Abstract
Introduction
QT interval dispersion, which reflects the regional heterogeneity of ventricular repolarization, increases during electroconvulsive therapy (ECT). Tpeak-Tend (TpTe) is considered a new marker of the transmural dispersion of ventricular repolarization (TDR). This study aimed to evaluate the effect of remifentanil on TpTe during ECT.
Methods
Forty-two patients who were scheduled to undergo ECT with American Society of Anesthesiologists physical status I or II randomly received 0.1 μg/kg remifentanil (group R: n = 21) or saline (group C: n = 21). After the induction of general anesthesia, we measured the TpTe, TpTe/QT, TpTe/QTc, TpTe/RR, TpTe/√RR and TpTe/3√RR every minute during ECT (QT: QT interval, QTc: corrected QT interval, RR: RR interval). Statistical analysis was performed using two-way analysis of variance (ANOVA).
Results
Immediately (T0) and 1 min (T1) after electrical stimulation, the RRs (group C: T0; 654.2 ± 145.9 ms, T1; 657.3 ± 114.8 ms, group R: T0; 849.6 ± 249.3 ms, T1; 885.4 ± 213.6 ms, p < 0.05) were significantly increased, while systolic (group C: T0; 177.1 ± 35 mmHg, group R: T0; 129 ± 27.2 mmHg, p < 0.05) and diastolic blood pressures (group C: T0; 107.1 ± 22.4 mmHg, T1; 101.3 ± 23.2 mmHg, group R: T0; 75.4 ± 19.3 mmHg, T1; 80.6 ± 18.3 mmHg, p < 0.05) were significantly decreased in group R compared to group C. The TpTe/RR was significantly lower at T1 in group R compared to group C (group C: 101.5 ± 28.2, group R: 76.8 ± 21.8, p < 0.05). However, there was no significant difference in TpTe, TpTe/QT, TpTe/QTc, TpTe/√RR or TpTe/3√RR between the two groups throughout the study.
Conclusion
Pretreatment with remifentanil suppressed the increase of TpTe/RR after electrical stimulation. Our results imply that remifentanil may lead to a decrease in TDR during ECT.
Trial Registration
This trial was registered with the University Hospital Medical Information Network (registration number: UMIN000051958).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Tpeak-Tend (TpTe) is considered a relatively new marker of the transmural dispersion of ventricular repolarization (TDR) |
No studies have reported the effects of remifentanil on TpTe during electroconvulsive therapy (ECT) |
To clarify the effect of remifentanil on TDR during ECT, we measured the TpTe in the present study |
What was learned from the study? |
Remifentanil suppressed the significant hemodynamic change during ECT |
Remifentanil significantly attenuated the increase in TpTe/RR (RR: RR interval) |
Our findings suggest that remifentanil likely reduces the TDR, a predictor of ventricular tachyarrhythmia |
Introduction
Electroconvulsive therapy (ECT) is usually performed to treat various psychiatric disorders when other treatments are unsuccessful. In most cases, this procedure is conducted under general anesthesia. Previous studies have demonstrated that remarkable hemodynamic changes, such as hypertension or tachycardia, are observed immediately after electrical stimulation in ECT because of the activation of the sympathetic nervous system [1, 2]. Most ECT-related deaths are due to adverse cardiovascular events following electrical stimulation [3, 4].
The dispersion of QT interval (QTD), defined as the difference between the maximum and minimum QT interval on 12-lead surface electrocardiography (ECG), is known to reflect the regional heterogeneity of ventricular repolarization of the myocardium. This marker has been proposed as a predictor of ventricular arrhythmia, which may lead to sudden cardiac death [5]. Our previous reports have suggested that QTD significantly increased after electrical stimulation [6, 7].
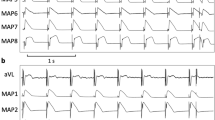
The interval from the peak to the end of the T wave (TpTe) on 12-lead electrocardiogram (ECG) is a comparatively novel parameter which reflects the transmural dispersion of ventricular repolarization (TDR). The TDR is strongly associated with the incidence of ventricular tachyarrhythmia [8], and its evaluation is beneficial for predicting the lethal arrhythmias in patients with coronary artery disease, Brugada syndrome, short QT and long QT syndromes [9]. Similar to QTD, TpTe is suggested to be a predictor of lethal ventricular arrhythmias, which may cause sudden death [10, 11]. The TpTe/QT ratio is also considered a marker of TDR and a noninvasive arrhythmogenic index of sudden cardiac death [12]. In addition, patients who developed ventricular tachycardia (VT) displayed prolonged TpTe/√RR (collected by RR interval) compared to patients who did not develop VT [13].
Remifentanil, an ultra-short-acting μ-opioid receptor agonist, is approved for general anesthesia and achieves predictable recovery because it allows fast emergence from anesthesia. Our previous study clarified that pretreatment with remifentanil significantly suppressed the increase of hemodynamic change and QTD during ECT [14]. The effectiveness of remifentanil against QTD, a marker of myocardial ventricular repolarization, during ECT has been demonstrated, but its effect on TpTe, a novel marker of TDR, has not been established. The purpose of this study is to investigate the change of TpTe during ECT and the effect of remifentanil on TpTp during ECT.
Methods
We included 44 patients with American Society of Anesthesiologists (ASA) physical status I or II, aged 20–65 years, who were scheduled to undergo modified electroconvulsive therapy (mECT). The study was approved by the ethics committee of Dokkyo Medical University (R-32-1) and registered with the University Hospital Medical Information Network (UMIN, registration number: UMIN000051958). We received written informed consent from all patients. All procedures were performed in accordance with the ethical standards of the Institutional and National Research Committee and the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. We excluded patients with cardiovascular, respiratory, metabolic or cerebrovascular diseases, and preoperative electrocardiogram (ECG) abnormalities. None of the patients had received premedication. The participants were randomly assigned to two groups: patients in group R (n = 22) received IV remifentanil (1 µg/kg) before the induction of general anesthesia, while those in group C (n = 22) received IV saline. In the operating room, standard monitoring of three-lead ECG signals, noninvasive measurement of arterial blood pressure and pulse oximetry were performed (DS-7780W; FUKUDA DENSHI Co., Ltd., Tokyo, Japan). After adequate preoxygenation, pretreatment with 1 µg/kg remifentanil or placebo (saline), intravenously injected, and anesthesia was induced with 1 mg/kg IV propofol. After confirming loss of consciousness, 1 mg/kg succinylcholine was intravenously administered. Subsequently, we performed assisted mask ventilation with 100% oxygen. An electrical stimulus was delivered via bitemporal electrodes using an ECT stimulator (Thymatron System; Somatics LLC, Lake Bluff, IL.). The magnitude of the energy setting for the ECT stimulus was predetermined according to age. We determined the efficacy of ECT using the tourniquet technique, which is based on the observation of convulsive movements of the distal leg. The seizures were detected using an electroencephalogram (EEG) monitor set in the electrical stimulator.
We measured the RR interval (RR), systolic blood pressure (sBP), diastolic blood pressure (dBP), TpTe, TpTe/QT (QT: QT interval), TpTe/QTc (QTc: corrected QT interval), TpTe/RR, TpTe/√RR or TpTe/3√RR before the induction of general anesthesia, after loss of consciousness (baseline), immediately after electrical stimulus (T0), and every 1 min to 7 min after electrical stimulus (T1–T7). Three-lead ECG signals were recorded using LRR-03 (GMS, Tokyo, Japan).
The primary outcome of the present study was the effect of remifentanil on TDR during ECT. Therefore, the primary endpoints of TDR were defined as TpTe, TpTe/QT, TpTe/QTc, TpTe/RR, TpTe/√RR or TpTe/3√RR in ECG during ECT. The secondary endpoints of our study were RR interval, sBP or dBP.
Statistical analysis
Statistical analyses were performed using Prism 6 software (GraphPad, La Jolla, CA, USA). Data were expressed as mean ± standard deviation (SD). Patient characteristics were analyzed using Student’s t-test and Fisher’s exact test. Changes in RR interval, sBP, dBP, TpTe, TpTe/QT, TpTe/QTc, TpTe/RR, TpTe/√RR (corrected by Bazzet formula) and TpTe/3√RR (corrected by Fridericia formula) were analyzed using two-way analysis of variance. When a significant overall effect was detected, Bonferroni’s post hoc test was conducted. In all analyses, the probability of detecting a significant difference was set at 5% (p < 0.05). A sample size of 18 subjects in each group was considered adequate, based on a previous study [15], to detect a difference of 10 and SD of 20 in the TpTe between the two groups at a power of 80%, with α = 0.05.
Results
Two patients were excluded from this study. A patient in group C was excluded because of ECG recording error, and one patient in group R was excluded because of remifentanil-induced anaphylaxis. Finally, we enrolled 42 patients (Fig. 1). There were no significant differences in age, sex, ASA physical status or body mass index (BMI) between the two groups (Table 1). Neither complications nor lethal arrhythmias were observed in this study. All patients received psychiatric medications (Table 2) .
Table 3 shows the values of the RR interval, systolic (sBP) and diastolic blood pressures (dBP) during the present measurement. The RR interval was significantly increased at T0 and T1 in group R compared with that in group C (group C: T0; 654.2 ± 144.9 ms, T1; 657.3 ± 114.8 ms, group R: T0; 849.6 ± 249.3 ms, T1; 885.4 ± 213.6 ms, p < 0.05). The sBP at T0 in group C was significantly increased compared with that in group R (group C: 177.1 ± 35 mmHg, group R: 129 ± 27.2 mmHg, p < 0.05). The dBP at T0 and T1 in group C significantly increased compared to that in group R (group C: T0; 107.1 ± 22.4 mmHg, T1; 101.3 ± 23.2 mmHg, group R: T0; 75.4 ± 19.3 mmHg, T1; 80.6 ± 18.3 mmHg, p < 0.05).
Table 4 shows the measurement values of TpTe, TpTe/QT, TpTe/QTc, TpTe/RR, TpTe/√RR and TpTe/3√RR during the ECT. The TpTe/RR at T1 in group R was significantly lower than that in group C (group C: T1; 101.5 ± 28.2, group R: T1; 76.8 ± 21.8). However, there were no significant differences in the TpTe, TpTe/QT, TpTe/QTc, TpTe/√RR and TpTe/3√RR values between the two groups during ECT.
Discussion
The present study demonstrated the effect of remifentanil on TpTe/RR, a maker of TDR and predictor of lethal ventricular arrhythmia during ECT.
TpTe as an index of TDR
The T peak represents complete repolarization of the epicardium and T end represents complete repolarization of the M cell action potential (mid-myocardium). Thus, the action potential duration of the longest M cell determines the TpTe interval and serves as a parameter of transmural dispersion of the whole ventricular repolarization [16, 17].
The efficacy of remifentanil on TDR using TpTe, TpTe/QT, TpTe/RR, TpTe/√RR and TpTe/3√RR during ECT has not been reported. Several studies have reported that TpTe has been established as a reliable parameter to determine the risk of ventricular arrythmia and sudden death [11, 18, 19]. The prolongation of TpTe leads to lethal arrhythmias such as ventricular tachycardia (VT) or ventricular fibrillation (VF) [13]. Similar to TpTe, TpTe/QT ratio is also considered a useful marker to evaluate TDR. Gupta et al. demonstrated that TpTe/QT ratio is independent of RR interval and more accurate compared to TpTe, especially under the conditions of long QT, short QT, Brugada syndrome and acute ST segment elevation [12].
A previous study suggested that TpTe/√RR corrected using the Bazzet formula or TpTe/3√RR corrected using the Fridericia formula is the preferred marker for sudden cardiac arrest risk [20]. However, significant efficacy of remifentanil on TpTe/√RR and TpTe/3√RR was not observed in the present study.
We measured the TpTe/RR (not using a 24-h Holter electrocardiogram) as an index of TDR, and the prolongation of this marker was significantly suppressed by remifentanil. It has been reported that the TpTe/RR slope measured from 24-h Holter electrocardiogram may be a predictor for sudden cardiac death [21]. Although the reliability of TpTe/RR (but not TpTe/RR slope) as a predictor for lethal arrhythmia has not been established, our result implied that TpTe/RR may reflect the TDR during ECT and may be suppressed by remifentanil. This contradiction between TpTe/RR and other parameters such as TpTe, TpTe/QT, TpTe/√RR and TpTe/3√RR remains uncertain. However, the difference of the drug for general anesthesia might affect the TDR in our observation. Further studies using other anesthetic agents such as pentobarbital are required to determine the reliability of TpTe/RR.
Efficacy of Remifentanil on Cardiovascular Systems
Our previous study demonstrated that QTD and QT interval was significantly increased during ECT [6]. Similar to TpTe, QTD has been considered as an index of ventricular arrhythmia, which may lead to sudden cardiac death [5]. In addition, we reported that remifentanil was effective in the suppression of QTD [14]. Thus, remifentanil might improve the TDR during ECT. Moreover, it is known that adding remifentanil reduced the consumption of propofol without adverse hemodynamic effects [22]. Generally, the benefits of adding remifentanil during ECT are as follows: (1) adequate seizure duration for convulsion therapy due to the reduction of propofol [23] and (2) attenuation of acute hemodynamic changes. In the present study, pretreatment with remifentanil attenuated the changes in RR interval, sBP and dBP after electrical stimulus. These results emphasize the efficacy of remifentanil for treating hemodynamic change during ECT.
Limitations
There are several limitations in our study. We observed the effect of remifentanil on the TpTe/RR but not TpTe/RR slope. Essentially, the TpTe/RR slope is evaluated using a 24-h Holter electrocardiogram. The TpTe/RR was measured just during the ECT in this protocol. Further verification over an extended period is required to assess the reliability of the TpTe/RR.
In several studies, the QT interval and QTD were significantly increased during ECT [6, 24]. In contrast, it has been reported that there was no significant change in QTD and TpTe during ECT [25]. In our results, TpTe, TpTe/QT, TpTe/√RR and TpTe/3√RR were not changed during ECT. This discrepancy might be attributed to differences in the anesthetic agents. We conducted the induction of general anesthesia using propofol for all patients. Kleinsasser et al. demonstrated that hemodynamic change and QT interval were more stable under propofol anesthesia compared to sevoflurane anesthesia [26]. Propofol was likely to suppress the changes in TDR during ECT. Further studies should be conducted using other anesthetic agents, such as barbiturates.
In many cases, the relationship between TpTe and lethal ventricular arrhythmia is assessed using a receiver-operating characteristic (ROC) curve. However, no lethal ventricular arrhythmia was observed in the present study. In addition, the sample size of our trial was inadequate to conduct a ROC curve. Hence, a ROC curve could not be constructed. Further studies to evaluate the cutoff value of TpTe associated with lethal ventricular arrhythmia is needed.
Conclusion
The current results suggest that pretreatment with remifentanil suppressed the prominent hemodynamic changes during ECT without any adverse effects. Moreover, remifentanil prevented the increase in TpTe/RR after the electrical stimulus. These results imply that remifentanil is beneficial for attenuating TDR caused by electrical stimuli. However, further verification of TDR during ECT is essential.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Suzuki Y, Miyajima M, Ohta K, et al. A triphasic change of cardiac autonomic nervous system during electroconvulsive therapy. J ECT. 2015;31:186–91.
Akutsu K, Shimizu T, Hamaguchi S, Yamaguchi S, Takasusuki T, Asahi K. Effect of remifentanil on cardiac autonomic activity changes during electroconvulsive therapy: a randomized controlled trial. Dokkyo Med J. 2023;2(2):158–63.
Sackeim HA, Dillingham EM, Prudic J, et al. Effect of concomitant pharmacotherapy on electroconvulsive therapy outcomes: short-term efficacy and adverse effects. Arch Gen Psychiatry. 2009;66:729–37.
Takagi S, Iwata K, Nakagawa A. Relationship between body mass index and blood pressure elevation during electroconvulsive therapy. J Clin Anesth. 2012;24:33–7.
Okin PM, Devereux RB, Howard BV, Fabsitz RR, Lee ET, Welty TK. Assessment of QT interval and QT dispersion for prediction of all-cause and cardiovascular mortality in American Indians: the strong heart study. Circulation. 2000;101:61–6.
Tezuka N, Egawa H, Fukagawa D, et al. Assessment of QT interval and QT dispersion during electroconvulsive therapy using computerized measurements. J ECT. 2010;26:41–6.
Yamaguchi S, Nagao M, Ikeda T, et al. QT dispersion and rate-corrected QT dispersion during electroconvulsive therapy in elderly patients. J ECT. 2011;27:183–8.
Haraguchi R, Ashihara T, Namba T, et al. Transmural dispersion of repolarization determines scroll wave behavior during ventricular tachyarrhythmias. Circ J. 2011;75:80–8.
Kilicaslan F, Tokatli A, Ozdag F, et al. Tp-e interval, Tp-e/QT ratio, and Tp-e/QTc ratio are prolonged in patients with moderate and severe obstructive sleep apnea. Pacing Clin Electrophysiol. 2012;35:966–72.
Kors JA, van Ritsema Eck HJ, van Herpen G. The meaning of the Tp-Te interval and its diagnostic value. J Electrocardiol. 2008;41:575–80.
Panikkath R, Reinier K, Uy-Evanado A, et al. Prolonged Tpeak-to-tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol. 2011;4:441–7.
Gupta P, Patel C, Patel H, et al. T(p-e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41:567–74.
Watanabe N, Kobayashi Y, Tanno K, et al. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol. 2004;37:191–200.
Kageyama M. Remifentanil prevents increase of QT dispersion during electroconvulsive therapy for psychiatric patients. Dokkyo J Med Sci. 2013;40:T37-45.
Kim SM, Hwang GS, Park JS, et al. The pattern of Tpeak-Tend and QT interval, and J wave during therapeutic hypothermia. J Electrocardiol. 2014;47:84–92.
Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928–36.
Antzelevitch C. Tpeak-tend interval as an index of transmural dispersion of repolarization. Eur J Clin Invest. 2001;31:555–7.
Rosenthal TM, Stahls PF 3rd, Abi Samra FM, et al. T-peak to T-end interval for prediction of ventricular tachyarrhythmia and mortality in a primary prevention population with systolic cardiomyopathy. Heart Rhythm. 2015;12:1789–97.
Zhu W, Huang X, Mei L, et al. The predictive value of Tp-Te interval, Tp-Te/QT ratio, and QRS-T angle of idiopathic ventricular tachycardia in patients with ventricular premature beats. Clin Cardiol. 2023;46:425–30.
Chua KC, Rusinaru C, Reinier K, et al. Tpeak-to-Tend interval corrected for heart rate: a more precise measure of increased sudden death risk? Heart Rhythm. 2016;13:2181–5.
Watanabe E, Arakawa T, Uchiyama T, et al. Prognostic significance of circadian variability of RR and QT intervals and QT dynamicity in patients with chronic heart failure. Heart Rhythm. 2007;4:999–1005.
Kadoi Y, Saito S. Effects of adding remifentanil to propofol anesthesia on systemic hemodynamics, cardiac output, and middle cerebral artery flow velocity during electroconvulsive therapy: a pilot study. J ECT. 2015;31:98–100.
Takekita Y, Suwa T, Sunada N, et al. Remifentanil in electroconvulsive therapy: a systematic review and meta-analysis of randomized controlled trials. Eur Arch Psychiatry Clin Neurosci. 2016;266:703–17.
Suzuki Y, Miyajima M, Ohta K, et al. Is prolongation of corrected QT interval associated with seizures induced by electroconvulsive therapy reduced by atropine sulfate? Pacing Clin Electrophysiol. 2017;40:1246–53.
Yilmaz Coşkun F, Elboğa G, Altunbaş G, Vuruşkan E, Uğur BK, Sucu M. Evaluation of ventricular repolarization features with Tp-e, Tp-e/QTc, JTc and JTd during electroconvulsive therapy. J Electrocardiol. 2018;51:440–2.
Kleinsasser A, Kuenszberg E, Loeckinger A, et al. Sevoflurane, but not propofol, significantly prolongs the Q-T interval. Anesth Analg. 2000;90:25–7.
Acknowledgements
We thank the participants of this study.
Medical writing/Editorial assistance
Writing assistance for the preparation of this article was provided by Editage (www.editage.jp). Financial support for this assistance was provided by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for the authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
No funding or sponsorship was received for this study or publication of this article. The journal’s Rapid Service and Open Access Fees were funded by the authors.
Author information
Authors and Affiliations
Contributions
Shigeki Yamaguchi: made substantial contributions to the conception, design of the work and revision of the manuscript drafts. Toshifumi Takasusuki: made substantial contributions to the data analysis and manuscript drafting. Kazuya Akutsu and Kozue Eda: made substantial contributions to data acquisition. All the authors have approved the submitted version of the manuscript and agreed to be accountable for any part of the work.
Corresponding author
Ethics declarations
Conflict of Interest
Kozue Eda, Kazuya Akutsu, Toshifumi Takasusuki and Shigeki Yamaguchi declare that they have no conflicts of interest.
Ethical Approval
The study was approved by the ethics committee of Dokkyo Medical University (R-32-1) and registered with the University Hospital Medical Information Network (UMIN, registration number: UMIN000051958). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Eda, K., Akutsu, K., Takasusuki, T. et al. Effect of Remifentanil on the Tpeak-Tend Interval During Electroconvulsive Therapy. Adv Ther 41, 262–270 (2024). https://doi.org/10.1007/s12325-023-02713-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02713-9