Abstract
Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV) are rare autoimmune diseases triggering inflammation of small vessels. This real-world analysis was focused on the most common AAV forms, granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), to describe patients’ demographic and clinical characteristics, therapeutic management, disease progression, and the related economic burden.
Methods
A retrospective analysis was conducted on administrative databases of a representative sample of Italian healthcare entities, covering approximately 12 million residents. Between January 2010 and December 2020, adult GPA patients were identified by payment waiver code or hospitalization discharge diagnosis, and MPA patients by payment waiver code with or without hospitalization discharge diagnosis. Clinical outcomes were evaluated through AAV-related hospitalizations, renal failure onset, and mortality. Economic analysis included healthcare resource utilization deriving from drugs, hospitalizations, and outpatient specialist services. The related mean direct costs year/patient were also calculated in patients stratified by presence/absence of glucocorticoid therapy and type of inclusion criterion (hospitalization/payment waiver code).
Results
Overall, 859 AAV patients were divided into GPA (n = 713; 83%) and MPA (n = 146; 17%) cohorts. Outcome indicators highlighted a clinically worse phenotype associated with GPA compared to MPA. Cost analysis during follow-up showed tendentially increased expenditures in glucocorticoid-treated patients versus untreated (overall AAV: €8728 vs. €7911; GPA: €9292 vs. €9143; MPA: €5967 vs. €2390), mainly driven by drugs (AAV: €2404 vs. €874; GPA: €2510 vs. €878; MPA: €1881 vs. €854) and hospitalizations.
Conclusion
Among AAV forms, GPA resulted in a worse clinical picture, higher mortality, and increased costs. This is the first real-world pharmaco-economic analysis on AAV patients stratified by glucocorticoid use on disease management expenditures. In both GPA and MPA patients, glucocorticoid treatment resulted in higher healthcare costs, mostly attributable to medications, and then hospitalizations, confirming the clinical complexity and economic burden for management of patients with autoimmune diseases under chronic immunosuppression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
ANCA-associated vasculitis (AAV) is a rare severe inflammatory disease associated with adverse outcomes damaging vital organs, including kidneys, often resulting in end-stage renal disease (ESRD). |
AAV is mainly managed with extended immunosuppression, usually glucocorticoid treatments, that is associated with significant complications, ultimately leading to a relevant clinical and economic burden. |
What was learned from the study? |
This Italian real-world study analyzed the two most common AAV forms, granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). GPA was more frequent and associated with a worse clinical phenotype, higher mortality, and cost burden. |
In both disease forms, steroid treatment and baseline patients’ characteristics (age, comorbidity profile) led to increased expenditures, mostly driven by drugs and then hospitalization. |
Taken together, our findings confirm the elevated costs for disease management of this patient population that could be related to both AAV forms, to older age and complex comorbidity profiles, and to the need for chronic immunosuppression. |
Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV) are a group of rare multisystem autoimmune diseases targeting small vessels, commonly characterized by granulomatous and neutrophilic tissue inflammation and by the presence of auto-antibodies directed against neutrophil antigens [1]. The term AAV comprises three distinct conditions: granulomatosis with polyangiitis (GPA, previously known as Wegener’s granulomatosis), microscopic polyangiitis (MPA), generally more frequent in older adults (average age at diagnosis 45–65 years for GPA and 50–60 years for MPA) [2, 3], and eosinophilic granulomatosis with polyangiitis (EGPA, formerly known as Churg–Strauss syndrome), the latter with an earlier onset, between the third and the fifth decade of life [4, 5]. All three disease forms can occur at any age, including in childhood, affect all ethnicities, and appear to be slightly more common in males [6,7,8].
The estimated prevalence worldwide is 200–400 cases per million people, with some differences according to the latitude, geographical location, and race [9]. In Europe, the respective yearly incidence rates for GPA, MPA, and EGPA range from 2.1 to 14.4, from 2.4 to 10.1, and from 0.5 to 3.7 per million people, respectively. The 5-year survival rates for GPA, MPA, and EGPA are reported to be 74–91%, 45–76%, and 60–97%, respectively [7].
The Italian National Institute of Health has codified GPA and MPA, the two most common phenotypes of AAV, as rare diseases (defined by a prevalence below 5 cases per 10,000 inhabitants), with a combined incidence in Italy of 10–18 cases/million inhabitants/year and a prevalence of 141 cases/million inhabitants [10].
The clinical manifestations of the AAV are highly variable, ranging from mild symptoms like a skin rash to fulminant multisystem disease [1, 5]. GPA features can include nasal crusting, stuffiness and epistaxis [11], uveitis [12], and lung and kidney involvement [1, 5, 6, 13]. MPA typically presents with more severe renal disease, together with rash and neuropathy [2, 3, 14].
The rarity and unpredictable clinical onset of the AAV makes the diagnosis challenging and often delayed more than 6 months in about one-third of patients [5]. In view of the multisystem involvement in AAV, a timely and reliable diagnostic path should be based on an interdisciplinary approach, a detailed patient clinical history, and examinations [5, 15].
The intent of the currently used therapies for AAV is to achieve durable remission, defined by European Vasculitis Society/European League against Rheumatism (EUVAS/EULAR) group [16] as “complete absence of active clinical disease”, using a recognized scoring tool, namely the Birmingham Vasculitis Activity Score (BVAS) [17]. The use of immunosuppressive agents like cyclophosphamide or rituximab combined with glucocorticoids (GCs) has brought significant benefits in terms of remission induction and prognosis, as AAV-treated patients show a 1-year mortality decreased by 80% compared to the untreated ones [5, 18]. However, iatrogenic side effects can occur with long-term treatments, as in the case of prolonged GC regimens, resulting in a significant clinical burden, organ damage, and worse comorbidity profile [19, 20]. Thus, the therapeutic interventions on AAV remain an open challenge, mainly in view of the high risk of relapsing disease and the wide interindividual variability in clinical phenotypes related to the multifactorial involvement of genetic and environmental factors, and the contribution of both innate and adaptive immune systems [21]. In the management of AAV patients, clinicians have always to deal with the delicate balance between avoiding disease recurrence and keeping a watchful eye on the clinical impact of immunosuppression, mostly based on the GC use [20]. In the front of the ongoing efforts of current pharmacological research to develop patient-tailored therapeutic approaches, other than non-specific immunosuppressive agents [22], the main risk for patients under life-long immunosuppression is that of infections and other concomitant diseases [23]. This condition leads to a growing need for pharmacological treatments, diagnostic tests, specialist visits, and hospitalizations with a significant economic burden for the National Health Systems (NHS) [24]. Few data are currently available in Italy regarding the healthcare resource consumption and related costs deriving from the management of AAV patients [25].
The present analysis in the Italian real-world setting was undertaken to investigate the most common forms of AAV, namely GPA and MPA, to assess the demographic and clinical characteristics of affected patients, to investigate their therapeutic management and disease progression, and to describe the current state-of-art regarding health resources consumption and the economic burden sustained by the Italian NHS.
Methods
Data Source
A retrospective database analysis was conducted by integrating administrative databases of a pool of geographically distributed Italian entities covering approximately 12 million health-assisted individuals. The following databases were used for the analysis: (1) demographic database, to extract patient demographic data, namely gender, age, and date of death; (2) pharmaceuticals database, for information on medicinal products reimbursed by the NHS, such as the Anatomical Therapeutic Chemical (ATC) code, number of packages, number of units per package, unit cost per package, and prescription date; (3) hospitalization database, to collect hospitalization data, such as discharge diagnosis codes classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), Diagnosis Related Group (DRG), and DRG-related charge (provided by the Italian NHS); (4) outpatient specialist services database, to record data on specialist visits and diagnostic tests (date and type of prescription, description activity, and laboratory test or specialist visit charge); and (5) payment waiver database, to gather active payment waiver codes by which patients are discharged from paying services/treatments in case of specific disease diagnoses.
To ensure privacy, an anonymous univocal numeric code was assigned to each participant, in full compliance with the European General Data Protection Regulation (GDPR) (2016/679). This patient code allowed the electronic linkage between the different databases. All the findings resulting from these analyses were produced as aggregated summaries, and never attributable to a single institution, department, doctor, individual, or individual prescribing behaviors. The study was performed in accordance with the principles of the Helsinki Declaration of 1964 and its later amendments, and approved by the local Ethics Committees of the Healthcare Departments involved. Informed consent was waived since, for organizational reasons, it was not possible to obtain it (pronouncement of the Data Privacy Guarantor Authority, General Authorization for personal data treatment for scientific research purposes—no. 9/2014).
Identification of Study Population
Inclusion and exclusion criteria Between January 2010 and December 2020 (inclusion period), adult (≥ 18 years) patients with AAV were identified based on the following criteria: at least one payment waiver code (RG0050/RG0070) or hospitalization (day hospital or regular admission) with primary or secondary discharge diagnosis of GPA (ICD-9-CM code: 446.4), or at least one payment waiver code (RG0020) for MPA, in association or not with hospitalization discharge diagnosis of MPA (ICD-9-CM code 446.0) (Table 1). The date of detection of one of the inclusion criteria was considered as the index date. The characterization period started 1 year before the index date (thus from 1 January 2009 onward). The follow-up period was all the available time after the index date, at least 1 year (maximum until 31 December 2021). Patients with no continuous inclusion during the study period were excluded.
Demographic and clinical characteristics For the whole sample population included in the study, and for patients divided by type of disease, GPA, and MPA, demographic and clinical characteristics were collected: age at the index date, age ranges (18–39 years, 40–59 years, 60–79 years, and over 80 years), gender (expressed as percentage of male sex), and presence of comorbidities evaluated through the Charlson Comorbidity Index (CCI), that assigns a score to each concomitant disease [26]. The patients were also characterized for their previous clinical history, collecting data on the most common drug prescriptions and causes of hospitalization.
Clinical outcomes After inclusion, all patients were evaluated for their therapeutic regimens by analyzing the most frequently prescribed drugs during follow-up. Moreover, outcomes were assessed using the following indicators: (1) AAV-related hospitalizations, with a primary discharge diagnosis for GPA (ICD-9-CM code: 446.4), or MPA (ICD-9-CM code: 446.0); (2) end-stage renal disease (ESRD), including dialysis and renal transplant (ICD-9-CM codes V42.0, V56.0, as primary or secondary diagnosis; 55.6, 39.95, 54.98, as primary or secondary procedures; or specialistic codes 39.95, 54.98); and (3) death.
Analysis of Healthcare Resource Use and Costs for the NHS
During the first year and all follow-up periods, healthcare resource utilization per alive patients was assessed in terms of the number of drug prescriptions (by considering any medication), hospital admissions (ordinary and day hospital), and outpatient specialist services (laboratory tests, specialistic visits, diagnostic procedures).
The related mean annual direct costs sustained by the NHS were then estimated in overall AAV, GPA, and MPA patients divided according to the presence or absence of GC treatment (ATC Code: H02AB) during follow-up. The evaluation of healthcare expenditures was then replicated on the patients further stratified by type of code used at inclusion, payment waiver code, or hospitalization code. Outliers (patients whose costs exceeded more than 3 times the standard deviation over the mean value) were excluded from all the analyses.
Statistical Analysis
A descriptive statistical analysis was used for continuous variables, presented as mean ± standard deviation, and categorical variables, presented as numbers and percentages. In some subgroups composed of less than four patients, data were not issuable (N.I.) for data privacy, since the results might be potentially referable to single individuals, in compliance with the Italian code for protection of personal data (“Codice in materia di protezione dei dati personali”, D. Lgs. 196/2003).
After adjusting for baseline-confounding variables using a generalized linear model (GLM), correlation analysis was applied to identify the potential predictors of increased healthcare costs, reporting 95% confidence intervals (CI) for regression coefficient, β, by adjusting for the variables assessed at baseline (age, gender, comorbidity index, use of GC, the inclusion criterion used for GPA and MPA detection). A p value below 0.05 was considered as statistically significant, and all the analyses were performed using STATA SE, version 17.0 (StataCorp, College Station, TX, USA).
Results
Identification of the Study Population
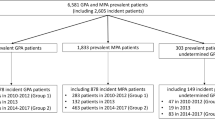
From the sample population of almost 12,000,000 health-assisted individuals followed at the participating healthcare units, 859 (0.01%) AAV patients meeting the inclusion criteria were identified, and then assigned to two mutually exclusive cohorts of 713 (83%) GPA patients or 146 (17%) MPA patients. Each cohort was further stratified according to the presence/absence of GC therapy. Regardless of the type of disease, the majority of patients received GC treatment: specifically, the proportion of GC-treated was 79.4% (566 out of 713) among GPA-affected patients, and 78.8% (115 out of 146) among MPA patients. The flowchart detailing the scheme of patients’ selection and numerosity of each subgroup is depicted in Fig. 1.
Baseline Characteristics of the Study Population
The baseline demographic and clinical features of the overall AAV population, and of the two study cohorts of GPA and MPA patients, are detailed in Table 2. Male gender was slightly underrepresented in the MPA patients, the age was on average around 57–58 years in all groups, and the CCI was relatively mild, ranging from 1.0 in MPA group to 1.3 among GPA patients (p < 0.01).
The detailed lists of the most commonly prescribed drugs and causes of hospital admission during the characterization period are reported, respectively, in Tables S1 and S2 in the supplementary material. In both GPA and MPA groups, antibacterials for systemic use, corticosteroids for systemic use, and drugs for acid-related disorders were found to be the most frequent medications (in a range between 73 and 84% of the patients). The same trend in drug prescription was also found at 1-year follow-up (Table S3). The most common causes of hospitalization were those related to the musculoskeletal system and connective tissue in both cohorts (above 14%), to the respiratory system in GPA patients (12.3%), and to the renal/urinary tract in MPA patients (16.4%).
Therapies and Clinical Outcomes during Follow-Up
At the first year of follow-up, systemic corticosteroids were administered in 79.4% of GPA and 78.8% of MPA patients, and immunosuppressive drugs in 38.1% of GPA and 39.7% of MPA patients (Table S3 of the supplementary material). Immunosuppressive agents were identified by the ATC code L04, which includes selective immunosuppressants, tumor necrosis factor alpha (TNF-α) inhibitors, interleukin inhibitors, calcineurin inhibitors, and other immunosuppressants.
Table 3 describes the clinical outcomes in the overall AAV patients, and in those divided according to disease form, GPA or MPA. The variables used to evaluate outcomes, namely AAV-related hospitalization, onset of ESRD, and mortality were assessed in terms of rates (number and percentage) and days-to-events (expressed as mean ± standard deviation). As reported in Table 3, the GPA cohort was characterized by a higher frequency of AAV hospitalization compared to MPA (28.5% vs. 13.0%, p < 0.001) and a rising trend in mortality rate (17.8% vs. 11.6%, p = 0.069). These outcome variables indicated GPA as a disease form with a worse phenotype, with the exception of ESRD that appeared with higher frequency but a later onset in MPA patients (10.2% vs. 15.8%, p = 0.054).
Total Mean Annual Direct Healthcare Costs during Follow-Up Period
The consumption of healthcare resources is reported in Table 4: compared to MPA, the GPA cohort was associated to higher numbers per alive patient of all drug prescriptions (p < 0.01), hospital admissions, both ordinary (p < 0.01) and day hospital (p < 0.01), and outpatient specialist services (not significant).
When analyzing the overall AAV population, the total annual mean healthcare cost per patient averaged €8564 (€9262 and €5207 for GPA and MPA patients, respectively). The GLM model adjusted for baseline covariates identified as potential predictors of increased healthcare costs the therapy with GCs (+ €1486.9; 95% CI 680.0–2293.7, p < 0.001), older age (+ €28.2 for each unitary year; 95% CI 7.8–48.6, p < 0.01), worse comorbidity profile assessed by CCI (+ €1142.45; 95% CI 546.6–1738.3, p < 0.001), and type of inclusion criterion (hospitalization with respect to payment waiver code) (Table S4 of the Supplementary Material).
A detailed analysis of annual mean healthcare costs was then performed during the follow-up period for overall AAV, GPA, and MPA cohorts divided by the presence/absence of at least one prescription of GCs (Fig. 2). The total healthcare direct costs tended to be increased in GC-treated patients (in agreement with the results obtained from the GLM regression model) in the overall AAV population, as well as in each disease cohort (GC-treated vs. GC-untreated: €8728 vs. €7911 for overall AAV, p = 0.540; €9292 vs. €9143 for GPA, p = 0.935; €5967 vs. €2390 for MPA, p < 0.05). In detail, GC-treated patients showed higher costs for drug expenses (GC-treated vs. GC-untreated: €2404 vs. €874 for overall AAV, p < 0.001; €2510 vs. €878 for GPA, p < 0.001; €1881 vs. €854 for MPA, p < 0.05) and for outpatient specialist services (GC-treated vs. GC-untreated: €1875 vs. €819 for overall AAV, p < 0.05; €1832 vs. €832 for GPA, p = 0.057; €2088 vs. €759 for MPA, p = 0.186). On the other hand, the presence of GC-treatment resulted in lowered costs (not significantly) for ordinary hospitalizations, but only in GPA and overall AAV patients (GC-treated vs. GC-untreated: €4175 vs. €5912 for overall AAV, p = 0.130; €4665 vs. €7078 for GPA, p = 0.078; €1829 vs. €681 for MPA, p = 0.107). Due to the fact that, in the current analysis, the mean annual costs related to MPA (included by payment waiver code) were lower than those estimated in GPA patients (included both by payment waiver code and by disease-specific hospitalization), the next step was to evaluate whether the inclusion criteria might have affected the healthcare costs. As shown in Fig. 3, total costs averaged €2044 (GC-untreated) and €4806 (GC-treated) in AAV-patients included by payment waiver code, and €19,856 (GC-untreated) and €16,949 (GC-treated) in AAV-patients included by hospitalization (with the most impactive item being that related to hospital stay expenditures).
Discussion
AAV represents a rare disease with a multifactorial etiology that can lead to organ failure and ultimately death [27]. This analysis of a real-life Italian clinical setting focused on the most common AAV forms, namely GPA and MPA, reported to have a later clinical onset, respectively 45–65 years and 50–60 years compared to EGPA, which presents more commonly between 30 and 50 years [2,3,4,5]. In line with these trends, our GPA and MPA patients were aged approximately 57–58 years, in general comparable between the two disease forms [28]. In contrast with previous studies [8], our population showed a slightly female predominance, but some divergences in demographic and epidemiological data of AAV are expected among studies conducted on different ethnicities and geographical areas [8, 9]. The sample of patients included here revealed a prominent occurrence of GPA, which accounted for 83% of overall AAV patients, consistent with recent real-world data by Quartuccio et al. referred to the northern Italian region, Friuli Venezia Giulia [25]. However, when comparing our findings with international reports, we noticed some discrepancies that, as mentioned above, are not surprising given the wide variability of AAV worldwide [9]. In the front of the increasing availability of AAV epidemiological data in US and Europe, there are few studies from central and Latin America, Asia, and Oceania, and often with contrasting results. In reports from a Peruvian population [29] and Asian countries [30], MPA was more frequent than GPA, while studies in south-eastern Australia found MPA as less common than GPA, similar to Europe [8, 31].
Evaluating the clinical outcomes in the two disease forms, GPA appeared to be associated with a worse phenotype, as documented by the higher rate and the shorter times to the occurrence of AAV-related hospitalizations and death. There are conflicting data on the severity of the two AAV conditions in the literature, but this can be again feasibly explained by the ample global variations of this rare disease in both epidemiological and clinical presentation. A recent real-word analysis by Bataille et al. on French health insurance databases (covering about 76% of the country population) revealed that MPA was associated with a 1.3-fold increase in standardized mortality ratio and more frequent renal, lung, and cardiac complications and urinary tract infections than GPA [32]. Another large 20-year US study on the medical records of patients with a diagnosis or suspected diagnosis of AAV in Minnesota between 1996 and 2015 described higher rates of mortality in patients with MPA or EGPA, but not patients with GPA, with respect to the general population [33].
In our sample, we found a greater frequency of ESRD in the MPA cohort, and this is an expected result, given that this form is known to be more commonly associated with renal involvement [3, 13]. Surprisingly, in spite of the larger proportion of MPA patients with renal failure, we noticed a later time of onset of ESRD in comparison with the GPA group. There might be several hypotheses behind this observation. One point might lie in the markedly smaller MPA cohort, composed of only 146 subjects, that might have affected its representativeness. Secondly, MPA patients showed more than doubled kidney-related hospitalizations compared to GPA ones. We can postulate that, since MPA patients are more prone to develop renal complications, as reported in the previously mentioned French study by Bataille and colleagues [32], they might have undergone a stricter surveillance by the nephrologists in an effort to slow the progression towards renal insufficiency. Nevertheless, any apparently unusual finding in AAV studies should be viewed with caution, in consideration of the unpredictable and highly variable clinical phenotypes of the three known pathological forms of AAV, namely GPA, MPA, and EGPA.
The issue of AAV treatment still remains the most puzzling question. While part of the patients achieve at least a temporary remission with the current standard of care, the extended recourse to GCs deserves much more attention from physicians in view of the clinical complexity of patients who have to receive life-long or prolonged immunosuppression [34]. Also, GCs, antibiotics, and drugs for gastrointestinal symptoms were the most prescribed medications in both GPA and MPA groups. As for other pathologies that require long-term therapy with GCs, the extensive use of systemic antibacterial drugs is plausibly due to the increased susceptibility to infections in immunosuppressed patients, as largely corroborated by the literature [35,36,37]. Prescriptions for the treatment of gastric acidity were observed in over 74% in GPA and 84% of MPA patients, and such elevated percentages might be feasibly explained by the protracted exposure to GCs [38], as well as by systemic vasculitis itself, often characterized by gastrointestinal symptoms [39, 40] and gut microbiota alterations [41].
To the best of our knowledge, this is the first study to analyze the economic burden of AAV patients stratified by GC therapy. We found that GC-treated patients were characterized by a trend towards increased overall higher healthcare costs, mainly driven by drug expenses and outpatient specialist services, but partly counterbalanced by lower costs for ordinary hospitalizations, except in the MPA group. Moreover, the GLM logistic model showed that healthcare costs were also predicted by the patients’ older age and their comorbidity profile. Although pharmaco-economic studies on the impact of GC therapy in AAV patients are lacking, we can postulate that GC-treated patients might have received clinical benefit in terms of a lower rate of remissions or complications requiring hospitalization, in front of a greater recourse to co-medications and specialist services, likely attributable to steroid-induced immune suppression [34,35,36,37], but also to baseline patient characteristics such as older age and complex clinical profiles. Moreover, regardless of the presence or not of treatment with GCs and the type of cost item, GPA affected the healthcare expenses to a larger extent compared with MPA, confirming that the worse clinical phenotype of GPA led to a markedly heavier impact on sustainability by the Italian NHS. This finding is consistent with the recently published data by Quartuccio et al., who estimated an annual cost per patient in the province of Udine of €5199 for GPA and €4771 for MPA [25]. A large study by Schmidt et al. on the economic burden of AAV was performed on almost the entire French population using the main public databases of the National Health Insurance. The analysis estimated a mean annual cost per patient of €26,560 for overall AAV incident patients, €24,570 for GPA only, and €30,464 MPA only, mostly burdened by expenses for hospitalizations (60%) and drugs (9%). The partial discrepancy with our data might lie in the different approach for the calculation of costs used in the French study, which included direct or indirect healthcare resource consumption, associated or not to disease management, in terms of medications, consultations, laboratory assays, medical devices/procedures, invalidity, transportation to healthcare facilities, and internal and external consultations in public hospitals [42]. When we further stratified the population by the type of inclusion criterion, the distribution of healthcare expenses across the various cost items highlighted some differences compared to the global cost analysis described above. Patients included by the payment waiver code were characterized by lower healthcare direct annual costs and the presence of GC treatment resulted in increased overall healthcare expenditure, mostly related to medications and ordinary hospitalizations. On the other hand, when examining the costs in patients included by hospitalization code, the mean annual costs were almost fourfold higher with respect to the payment waiver code-included patients. In particular, the total costs were tendentially lower in GC-treated patients, with ordinary hospitalizations as the most impactive item, but this might be explained by the use of hospitalization codes to carry out this sub-analysis.
The limitations of the present analysis mainly lie in its retrospective observational design and the recourse to data taken from administrative databases that could lack some information. Thus, there might have been incomplete documentation on comorbidities and their severity, since pre-existing concomitant diseases were extrapolated in the year prior the index date using drug treatments and hospitalizations as proxy for diagnosis, thus untreated or non-hospitalized comorbidities were not captured. Moreover, differently from RCTs, in observational retrospective real-world analyses, patients meeting the inclusion criteria are consecutively selected from the administrative databases, so variables like age and sex distributions are beyond the control of the investigators. For this reason, patient characteristics could not be matched a priori between the GPA and MPA cohorts. Indeed, while mean age was comparable, there was a slight non-statistically significant imbalance in gender distribution, as males were more represented among GPA patients compared to those affected by MPA. At enrolment, a significant difference between the groups emerged for CCI, suggesting that GPA patients are burdened by a more complex comorbidity profile, consistent with the overall message of this analysis. Moreover, the data should be interpreted by considering that GPA patients were included by hospitalization diagnosis or payment waiver code, while MPA patients were included by payment waiver code, independently of the presence of a hospitalization diagnosis. This criterion was adopted since, for MPA, the hospitalization code ICD-9-CM 446.0 is not disease-specific (it is a comprehensive code for “Polyarteritis nodosa and allied conditions”); thus, to overcome the patient selection bias, the specific payment waiver code was considered. This approach could have led to an underestimation of MPA patients. Lastly, the GLM model was developed to evaluate predictors of costs among baseline confounding variables; however, additional covariates not assessed in the present analysis could have been impacted the economic outcomes.
Conclusions
The present real-world analysis confirmed that AAV is associated with unfavorable clinical and economic outcomes. In our sample, consisting of patients affected by the two most common forms of the disease, GPA and MPA, the first was more frequent and associated with a worse phenotype, a trend of higher mortality, and a heavier burden in terms of healthcare resource consumption and related direct expenses sustained by the Italian NHS. Here, we conducted for the first time a pharmaco-economic investigation on AAV patients stratified by type of disease, GPA versus MPA, by the presence/absence of steroid treatment, and by the type of code used at inclusion, namely payment waiver code or hospitalization code. The emerging message of these analyses and subanalyses was that drug expenses and hospitalization were commonly the most impactive cost item on the overall burden, confirming the clinical complexity of patients under chronic or prolonged immunosuppression with GCs. Further efforts are needed to optimize AAV management, with the goal of ameliorating the clinical outcomes of AAV patients and alleviating the resulting economic burden for the NHS.
Data Availability
All the data that support the results of this study are available upon reasonable request from CliCon S.r.l. which is the body entitled of data treatment and analysis by the participating healthcare units.
References
Almaani S, Fussner LA, Brodsky S, Meara AS, Jayne D. ANCA-associated vasculitis: an update. J Clin Med. 2021;10(7):1446.
Microscopic polyangiitis. https://www.vasculitisfoundation.org/education/forms/microscopic-polyangiitis/. Accessed 10 Jan 2023.
Chung SA, Seo P. Microscopic polyangiitis. Rheum Dis Clin North Am. 2010;36(3):545–58.
Wojciechowska J, KręCicki T. Clinical characteristics of patients with granulomatosis with polyangiitis and microscopic polyangiitis in ENT practice: a comparative analysis. Acta Otorhinolaryngol Ital. 2018;38(6):517–27.
Yates M, Watts R. ANCA-associated vasculitis. Clin Med (Lond). 2017;17(1):60–4.
Hunter RW, Welsh N, Farrah TE, Gallacher PJ, Dhaun N. ANCA associated vasculitis. BMJ. 2020;14(369): m1070.
Robson J, Doll H, Suppiah R, et al. Damage in the ANCA-associated vasculitides: long-term data from the European vasculitis study group (EUVAS) therapeutic trials. Ann Rheum Dis. 2015;74(1):177–84.
Watts RA, Mahr A, Mohammad AJ, Gatenby P, Basu N, Flores-Suárez LF. Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Nephrol Dial Transplant. 2015;30(Suppl 1):i14–22.
Gatenby PA, Lucas RM, Engelsen O, Ponsonby AL, Clements M. Antineutrophil cytoplasmic antibody-associated vasculitides: could geographic patterns be explained by ambient ultraviolet radiation? Arthritis Rheum. 2009;61(10):1417–24.
https://www.orpha.net/consor/cgi-bin/OC_Exp.php?Lng=IT&Expert=700. Accessed 10 Jan 2023.
Düzgün N, Umudum H. Chronic large nasal bloody crusting and recurrent episcleritis: Limited granulomatosis with polyangiitis. Eur J Rheumatol. 2019;6(1):65–6.
Sfiniadaki E, Tsiara I, Theodossiadis P, Chatziralli I. Ocular manifestations of granulomatosis with polyangiitis: a review of the literature. Ophthalmol Ther. 2019;8(2):227–34.
Lin CY, Chen HA, Chang TW, et al. Time-dependent risk of mortality and end-stage kidney disease among patients with granulomatosis with polyangiitis. Front Med (Lausanne). 2022;10(9): 817204.
Hashmi MF, Jain V, Tiwari V. Microscopic Polyangiitis. [Updated 2022 Nov 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK531484/ Accessed 10 Jan 2023.
Koh JH, Kemna MJ, Cohen Tervaert JW, Kim WU. Editorial: can an increase in antineutrophil cytoplasmic autoantibody titer predict relapses in antineutrophil cytoplasmic antibody-associated vasculitis? Arthritis Rheumatol. 2016;68(7):1571–3.
Hellmich B, Flossmann O, Gross WL, et al. EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti-neutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis. 2007;66(5):605–17.
Mukhtyar C, Lee R, Brown D, et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis. 2009;68(12):1827–32.
Dirikgil E, van Leeuwen JR, Bredewold OW, et al. ExploriNg DUrable remission with rituximab in ANCA-associatEd vasculitis (ENDURRANCE trial): protocol for a randomised controlled trial. BMJ Open. 2022;12(9): e061339.
Speer C, Altenmüller-Walther C, Splitthoff J, et al. Glucocorticoid maintenance therapy and severe infectious complications in ANCA-associated vasculitis: a retrospective analysis. Rheumatol Int. 2021;41(2):431–8.
Floyd L, Morris A, Joshi M, Dhaygude A. Glucocorticoid therapy in ANCA vasculitis: using the glucocorticoid toxicity index as an outcome measure. Kidney360. 2021;2(6):1002–10.
Trivioli G, Marquez A, Martorana D, et al. Genetics of ANCA-associated vasculitis: role in pathogenesis, classification and management. Nat Rev Rheumatol. 2022;18(10):559–74.
Puéchal X, Guillevin L. How best to manage relapse and remission in ANCA-associated vasculitis [published online ahead of print, 2022 Sep 14]. Expert Rev Clin Immunol. 2022. https://doi.org/10.1080/1744666X.2022.2122954.
Zhang N, Sun J, Ji C, Bao X, Yuan C. Predicting bacterial infection risk in patients with ANCA-associated vasculitis in southwest China: development of a new nomogram. Clin Rheumatol. 2022. https://doi.org/10.1007/s10067-022-06314-9.
Watts RA, Robson J, Pearce F. The global burden of anti-neutrophil cytoplasmic antibody vasculitis: high but unquantified. Rheumatology (Oxford). 2017;56(9):1439–40.
Quartuccio L, Treppo E, Valent F, De Vita S. Healthcare and economic burden of ANCA-associated vasculitis in Italy: an integrated analysis from clinical and administrative databases. Intern Emerg Med. 2021;16(3):581–9.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
McDermott G, Fu X, Cook C, et al. The effect of achieving serological remission on subsequent risk of relapse, end-stage renal disease and mortality in ANCA-associated vasculitis: a target trial emulation study. Ann Rheum Dis. 2022;81(10):1438–44.
Liao QQ, Ren YF, Zhu KW, et al. Long-term prognostic factors in patients with antineutrophil cytoplasmic antibody-associated vasculitis: a 15-year multicenter retrospective study. Front Immunol. 2022;13: 913667.
Sánchez Torres A, Acevedo Vásquez E, Sánchez Schwartz C, et al. Epidemiología de las vasculitis sistémicas primarias en una población latinoamericana. Reumatologia. 2005;21:145–50.
Naidu GSRSNK, Misra DP, Rathi M, Sharma A. Is granulomatosis with polyangiitis in Asia different from the West? Int J Rheum Dis. 2019;22(Suppl 1):90–4.
Ormerod AS, Cook MC. Epidemiology of primary systemic vasculitis in the Australian Capital Territory and south-eastern New South Wales. Intern Med J. 2008;38(11):816–23.
Bataille PM, Durel CA, Chauveau D, Panes A, Thervet ÉS, Terrier B. Epidemiology of granulomatosis with polyangiitis and microscopic polyangiitis in adults in France. Autoimmun. 2022;133: 102910.
Berti A, Cornec D, Crowson CS, Specks U, Matteson EL. The epidemiology of antineutrophil cytoplasmic autoantibody-associated vasculitis in olmsted county, minnesota: a twenty-year US population-based study. Arthritis Rheumatol. 2017;69(12):2338–50.
Yasir M, Goyal A, Sonthalia S. Corticosteroid Adverse Effects. [Updated 2022 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK531462/. Accessed 10 Jan 2023.
Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954–63.
Fardet L, Petersen I, Nazareth I. Common infections in patients prescribed systemic glucocorticoids in primary care: a population-based cohort study. PLoS Med. 2016;13(5): e1002024.
Riley TR, George MD. Risk for infections with glucocorticoids and DMARDs in patients with rheumatoid arthritis. RMD Open. 2021;7(1): e001235.
Hernández-Díaz S, Rodríguez LA. Steroids and risk of upper gastrointestinal complications. Am J Epidemiol. 2001;153(11):1089–93.
Ledó N, Pethő ÁG. Gastrointestinal symptoms as first remarkable signs of ANCA-associated granulomatosis with polyangiitis: a case report and reviews. BMC Gastroenterol. 2021;21(1):158.
Gendreau S, Porcher R, Thoreau B, French Vasculitis Study Group, et al. Characteristics and risk factors for poor outcome in patients with systemic vasculitis involving the gastrointestinal tract. Semin Arthritis Rheum. 2021;51(2):436–41.
Hatemi I, Hatemi G, Çelik AF. Systemic vasculitis and the gut. Curr Opin Rheumatol. 2017;29(1):33–8.
Schmidt A, Genreau M, Panes A, Spearpoint P, Champs FO, Bataille P. Economic burden of Granulomatosis with Polyangiitis (GPA) and Microscopic Polyangiitis (MPA) for ANCA-associated vasculitis (AAV) patients in France. Poster PRO54, ISPOR Europe 2020, 16–19 November 2020, Milan, Italy. https://static.hevaweb.com/web/PDF/fa9f094004af5-vifor-gpa-mpa-poster-ispor2020-v1r2i1.pdf. Acessed 30 June 2023
Authorship
All the listed coauthors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take full responsibility for the integrity of the work, and have approved this version to be published.
Prior Presentation
Poster presentation at the Congress ISPOR Europe 2022, Vienna, Austria (Value in Health, Volume 25, Issue 12S, December 2022).
Funding
Vifor Pharma Group purchased the study report that is the basis for this manuscript and will support the journal’s rapid service and open access fees. This manuscript was developed with Vifor and CliCon S.r.l. Società Benefit. The agreement signed by CliCon and Vifor does not create any entityship, joint venture or any similar relationship between parties. CliCon S.r.l. is an independent company. Neither CliCon S.r.l. nor any of their representatives are employees of Vifor for any purpose.
Author information
Authors and Affiliations
Contributions
Conceptualization and study design: Luca Degli Esposti, Melania Dovizio, Valentina Perrone; Data curation: Margherita Andretta, Marcello Bacca, Antonietta Barbieri, Fausto Bartolini, Arturo Cavaliere, Alessandro Chinellato, Andrea Ciaccia, Mariarosaria Cillo, Rita Citraro, Alberto Costantini, Stefania Dell’Orco, Fulvio Ferrante, Simona Gentile, Stefano Grego, Daniela Mancini, Rossella Moscogiuri, Elena Mosele, Romina Pagliaro, Cataldo Procacci, Davide Re, Fiorenzo Santoleri, Loredana Ubertazzo, Adriano Vercellone; Statistical analysis: Chiara Veronesi; Writing – original draft, review & editing: Melania Dovizio, Valentina Perrone; Supervision: Luca Degli Esposti, Antonio Ramirez de Arellano, Giuseppe Gigliotti, Luca Quartuccio; Validation: Luca Degli Esposti, Giuseppe Gigliotti, Luca Quartuccio. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Antonio Ramirez de Arellano is an employees of Vifor Pharma Group. Luca delgi Esposti, Melania Dovizio, Valentina Perrone, Chiara Veronesi, Margherita Andretta, Marcello Bacca, Antonietta Barbieri, Fausto Bartonili, Arturo Cavaliere, Alessandro Chinellato, Andrea Ciaccia, Mariarosaria Cillo, Rita Citraro, Alberto Costantini, Stephania Dell’Orco, Fulvio Ferrante, Sinoma Gentile, Daniela Mancini, Rossella Moscogiuri, Elena Mosele, Romania Pagliaro, Cataldo Procacci, Davide Re, Fiorenzo Santoleri, Lorendana Ubertazzo, Adriano Vercellone, Giuseppe Gigliotti, and Luca Quartuccio have no competing interest to disclose.
Ethical Approval
The study was performed in accordance with the principles of Helsinki Declaration of 1964 and its later amendments and approved by the local Ethics Committees of the Healthcare Departments involved: Comitato Etico Lazio 1 (Prot N 1080/CE Lazio 1, 23/09/2020); Comitato Etico Interaziendale AOAL (Prot N 0008668, 20/04/2021); Comitato Etico per le Sperimentazioni Cliniche della Provincia di Vicenza (Prot N 1627, 20/10/2020); Comitato Etico per le Sperimentazioni Cliniche della Provincia di Vicenza (Prot N 0036999, 28/04/2021); Comitato Etico per la Sperimentazione Clinica della provincia di Venezia (Prot N 05.08, 08/10/2020); Comitato Etico Regionale CER Umbria (Prot N 19414/20/ON, 16/09/2020); Comitato Etico per le province di L'Aquila e Teramo (Prot N 11, 24/03/2021); Comitato Indipendente di Etica Medica ASL Brindisi(Prot N 48144, 28/05/2021); Comitato Etico Interaziendale Campania SUD (Prot N 0165790, 04/11/2020); Comitato Etico Lazio 2 (Prot N 0216084/2020, 16/12/2020); Comitato Etico Lazio 1 (Prot N 1166/CE Lazio 1, 12/10/2020); Comitato Etico Lazio 1 (Prot N 1079/CE Lazio 1, 23/09/2020); Comitato Etico delle Province di Chieti e Pescara (Prot N 07,18/03/2021); Comitato Etico Interaziendale Campania SUD (Prot N 0133202, 07/09/2020); Comitato Etico Azienda Sanitaria Regionale Molise (Prot N 101125/2020, 20/10/2020); Comitato Etico Regionale CER Liguria (Prot N 52/2021, 14/06/2021); Comitato Etico Lazio 2 (Prot N 0179046/2020, 28/10/2020); Comitato Etico Interprovinciale Area 1 (ASL Foggia, ASL BAT, Prot N 63/seqCE/20, 03/12/2020); Comitato Etico sezione area centro—Regione Calabria (Prot N 212, 23/07/2020); Comitato Etico Interprovinciale Area 1 (ASL Foggia, ASL BAT, Prot N 63/seqCE/20, 03/12/2020); Comitato Indipendente di Etica Medica ASL Brindisi (Prot N 48148, 28/05/2021).
Informed Consent Statement
According to the pronouncement of the Data Privacy Guarantor Authority (General Authorization for personal data treatment for scientific research purposes – n.9/2014, December 11th – published on the Official Gazette n. 301 on December 30th, 2014) data treatment is authorized without patient Informed Consent, when the collection is impossible due to organizational reasons.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Degli Esposti, L., Dovizio, M., Perrone, V. et al. Profile, Healthcare Resource Consumption and Related Costs in ANCA-Associated Vasculitis Patients: A Real-World Analysis in Italy. Adv Ther 40, 5338–5353 (2023). https://doi.org/10.1007/s12325-023-02681-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02681-0