 2012. Accessed 12 June 2023.
US Food and Drug Administration. What are “biologics” questions and answers. https://www.fda.gov/about-fda/center-biologics-evaluation-and-research-cber/what-are-biologics-questions-and-answers. Published 2018. Updated 6 Feb 2018. Accessed 12 June 2023.
US Food and Drug Administration. Considerations in demonstrating interchangeability with a reference product guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-demonstrating-interchangeability-reference-product-guidance-industry. Published 2019. Updated 6 May 2020. Accessed 12 June 2023.
US Food and Drug Administration. Biosimilar and interchangeable biologics: more treatment choices. https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices#:~:text=An%20interchangeable%20biosimilar%20product%20is,biosimilar%20and%20interchangeable%20biosimilar%20medications. Published 2021. Updated 17 Aug 2023. Accessed 7 Mar 2023.
US Food and Drug Administration. Generic drugs: questions & answers. https://www.fda.gov/drugs/frequently-asked-questions-popular-topics/generic-drugs-questions-answers#q1. Published 2021. Updated 16 Mar 2021. Accessed 12 June 2023.
US Food and Drug Administration. Biological product innovation and competition. https://www.fda.gov/drugs/biosimilars/biological-product-innovation-and-competition. Published 2023. Updated 7 Mar 2023. Accessed 12 June 2023.
Baumgart DC, Misery L, Naeyaert S, Taylor PC. Biological therapies in immune-mediated inflammatory diseases: can biosimilars reduce access inequities? Front Pharmacol. 2019;10:279.
St Clair-Jones A, Prignano F, Goncalves J, Paul M, Sewerin P. Understanding and minimising injection-site pain following subcutaneous administration of biologics: a narrative review. Rheumatol Ther. 2020;7(4):741–57.
Usach I, Martinez R, Festini T, Peris JE. Subcutaneous injection of drugs: literature review of factors influencing pain sensation at the injection site. Adv Ther. 2019;36(11):2986–96.
Aronson R. The role of comfort and discomfort in insulin therapy. Diabetes Technol Ther. 2012;14(8):741–7.
Dashiell-Aje E, Harding G, Pascoe K, DeVries J, Berry P, Ramachandran S. Patient evaluation of satisfaction and outcomes with an autoinjector for self-administration of subcutaneous belimumab in patients with systemic lupus erythematosus. Patient. 2018;11(1):119–29.
Fleischmann RM, Bock AE, Zhang W, et al. Usability study of PF-06410293, an adalimumab biosimilar, by prefilled pen: Open-label, single-arm, sub-study of a phase 3 trial in patients with rheumatoid arthritis. Rheumatol Ther. 2022;9(3):839–50.
Ghil J, Zielinska A, Lee Y. Usability and safety of SB5 (an adalimumab biosimilar) prefilled syringe and autoinjector in patients with rheumatoid arthritis. Curr Med Res Opin. 2019;35(3):497–502.
Hammer HB, Uhlig T, Kvien TK, Lampa J. Pain catastrophizing, subjective outcomes, and inflammatory assessments including ultrasound: results from a longitudinal study of rheumatoid arthritis patients. Arthritis Care Res (Hoboken). 2018;70(5):703–12.
Kivitz A, Cohen S, Dowd JE, et al. Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther. 2006;28(10):1619–29.
Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care. 2010;33(2):240–5.
Roszkiewicz J, Swacha Z, Smolewska E. Prefilled pen versus prefilled syringe: a pilot study evaluating two different methods of methotrexate subcutaneous injection in patients with JIA. Pediatr Rheumatol Online J. 2020;18(1):64.
Verdun di Cantogno E, Russell S, Snow T. Understanding and meeting injection device needs in multiple sclerosis: a survey of patient attitudes and practices. Patient Prefer Adherence. 2011;5:173–80.
Vermeire S, D’Heygere F, Nakad A, et al. Preference for a prefilled syringe or an auto-injection device for delivering golimumab in patients with moderate-to-severe ulcerative colitis: a randomized crossover study. Patient Prefer Adherence. 2018;12:1193–202.
Pfizer Inc. ABRILADATM (adalimumab-afzb) [prescribing information]. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761118. Published 2023. Updated Apr 2024 (pending). Accessed 10 Jan 2024.
Amgen Inc. AmjevitaTM (adalimumab-atto) [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761024s015lbl.pdf. Published 2023. Updated Aug 2023. Accessed 7 Nov 2023.
Boehringer Ingelheim Pharmaceuticals Inc. CYLTEZO® (adalimumab-adbm) [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761058s018lbl.pdf. Published 2023. Updated June 2023. Accessed 7 Nov 2023.
Samsung Bioepis Co. Ltd. HadlimaTM (adalimumab-bwwd) [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761059s008lbl.pdf. Published 2023. Updated July 2023. Accessed 7 Nov 2023.
Mylan Pharmaceuticals Inc. FKKBCL, Biocon Biologics Ltd,. Hulio® (adalimumab-fkjp) [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761154s005lbl.pdf. Published 2023. Updated Aug 2023. Accessed 7 Nov 2023.
Sandoz Inc. Hyrimoz® (adalimumab-adaz) [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761071s016lbl.pdf. Published 2023. Updated Sept 2023. Accessed 7 Nov 2023.
Fresenius Kabi. IDACIO® (adalimumab-aacf) [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761255s003lbl.pdf. Published 2023. Updated Oct 2023. Accessed 7 Nov 2023.
Celltrion Inc. Yuflyma® (adalimumab-aaty) [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761219s001lbl.pdf. Published 2023. Updated Sept 2023. Accessed 7 Nov 2023.
Coherus BioSciences Inc. YUSIMRYTM (adalimumab-aqvh) [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761216s004lbl.pdf. Published 2023. Updated Sept 2023. Accessed 7 Nov 2023.
Dennis M. More Humira biosimilars launch in US, including first interchangeable. https://firstwordpharma.com/story/5757198?aid=5757198. Published 2023. Updated 03 July 2023. Accessed 14 Aug 2023.
Pfizer Inc. FDA grants interchangeable designation to pfizer’s biosimilar ABRILADA™. https://www.pfizer.com/news/announcements/fda-grants-interchangeable-designation-pfizers-biosimilar-abriladatm#:~:text=Starting%20in%20late%20October%202023,below%20the%20Humira%20list%20price.&text=ABRILADA%20is%20a%20citrate%2Dfree,received%20FDA%20app. Published 2023. Updated 5 Oct 2023. Accessed 20 Oct 2023.
Alvotech. Alvotech provides U.S. regulatory update on AVT02, a high-concentration interchangeable biosimilar candidate to Humira® (adalimumab). https://investors.alvotech.com/news-releases/news-release-details/alvotech-provides-us-regulatory-update-avt02-high-concentration. Published 2023. Updated 20 Sept 2023. Accessed 20 Oct 2023.
ClinicalTrials.gov. A comparative study between ABP 501 and Humira® in participants with moderate to severe plaque psoriasis. https://clinicaltrials.gov/study/NCT05073315. Published 2021. Updated 5 Jan 2023. Accessed 12 June 2023.
US Food and Drug Administration. Purple Book database of licensed biological products. https://purplebooksearch.fda.gov/. Published 2020. Updated 24 Oct 2023. Accessed 7 Nov 2023.
Jeremias S. Coherus rep discusses interchangeable ophthalmology biosimilar, previews onpro competitor. https://www.centerforbiosimilars.com/view/coherus-rep-previews-interchangeable-ranibizumab-adalimumb-launches-on-pro-competitor?seriesVid=4. Published 2022. Updated 28 Sept 2022. Accessed 12 June 2023.
Celltrion Healthcare. Celltrion USA announces US FDA approval of Yuflyma® (adalimumab-aaty), a high-concentration and citrate-free formulation of Humira® (adalimumab) biosimilar. https://www.celltrion.com/en-us/company/media-center/press-release/2102?page=1&searchWord=Celltrion+USA+Announces+U.S.+FDA+Approval+of+Yuflyma%C2%AE+%28adalimumab-aaty%29%2C+a+High-Concentration+and+Citrate-Free+Formulation+of+Humira%C2%AE+%28adalimumab%29+Biosimilar&selectType=. Published 2023. Updated 24 May 2023. Accessed 11 Jan 2024.
ClinicalTrials.gov. Hulio interchangeability to Humira®, comparing pharmacokinetics, efficacy, safety and immunogenicity. https://clinicaltrials.gov/ct2/show/NCT05637515. Published 2022. Updated 13 Oct 2023. Accessed 12 June 2023.
Jeremias S. FDA approves idacio, the eighth adalimumab biosimilar. https://www.centerforbiosimilars.com/view/fda-approves-idacio-the-eighth-adalimumab-biosimilar. Published 2022. Updated 14 Dec 2022. Accessed 20 Oct 2023.
Fleischmann RM, Saikali W, Lakhanpal S, et al. Multiple switching between the biosimilar adalimumab PF-06410293 and reference adalimumab in patients with active rheumatoid arthritis: a phase 3, open-label, randomised, parallel-group study. Lancet Rheumatol. 2023;5(9):E532–41.
Samsung Bioepis Co. Ltd., Organon. Application (sBLA) for Interchangeability Designation for HADLIMA™ (adalimumab-bwwd), a Biosimilar to Humira®. https://www.organon.com/news/samsung-bioepis-organon-announce-fda-acceptance-of-supplemental-biologics-license-application-sbla-for-interchangeability-designation-for-hadlima-adalimumab-bwwd-a-biosimilar-to-humira/. Published 2023. Updated 7 Nov 2023. Accessed 12 Nov 2023.
Karow AR, Bahrenburg S, Garidel P. Buffer capacity of biologics–from buffer salts to buffering by antibodies. Biotechnol Prog. 2013;29(2):480–92.
Sek D. Breaking old habits: Moving away from commonly used buffers in pharmaceuticals. European pharmaceutical review. https://www.europeanpharmaceuticalreview.com/article/13699/breaking-old-habits-moving-away-from-commonly-used-buffers-in-pharmaceuticals. Published 2012. Updated 10 July 2012. Accessed 27 Feb 2023.
Falconer RJ. Advances in liquid formulations of parenteral therapeutic proteins. Biotechnol Adv. 2019;37(7): 107412.
Laursen T, Hansen B, Fisker S. Pain perception after subcutaneous injections of media containing different buffers. Basic Clin Pharmacol Toxicol. 2006;98(2):218–21.
Tapete G, Bertani L, Pieraccini A, et al. Effectiveness and safety of nonmedical switch from adalimumab originator to SB5 biosimilar in patients with inflammatory bowel diseases: twelve-month follow-up from the TABLET registry. Inflamm Bowel Dis. 2022;28(1):62–9.
Gazerani P, Wang K, Cairns BE, Svensson P, Arendt-Nielsen L. Effects of subcutaneous administration of glutamate on pain, sensitization and vasomotor responses in healthy men and women. Pain. 2006;124(3):338–48.
Bolge SC, Goren A, Tandon N. Reasons for discontinuation of subcutaneous biologic therapy in the treatment of rheumatoid arthritis: a patient perspective. Patient Prefer Adherence. 2015;9:121–31.
Salaffi F, Di Carlo M, Farah S, Carotti M. Adherence to subcutaneous anti-TNFalpha agents in patients with rheumatoid arthritis is largely influenced by pain and skin sensations at the injection site. Int J Rheum Dis. 2020;23(4):480–7.
Rosembert D, Malaviya A, How J, et al. P505 different failure rates after non-medical switching of 744 patients from adalimumab originator to 2 different adalimumab biosimilars at Cambridge University Hospitals, UK: real-world experience. J Crohns Colitis. 2020;14(1):S438–9.
Nash P, Vanhoof J, Hall S, et al. Randomized crossover comparison of injection site pain with 40 mg/0.4 or 0.8 mL formulations of adalimumab in patients with rheumatoid arthritis. Rheumatol Ther. 2016;3(2):257–70.
Yoshida T, Otaki Y, Katsuyama N, Seki M, Kubota J. New adalimumab formulation associated with less injection site pain and improved motivation for treatment. Mod Rheumatol. 2019;29(6):949–53.
Bergman M, Patel P, Chen N, Jing Y, Saffore CD. Evaluation of adherence and persistence differences between adalimumab citrate-free and citrate formulations for patients with immune-mediated diseases in the United States. Rheumatol Ther. 2021;8(1):109–18.
Gely C, Marin L, Gordillo J, et al. Impact of pain associated with the subcutaneous administration of adalimumab. Gastroenterol Hepatol. 2020;43(1):9–13.
AbbVie Inc. HUMIRA® (adalimumab) injection, for subcutaneous use [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125057s401lbl.pdf. Published 2017. Updated Apr 2017. Accessed 8 Nov 2023.
Pfizer Canada ULC. Abrilada® adalimumab injection [product monograph]. https://webfiles.pfizer.com/file/29c68fba-e640-4f00-bb88-c288693a9c1a?referrer=ccb731e5-4f2d-4f4a-b2dc-e5e912145fc6. Published 2022. Updated 14 Nov 2022. Accessed 8 Nov 2023.
Amgen Inc. AmjevitaTM (adalimumab-atto) [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761024lbl.pdf. Published 2016. Updated Sept 2016. Accessed 8 Nov 2023.
Arthritis Foundation. 2023 adalimumab (Humira®) biosimilars information. https://www.arthritis.org/getmedia/1b3c2a5f-8e47-4c60-a321-3c05aa8bd79f/HumiraBiosimilars_0923.pdf. Published 2023. Updated Sept 2023. Accessed 12 Nov 2023.
Boehringer Ingelheim Pharmaceuticals Inc. CYLTEZO® (adalimumab-adbm) [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761058lbl.pdf. Published 2017. Updated Aug 2017. Accessed 8 Nov 2023.
Samsung Bioepis Co. Ltd. Hadlima® adalimumab injection [product monograph]. https://pdf.hres.ca/dpd_pm/00068763.PDF. Published 2022. Updated 14 Dec 2022. Accessed 8 Nov 2023.
Celltrion Healthcare. Yuflyma™ adalimumab injection [product monograph]. https://pdf.hres.ca/dpd_pm/00064135.PDF. Published 2021. Updated 24 Dec 2021. Accessed 8 Nov 2023.
Coherus BioSciences Inc. Coherus launches YUSIMRY™, a biosimilar of Humira®, at $995 per carton in U.S. https://investors.coherus.com/news-releases/news-release-details/coherus-launches-yusimrytm-biosimilar-humirar-995-carton-us#. Published 2023. Updated 03 July 2023. Accessed 14 Aug 2023.
US Food and Drug Administration. Yusimry product quality review. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761216Orig1s000ChemR.pdf. Published 2021. Updated 19 Sept 2021. Accessed 8 Nov 2023.
ClinicalTrials.gov. A study in healthy people to test how 2 different formulations of BI 695501 are taken up by the body when given as an injection. https://www.clinicaltrials.gov/ct2/show/NCT05203289. Published 2022. Updated 21 Sept 2022. Accessed 12 June 2023.
Coherus BioSciences Inc. Coherus BioSciences (CHRS) Q2 2022 earnings call transcript. https://www.nasdaq.com/articles/coherus-biosciences-chrs-q2-2022-earnings-call-transcript. Published 2022. Updated 6 Aug 2022. Accessed 8 Nov 2023.
Heise T, Nosek L, Dellweg S, et al. Impact of injection speed and volume on perceived pain during subcutaneous injections into the abdomen and thigh: a single-centre, randomized controlled trial. Diabetes Obes Metab. 2014;16(10):971–6.
Zijlstra E, Jahnke J, Fischer A, Kapitza C, Forst T. Impact of injection speed, volume, and site on pain sensation. J Diabetes Sci Technol. 2018;12(1):163–8.
Anderson G, Meyer D, Herrman CE, et al. Tolerability and safety of novel half milliliter formulation of glatiramer acetate for subcutaneous injection: an open-label, multicenter, randomized comparative study. J Neurol. 2010;257(11):1917–23.
Samsung Bioepis Co. Ltd. HadlimaTM instructions for use. https://www.organon.com/product/usa/pi_circulars/h/hadlima/hadlima_ifu.pdf. Published 2022. Updated Dec 2022. Accessed 8 Nov 2023.
Fresenius Kabi. How to administer Idacio® using the syringe. https://www.idaciohcp.com/get-started-on-idacio#syringe-video. Published 2023. Accessed 8 Nov 2023.
Mylan Pharmaceuticals Inc. FKKBCL, Biocon Biologics Ltd, Biocon Biologics Ltd,. Instructions for use Hulio® pen. https://www.hulio.com/how-to-use. Published 2022. Updated July 2022. Accessed 8 Nov 2023.
Coherus BioSciences Inc. Instructions for use YusimryTM prefilled syringe. https://assets.website-files.com/6483738bedd883892f331b8d/649f37c40a0abc6289e97462_PrefilledSyringeInstructionsForUse.pdf. Published 2021. Updated Dec 2021. Accessed 8 Nov 2023.
Coherus BioSciences Inc. Instructions for use: YusimryTM Pen. https://assets.website-files.com/6483738bedd883892f331b8d/6483738bedd883892f331c24_IFU_PMD-0044_R01-24b6d636.pdf. Published 2023. Updated Feb 2023. Accessed 8 Nov 2023.
Biosimilar Collaborations Ireland Limited. Hulio® adalimumab injection [product monograph]. https://health-products.canada.ca/dpd-bdpp/info?lang=eng&code=99197. Published 2023. Updated 20 July 2023. Accessed 13 Dec 2023.
Mylan Pharmaceuticals Inc. FKKBCL, Biocon Biologics Ltd, Biocon Biologics Ltd,. Instructions for use Hulio® single-dose prefilled syringe. https://www.hulio.com/pdf/instructions-for-use-prefilled-syringe.pdf. Published 2022. Updated July 2022. Accessed 13 Dec 2023.
van den Bemt BJF, Gettings L, Domanska B, Bruggraber R, Mountian I, Kristensen LE. A portfolio of biologic self-injection devices in rheumatology: how patient involvement in device design can improve treatment experience. Drug Deliv. 2019;26(1):384–92.
Kivitz A, Segurado OG. HUMIRA pen: a novel autoinjection device for subcutaneous injection of the fully human monoclonal antibody adalimumab. Expert Rev Med Devices. 2007;4(2):109–16.
Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23(1–2):37–43.
Jaber A, Bozzato GB, Vedrine L, Prais WA, Berube J, Laurent PE. A novel needle for subcutaneous injection of interferon beta-1a: effect on pain in volunteers and satisfaction in patients with multiple sclerosis. BMC Neurol. 2008;8:38.
US Food and Drug Administration. Recommendations for labeling medical products to inform users that the product or product container is not made with natural rubber latex. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/recommendations-labeling-medical-products-inform-users-product-or-product-container-not-made-natural. Published 2014. Updated Dec 2014. Accessed 12 Nov 2023.
Arthritis Foundation. Ease of use products. https://www.arthritis.org/partnership/ease-of-use-products. Published 2023. Accessed 12 Nov 2023.
Pfizer Inc. AbriladaTM (adalimumab-afzb) injection [product information]. https://abrilada.pfizerpro.com/product-information. Published 2024. Accessed 24 Jan 2024.
Bayas A, Japp G, Fulda U, Kallmann B. Injection devices in the basic therapy of multiple sclerosis. Survey among neurologists, MS nurses and patients. Nervenheilkunde. 2010;29:57–62.
Park D, Yun J, Hwang SJ, Park SJ. Evaluation of physicochemical and biological stability of 36-months-aged SB5 (adalimumab biosimilar) for 4 weeks at room temperature. Adv Ther. 2019;36(2):442–50.
Shin YK, Han WY, Kim SJ, et al. Investigation of the physicochemical and biological stability of the adalimumab biosimilar CT-P17. Adv Ther. 2021;38(11):5609–22.
US Food and Drug Administration. Fact sheets: interchangeable biological products. https://www.fda.gov/drugs/biosimilars/multimedia-education-materials. Updated 24 Oct 2023. Accessed 12 June 2023.
Gibofsky A, Evans C, Strand V. Provider and patient knowledge gaps on biosimilars: insights from surveys. Am J Manag Care. 2022;28(12 Suppl):S227–33.
Wilde S, Schapiro L, Fletcher M, Pearson C. Understanding stakeholder perception of biosimilars. https://www.norc.org/content/dam/norc-org/pdfs/20210405_AV%20-%20NORC%20Biosimilars%20Final%20Report.pdf. Published 2021. NORC. Updated Apr 2021. Accessed 14 June 2023.
US Food and Drug Administration. Labeling for biosimilar and interchangeable biosimilar products. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/labeling-biosimilar-and-interchangeable-biosimilar-products. Published 2023. Updated 18 Sept 2023. Accessed 20 Oct 2023.
Medical Writing and Editorial Assistance.
Medical writing support was provided by Elyse Smith, PhD, of Engage Scientific Solutions and was funded by Pfizer.
Funding
This work was funded by Pfizer, including the journal’s Rapid Service Fee and Open Access Fee.
Author information
Authors and Affiliations
Contributions
Ann Wicker and Mark Latymer equally contributed to conception of the work. Jessica R. Allegretti, Jessica H. Brady, Ann Wicker, Mark Latymer, and Alvin Wells contributed to manuscript revision, take responsibility for the integrity of the work as a whole, and read and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
Jessica R. Allegretti reports consulting fees from AbbVie Inc, Adiso Therapeutics, Bristol-Myers Squibb, Ferring, Finch Therapeutics, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Pfizer, Roivant Sciences and Seres Therapeutics; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AbbVie Inc, Bristol-Myers Squibb, and Janssen Pharmaceuticals; payment for expert testimony from Finch Therapeutics; and participation on a Data Safety Monitoring Board or Advisory Board for Merck. Jessica H. Brady has no disclosures to report. Mark Latymer and Ann Wicker are full-time employees of and hold stock or options in Pfizer. Alvin Wells reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AbbVie Inc, Alexion Pharmaceuticals, Amgen Inc, AstraZeneca, Aurinia Pharmaceuticals Inc, Eli Lilly and Company, GSK plc and UCB.
Ethical Approval
This article is based on previously conducted studies and other sources; it does not contain any studies with human participants or animals performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Allegretti, J.R., Brady, J.H., Wicker, A. et al. Relevance of Adalimumab Product Attributes to Patient Experience in the Biosimilar Era: A Narrative Review. Adv Ther 41, 1775–1794 (2024). https://doi.org/10.1007/s12325-024-02818-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02818-9