Abstract
Introduction
Ethnicity differences are an important determinant in the clinical manifestation of Parkinson’s disease (PD), but they are not yet widely recognized, particularly regarding the response to dopaminergic medications. The aim of this paper is to analyze the efficacy and safety of safinamide in Chinese patients with PD in the pivotal studies SETTLE and XINDI compared to the non-Chinese population of the SETTLE trial.
Methods
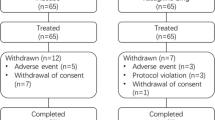
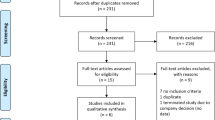
SETTLE (NCT00627640) and XINDI (NCT03881371) were phase III, randomized, double-blind, placebo-controlled, multicenter trials. Patients received safinamide or placebo as add-on to levodopa. The primary efficacy endpoint was the change in the mean total daily OFF time. Secondary efficacy endpoints included total daily ON time, ON time with no/non-troublesome dyskinesia, Unified Parkinson’s Disease Rating Scale, and Parkinson’s Disease Questionnaire-39 items. Safety was evaluated through the frequency of adverse events. Data from 440 non-Chinese and 109 Chinese patients in the SETTLE study, and 305 Chinese patients in the XINDI trial were considered for this post hoc analysis.
Results
Significant positive results were seen in favor of safinamide in all populations for the primary and secondary endpoints, with no differences in terms of magnitude. No “treatment by ethnicity” interaction was detected for any parameters, confirming the homogeneity of treatment effects between different populations. The safety and tolerability of safinamide in Chinese patients were similar to those in the other ethnic groups, without unexpected adverse reactions.
Conclusions
Safinamide was shown to improve PD symptoms and quality of life in different ethnic populations, without any treatment by race interaction. Further studies are warranted to investigate potential differences in a real-life situation.
Trial Registration Number
SETTLE (NCT00627640) and XINDI (NCT03881371).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out his study? |
The clinical manifestation of Parkinson’s disease and the response to treatments may be different between ethnic groups, and in particular in Chinese subjects compared to other populations |
The aim of this study was to investigate the effects of safinamide in Chinese and non-Chinese patients through the data of two pivotal studies, one performed in Europe, Asia, Pacific, and North America, the second in China |
What was learned from the study? |
Safinamide improved motor symptoms and motor fluctuations in different populations without any ethnicity interaction. No differences were detected in terms of safety and tolerability |
Large real-life trials in different ethnic populations are warranted to confirm these findings |
Introduction
Parkinson’s disease (PD) is a major neurodegenerative disorder characterized by a progressive loss of nigrostriatal dopaminergic neurons leading to a dopamine deficiency. Classical motor features of PD include tremor, bradykinesia, rigidity, and gait and postural instability [1]. The disease is also associated with several non-motor symptoms, such as fatigue, pain, mood disorders, sleep disturbances, and cognitive dysfunction, with a strong impact on patients’ daytime activities and well-being [2]. Beyond dopamine, other neurotransmitters are known to be involved: glutamate plays important roles in the pathogenesis of primary symptoms, motor fluctuations, dyskinesia, and neuronal cell loss [3]. The prevalence of PD increases with age, and the number of individuals with PD over age 50 is expected to double by 2030 causing a serious socioeconomic burden in the future aging society and an increasing demand for new PD therapies [4, 5]. The epidemiology of PD among various ethnic groups has been poorly studied: some preliminary data suggest that the prodromal risk of developing PD and the clinical symptom expression may vary between different ethnic groups; Chinese patients, for example, are more likely to experience dyskinesia and depression than non-Chinese or other populations [6, 7].
Safinamide is a unique treatment modulating both dopaminergic and glutamatergic systems. The glutamatergic mechanism of action is different from that of amantadine: safinamide, in fact, has an indirect effect on the glutamate release through the blockade of sodium channels, while amantadine has a direct effect due to the N-methyl-d-aspartate (NMDA) receptor antagonism [8].
The metabolism of safinamide is not dependent on cytochrome P (CYP) enzymes, is not influenced by any known genetic polymorphisms, and is not influenced by weight, race, age, or gender [9].
Results from pivotal studies showed that safinamide has positive effects on both motor [10,11,12,13,14] and non-motor functions [15,16,17] in patients with PD, with the same efficacy in both genders [18]. A previous publication [19] has described a post hoc analysis of the SETTLE study [12] dividing the patients into two groups, Asian-Pacific and non-Chinese. The Asian-Pacific subjects came from different countries (Australia, Hong Kong, India, Korea, Malaysia, Singapore, Taiwan, Thailand, and New Zealand), but not from one unique ethnic group. The aim of this paper is to describe the results of new additional analyses comparing the non-Chinese population of the SETTLE study with the Chinese subjects of the SETTLE and the XINDI trials, aiming to confirm, as seen in a previous pharmacokinetic/pharmacodynamic study [20], that there are no differences regarding the efficacy and safety of safinamide between different populations. The two pivotal studies (SUCCESS and XINDI) have been chosen because the patients’ characteristics and the design are similar and the dosing regimens are identical (safinamide or placebo administered at 50 mg/day for 15 days, then at 100 mg/day). The study 016 [10] was excluded because of a different dosing regimen, with two fixed dose levels (50 and 100 mg/day), and because there were no Chinese patients.
Methods
Study Design and Study Population
SETTLE (NCT00627640) and XINDI (NCT03881371) were phase III, double-blind, multicenter studies in patients with PD and motor fluctuations. The SETTLE trial enrolled patients in 21 countries of Europe, Asia, Pacific, and North America, while the XINDI study enrolled only patients in China. Patients with a diagnosis of idiopathic PD based on medical history and neurological examination [21] of more than 3 years duration, a Hoehn and Yahr (H&Y) stage 1–4 [22], and daily OFF time ≥ 1.5 h (excluding morning akinesia), were randomized to receive safinamide or placebo as add-on to levodopa (L-dopa). Patients with severe, disabling peak-dose or biphasic dyskinesia, wide or unpredictable fluctuations, cognitive or psychiatric problems were excluded. The efficacy was assessed by the changes in “OFF” and “ON” time from the patient diaries, the Unified Parkinson’s Disease Rating Scale (UPDRS) [23], and the Parkinson’s Disease Questionnaire-39 items (PDQ-39) [24]. Previous and concomitant medications were coded using the World Health Organization Drug Dictionary (WHO-DD) [25] and the adverse events (AEs) with the Medical Dictionary for Regulatory Activities (MedDRA) version 23.1 [26]. All AEs and serious adverse events (SAEs) were followed up until resolution. The studies were conducted in compliance with the last version of the Declaration of Helsinki and the Good Clinical Practices [27] and after the signature of a written informed consent by the patients and were approved by local ethics committees and national health authorities. Full details of the trials have been reported by Shapira et al. [12] and Wei et al. [14] and are also available at ClinicalTrials.gov.
Statistical Methods
Statistical analyses were performed using SAS® for Windows release 9.4 (SAS Institute, Inc., Cary, NC, USA), with two-sided tests at the significance level of α = 0.05. Demographic data were retrieved during the baseline visit from the patient’s history and hospital clinical records. Categorical variables were described as the number and percentage of subjects, while continuous variables were described by means of descriptive statistics. Conventional chi-square test or Fisher’s exact test, respectively, was used to detect any difference between subgroups. Efficacy endpoints were reported by the least-squares mean (LSM) for treatment differences and two-tailed 95% confidence intervals (CIs) using the latest result computed on the basis of the numbers of patients with non-missing observations. The p values versus placebo were calculated using analysis of covariance (ANCOVA) with treatment and center as independent factor, baseline values as covariate, and the change from baseline as dependent variable. The results of the efficacy outcomes were compared between ethnicities using ANCOVA with baseline values, country, body weight, disease duration, H&Y stage, and anti-PD medications other than L-dopa as covariates. The incidence of adverse events vs placebo were analyzed using Fisher’s exact test, while differences between the populations’ subgroups were compared through logistic regression using country, body weight, disease duration, H&Y stage, and anti-PD medications other than L-dopa as covariates.
Results
Demography
As shown in Table 1, the study populations consisted of 440 non-Chinese and 109 Chinese patients in the SETTLE study, and 305 Chinese patients in the XINDI trial. As written previously, data of the SETTLE non-Chinese population were compared with those of the Chinese patients in the SETTLE and XINDI trials, considered as distinct subgroups of patients. There were no differences at baseline between ethnic groups in the demographic and clinical characteristics except for the mean total daily levodopa dose that was higher in the SETTLE compared to the XINDI study (SETTLE non-Chinese population: 776.5 ± 423.8 mg; SETTLE Chinese subgroup: 756.4 ± 384.5 mg; XINDI: 510.0 ± 185.0 mg; p value for interaction between subgroups of patients = 0.0371).
Efficacy
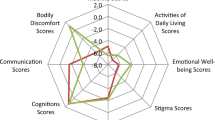
Changes from baseline to end of study in the efficacy parameters, comparing safinamide to placebo, are reported in Table 2. Significant positive results were seen in favor of safinamide in the three populations for all parameters analyzed. There was a statistically significant reduction of the OFF time (primary endpoint) with an LSM difference versus placebo of − 1.07 h (p < 0.0001) in the non-Chinese population of the SETTLE study, − 0.95 h (p = 0.0321) in the Chinese patients in the SETTLE, and − 1.10 h (p < 0.0001) in the Chinese subjects of the XINDI trial. Regarding the other main secondary efficacy endpoints, safinamide, compared with placebo, showed statistically significant improvements in the total daily ON time (SETTLE non-Chinese population: LSM difference + 0.86 h, p = 0.0096; SETTLE Chinese subgroup: LSM difference + 0.90 h, p = 0.0034; XINDI: LSM difference + 0.89 h, p = 0.0049), total daily ON time with no/non-troublesome dyskinesia (SETTLE non-Chinese population: LSM difference + 1.01 h, p < 0.0001; SETTLE Chinese subgroup: LSM difference + 0.93 h, p = 0.0459; XINDI: LSM difference + 1.07 h, p = 0.0021), UPDRS part III scores (SETTLE non-Chinese population: LSM difference − 2.63, p = 0.0142; SETTLE Chinese subgroup: LSM difference − 2.82, p = 0.0040; XINDI: LSM difference − 3.80, p = 0.0002), PDQ-39 summary of the index score (SETTLE non-Chinese population: LSM difference − 2.63, p = 0.006; SETTLE Chinese subgroup: LSM difference − 2.98, p = 0.0049; XINDI: LSM difference − 3.36, p = 0.0033), and the PDQ-39 subscale scores for mobility (SETTLE non-Chinese population: LSM difference − 4.86, p = 0.001; SETTLE Chinese subgroup: LSM difference − 4.39, p = 0.0190; XINDI: LSM difference − 4.62, p = 0.0038), activities of daily living (SETTLE non-Chinese population: LSM difference − 4.59, p = 0.006; SETTLE Chinese subgroup: LSM difference − 5.03, p = 0.0035; XINDI: LSM difference − 5.81, p = 0.0012), emotional well-being (SETTLE non-Chinese population: LSM difference − 3.66, p = 0.019; SETTLE Chinese subgroup: LSM difference − 3.76, p = 0.0036; XINDI: LSM difference − 5.23, p = 0.0047), and stigma (SETTLE non-Chinese population: LSM difference − 2.76, p = 0.061; SETTLE Chinese subgroup: LSM difference − 2.52, p = 0.099; XINDI: LSM difference − 4.74, p = 0.0275).
The p value for the “treatment by ethnicity” interaction was non-significant for all parameters, confirming the homogeneity of treatment effects between different populations.
Stratifications according to the administration of baseline medications as add-on to levodopa other than safinamide or placebo were not performed since concomitant multiple adjunctive treatments were administered and subgroups partly overlapped.
Adverse Events and Serious Adverse Events
As reported in Table 3, the percentage of patients experiencing adverse events (AEs) and serious adverse events (SAEs) was similar among the three subgroup of patients. No significant differences were detected in the percentage of patients experiencing AEs/SAEs related to the investigational medicinal product (IMP) or leading to withdrawal from the studies. The p value for the “treatment by ethnicity” interaction was non-significant for all these data. As reported by Wei et al. [14], the slight difference in the incidence of AEs observed in the XINDI study between safinamide and placebo was not statistically significant. The majority of AEs/SAEs were rated as mild or moderate, were completely resolved at the end of the study, and were those described in the patients’ leaflet. The most frequent AEs were dyskinesia, fall, urinary tract infection, nausea, and headache. Dyskinesia was observed with higher prevalence in all subjects receiving safinamide (SETTLE non-Chinese population: 14.6%; SETTLE Chinese subgroup: 13.5%; XINDI: 11.9%) compared with placebo (SETTLE non-Chinese population: 5.4%; SETTLE Chinese subgroup: 6.0%; XINDI: 3.9%), but was generally of mild or moderate intensity, transient, and did not lead to withdrawal from the study or IMP interruption. Drugs that increase the dopaminergic tone are known to increase dyskinesia; however, most patients with PD who complained of dyskinesia had presented this motor complication since the beginning of the study with no further aggravation. Moreover, as seen in previous pivotal trials, safinamide did not deteriorate ON time with troublesome dyskinesia [10, 11].
Discussion
The role of ethnicity in PD is poorly understood and underinvestigated, despite differences observed in epidemiology, clinical manifestation, and response to treatments. Several factors might play a role, such as pharmacogenetic, sociocultural, and environmental [28]. This is the first publication that analyzes potential differences, after safinamide administration, comparing Chinese patients with PD with other ethnic populations from the USA, Europe, Asia, and Pacific.
Safinamide significantly improved motor fluctuations and motor symptoms in all subjects, without any significant interaction between treatment and race. The improvements observed with the UPDRS part III (motor score) were not only statistically but also clinically significant according to the criteria of Shulman et al. [29] and were confirmed by the positive results seen in the mobility domain of the PDQ-39 scale. These results may be explained by the dual mechanism of action of safinamide, dopaminergic (MAO-B inhibition) and non-dopaminergic (glutamate modulation). Safinamide prevents the degradation of dopamine, thereby raising its levels and prolonging its effects. This mechanism is expected to improve both the quality and the duration of fluctuations. Moreover, glutamate is known to be involved, together with dopamine and other neurotransmitters, in the deterioration of motor symptoms and in the development of motor complications [30, 31]. Significant improvements were seen in three other PDQ-39 domains—activities of daily living, emotional well-being, and stigma—and were reflected by a general improvement of patient’s quality of life, as shown by the PDQ-39 summary of index scores. The benefit seen in the emotional well-being domain of the PDQ-39 score, in particular, is consistent with previous published data on the efficacy of safinamide on depression and apathy [16, 32]. Despite a different baseline levels of levodopa dosages, which could reflect different therapeutic strategies, the L-dopa equivalent daily dose (LEDD) did not changed during the study periods [12, 14], confirming that safinamide treatment does not require an increase of L-dopa dose [33]. Overall, the safety profile of safinamide in Chinese patients was similar to that in the other ethnicities, without any unexpected adverse reaction. Adverse events occurred with a similar frequency in both safinamide and placebo groups except for dyskinesia, which was prevalent with safinamide, although non-significant. Its incidence resembled that reported for rasagiline and opicapone in patients with fluctuating PD [34,35,36,37]. Previous trials have shown that safinamide does not deteriorate the ON time with troublesome dyskinesia and improves dyskinesia scales [11, 38], and therefore this frequency difference could be due to the increase of the dopaminergic tone mitigated by the glutamatergic modulation of the drug.
There are some limitations to be considered in this post hoc analysis. The original trials were not designed nor powered to investigate differences between ethnic subgroups and did not consider genetic and socioeconomic factors. The results could be also limited by the short-term treatment duration, the eligibility criteria, and the frequency of visits that do not reflect the routine clinical practice. There was only one pre-specified dosage level of safinamide and data were compared versus placebo, without a direct comparison with another active treatment. These findings should therefore be considered as exploratory and must be confirmed in larger real-life trials in different ethnic populations.
Conclusions
Increased knowledge on the role of ethnicity in PD may help to evaluate more appropriately symptom expression and treatment response, improve the diagnosis, and prescribe personalized medicines. Our post hoc analyses of the SETTLE and XINDI studies have shown that safinamide was effective in improving motor symptoms and motor fluctuations in different populations without any ethnicity interaction, and with a favorable safety profile. Further epidemiological studies are needed to investigate the effects of safinamide in different ethnic populations and in usual care setting.
Data Availability
The data supporting the conclusions of this article will be made available by the corresponding author on reasonable request. Full details of the trials SUCCESS and XINDI have been reported by Shapira et al. [12] and Wei et al. [14], respectively, and are also available at ClinicalTrials.gov. (SETTLE: NCT00627640; XINDI: NCT03881371).
References
Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76.
Chaudhuri KR, Shapira AHV. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8(5):464–74.
Chase TN, Bibbiani F, Oh JD. Striatal glutamatergic mechanisms and extrapyramidal movement disorders. Neurotox Res. 2003;5:139–46.
Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson’s disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–6.
Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. https://doi.org/10.1038/nrdp.2017.13.
Rana AQ, Athar A, Owlia A, et al. Impact of ethnicity on non-motor symptoms of Parkinson’s disease. J Parkinsons Dis. 2012;2:281–5.
Li HJ, Zhang MF, Chen MX, et al. Validation of the nonmotor symptoms questionnaire for Parkinson’s disease: results from a Chinese pilot study. Int J Neurosci. 2015;125:929–35.
Jost WH. A critical appraisal of MAO-B inhibitors in the treatment of Parkinson’s disease. J Neural Transm. 2022;129:723–36.
Onofrj M, Bonanni L, Thomas A. An expert opinion on safinamide in Parkinson’s disease. Expert Opin Investig Drugs. 2007;17(7):1115–25.
Borgohain R, Szasz J, Stanzione P, et al. Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov Disord. 2014;29(2):229–37.
Borgohain R, Szasz J, Stanzione P, et al. Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson’s disease. Mov Disord. 2014;29(10):1273–80.
Schapira AHV, Fox SH, Hauser RA, et al. Assessment of safety and efficacy of safinamide as a levodopa adjunct in patients with Parkinson’s disease and motor fluctuations: a randomized clinical trial. JAMA Neurol. 2017;74:216–24.
Hattori N, Tsuboi Y, Yamamoto A, Sasagawa Y, Nomoto M. Efficacy and safety of safinamide as an add-on therapy to L-dopa for patients with Parkinson’s disease: a randomized, double-blind, placebo-controlled, phase II/III study. Parkinsonism Relat Disord. 2020;75:17–23.
Wei Q, Tan Y, Xu P, et al. The XINDI study: a randomized phase III clinical trial evaluating the efficacy and safety of safinamide as add-on therapy to levodopa in Chinese Parkinson’s disease patients with motor fluctuations. CNS Drugs. 2022;36(11):1217–27.
Cattaneo C, Barone P, Bonizzoni E, Sardina M. Effects of safinamide on pain in fluctuating Parkinson’s disease patients: a post-hoc analysis. J Parkinsons Dis. 2017;7(1):95–101.
Cattaneo C, Müller T, Bonizzoni E, et al. Long-term effects of safinamide on mood fluctuations in Parkinson’s disease. J Parkinsons Dis. 2017;7(4):629–34.
Cattaneo C, Kulisevsky J, Tubazio V, Castellani P. Long-term efficacy of safinamide on Parkinson’s disease chronic pain. Adv Ther. 2018;35(4):515–22.
Pellecchia MT, Picillo M, Russillo MC, et al. Efficacy of safinamide and gender differences during routine clinical practice. Front Neurol. 2021;12:756304. https://doi.org/10.3389/fneur.2021.756304.
Bhidayasiri R, Ishida T, Kamei T, et al. Safinamide as an adjunct to levodopa in Asian and Caucasian patients with Parkinson’s disease and motor fluctuations: a post-hoc analysis of the SETTLE study. J Mov Disord. 2023;16(2):180–90.
Loprete L, Leuratti C, Cattaneo C, Thapar MM, Farrell C, Sardina M. Population pharmacokinetic and pharmacodynamic analyses of safinamide in subjects with Parkinson’s disease. Pharma Res Per. 2016;4(5):e00251. https://doi.org/10.1002/prp2.251.
National Collaborating Centre for Chronic Conditions. Parkinson’s disease: national clinical guideline for diagnosis and management in primary and secondary care. London: Royal College of Physicians; 2006.
Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–42.
Fahn S, Elton R, Members of the UPDRS Development Committee. The unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent developments in Parkinson’s disease, vol. 2. Florham Park: McMellam Health Care Information; 1987. p. 153–63.
Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well-being for individuals with Parkinson’s disease. Qual Lif Res. 1995;4:241–8.
World Health Organization-Drug Dictionary (WHO-DD), Uppsala Monitoring Centre; 2020.
Medical Dictionary for Regulatory Activities (MedDRA) version 23.1, September 2020.
ICH Topic E10. Note for guidance on choice of control groups in clinical trials. CPMP/ICH/364/96 January 2001.
Ben-Joseph A, Marshall CR, Lees AJ, Noyce A. Ethnic variation in the manifestation of Parkinson’s disease: a narrative review. J Parkinsons Dis. 2020;10(1):31–45.
Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The clinically important difference on the unified Parkinson’s disease rating scale. Arch Neurol. 2010;67(1):64–70.
Blandini F, Porter RH, Greenamyre JT. Glutamate and Parkinson’s disease. Mol Neurobiol. 1996;12:73–94.
Pagonabarraga J, Tinazzi M, Caccia C, Jost WH. The role of glutamatergic neurotransmission in the motor and non-motor symptoms in Parkinson’s disease: clinical cases and a review of the literature. J Clin Neurosci. 2021;90:178–83.
Kulisevsky J, Martinez-Horta S, Campolongo A, et al. A randomized clinical trial to evaluate the effects of safinamide on apathetic non-demented patients with Parkinson’s disease. Front Neurol. 2022;13:866502. https://doi.org/10.3389/fneur.2022.86652.
Cattaneo C, Sardina M, Bonizzoni E. Safinamide as add-on therapy to levodopa in mid- to late-stage Parkinson’s disease fluctuating patients: post-hoc analyses of studies 016 and SETTLE. J Parkinsons Dis. 2016;6(1):165–73.
Parkinson Study Group. A randomized placebo-controlled trial of rasagiline in levodopa-treated patients with Parkinson’s disease and motor fluctuations: the PRESTO study. Arch Neurol. 2005;62:241–8.
Zhang Z, Shao M, Chen S, et al. Adjunct rasagiline to treat Parkinson’s disease with motor fluctuations; a randomized, double-blind study in China. Transl Neurodegener. 2018;7:14. https://doi.org/10.1186/s40035-018-0119-7.
Ferreira JJ, Lees A, Rocha JF, et al. Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: a randomized, double-blind, controlled trial. Lancet Neurol. 2016;15(2):154–65.
Lees AJ, Ferreira J, Rascol O, et al. Opicapone as adjunct to levodopa therapy in patients with Parkinson’s disease and motor fluctuations: a randomized clinical trial. JAMA Neurol. 2017;74(2):197–206.
Cattaneo C, La Ferla R, Bonizzoni E, Sardina M. Long-term effects of safinamide on dyskinesia in mid- to late-stage Parkinson’s disease: a post-hoc analysis. J Parkinsons Dis. 2015;5(3):475–81.
Acknowledgements
The authors thanks the patients involved in the trials SUCCESS and XINDI, their families and caregivers, the investigators, and the staff of all the investigational sites. The list of study investigators is provided in the Supplementary Material.
Funding
Financial support for the paper, the journal’s Rapid Service and the Open Access were provided by Zambon SpA; Zambon SpA was not involved in the analysis, interpretation of data, writing of this article, or the decision to submit it for publication.
Author information
Authors and Affiliations
Contributions
Carlo Cattaneo and Jaime Kulisevsky contributed to analyzing the data, interpreting the findings, writing and reviewing the paper, collecting and analyzing the literature, designing the tables, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Carlo Cattaneo is an employee of Zambon SpA; Jaime Kulisevsky has received compensation for consultancy and speaker related activities from Zambon SpA.
Ethical Approval
This article is based on the data of the previously conducted studies SUCCESS and XINDI that have been approved in all countries by ethical national committees and national health authorities and were performed according to the guidelines of the Declaration of Helsinki. The authors received permission to access the data. No new studies with human participants have been performed for this publication. Written informed and privacy consents to participate in the studies SUCCESS and XINDI, collect, analyze, and report anonymized and aggregated data were obtained from all patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cattaneo, C., Kulisevsky, J. The Effects of Safinamide in Chinese and Non-Chinese Patients with Parkinson’s Disease. Adv Ther 41, 638–648 (2024). https://doi.org/10.1007/s12325-023-02736-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02736-2