Abstract
Introduction
Cardiovascular (CV) events are the leading cause of death in prostate cancer. Men with prostate cancer are likely to have CV risk factors and use CV-related concomitant medications. In the phase 3 HERO study, a 54% lower incidence of major adverse cardiac events was reported in men treated with the oral gonadotropin-releasing hormone (GnRH) receptor antagonist, relugolix, vs leuprolide. Herein, we characterize the impact of concomitant CV therapies on efficacy and safety in the HERO study.
Methods
In HERO, 930 men with advanced prostate cancer (APC) were randomized 2:1 and treated with relugolix (120 mg orally once daily; after single 360 mg loading dose) or leuprolide (injections every 3 months) for 48 weeks. Subgroups analyzed included men who received antihypertensives, antithrombotics, or lipid-modifying therapies (LMAs), as well as the most common drug classes (> 10%) and single most common agent within each class. Assessments included sustained testosterone suppression to castrate levels (< 50 ng/dL) through 48 weeks and safety.
Results
Antihypertensives, antithrombotics, and LMAs were utilized by 52.7%, 39.1%, and 39.6% of men in HERO, respectively. In the main subgroups, point estimates for sustained castration rates were generally consistent with overall estimates of relugolix and leuprolide observed in the overall population. Sustained castration rates were also mostly consistent for men taking the most common drug classes and individual agents in each class (losartan [n = 103]: relugolix, 95.4% vs leuprolide, 80.6%; amlodipine [n = 229]: 97.2% vs 85.5%; metoprolol [n = 88]: 95.7% vs 86.9%; acetylsalicylic acid [n = 259]: 97.0% vs 92.1%; clopidogrel [n = 43]: 96.4% vs 86.7%; simvastatin [n = 78]: 98.0% vs 87.3%). Incidence and types of adverse events (AEs) among men who received these medications were mostly consistent with overall population results, with some increases in grade ≥ 3 and fatal AEs.
Conclusion
Relugolix suppressed testosterone and was generally well tolerated when given with concomitant CV agents.
Trial Registration
Clinical Trial ID NCT03085095.
Prior Presentation
Data presented at 15th Annual Genitourinary Cancers Symposium; February 17–19, 2022, San Francisco, CA, USA [Abstract 101, Poster board E11]. The published abstract from this presentation can be found at https://ascopubs.org/doi/10.1200/JCO.2022.40.6_suppl.101.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Cardiovascular (CV) events are the leading cause of death in prostate cancer and men with prostate cancer are likely to have CV risk factors and use CV-related concomitant medications. |
We characterized the impact of concomitant CV therapies on efficacy and safety in the HERO study, in which 52.7%, 39.1%, and 39.6% of men utilized antihypertensives, antithrombotics, and lipid-modifying agents, respectively. |
What was learned from this study? |
In the main subgroups analyzed in our analysis, point estimates for sustained castration rates were generally consistent with overall estimates of relugolix and leuprolide observed in the overall population. |
Incidence and types of adverse events (AEs) among men who received these medications were mostly consistent with overall population results, with some increases in grade ≥ 3 and fatal AEs. |
Overall, relugolix suppressed testosterone and was generally well tolerated when given with concomitant CV agents. |
Introduction
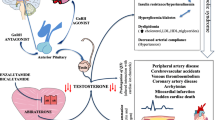
Cardiovascular (CV) events are the leading cause of death in patients with prostate cancer, and at least two-thirds of men with prostate cancer are reported as having known CV risk factors, such as obesity, diabetes, hypertension, and hyperlipidemia [1,2,3]. As such, many men with prostate cancer use concomitant CV-related medications. The concern with CV events in men with prostate cancer is noteworthy as a number of reports suggest that the luteinizing hormone-releasing hormone (LHRH) agonist leuprolide is associated with an increased risk of cardiovascular events in men with prostate cancer [4, 5] and its prescribing information contains warnings for increased risk of myocardial infarction, sudden cardiac death, and stroke [6]. Several prior studies have shown the risk of major cardiovascular and cerebrovascular events using gonadotropin-releasing hormone (GnRH) antagonists is significantly lower compared with LHRH agonists [7, 8], although this is not considered definitive because other studies have not shown a lower risk [4, 9, 10].
In December 2020, the US Food and Drug Administration approved the first oral GnRH antagonist, relugolix, for the treatment of adult patients with advanced prostate cancer, followed by similar approvals in the European Union and Japan. The approvals were based, in part, on results from the phase 3 HERO study, in which 934 men requiring at least 1 year of androgen deprivation therapy (ADT) were randomized (2:1) to receive relugolix 360 mg oral loading dose on the first day, followed by daily oral doses of 120 mg, or leuprolide acetate 22.5 mg injection subcutaneously every 3 months for 48 weeks [3]. In this pivotal trial, relugolix demonstrated suppression of testosterone to castrate levels in 96.7% of patients from day 29 through 48 weeks, which was superior to leuprolide [3]. The most common adverse events (AEs) observed in relugolix-treated patients were hot flash, fatigue, constipation, diarrhea, and arthralgia. In addition, a 54% lower risk of major adverse cardiovascular events (MACE), defined as non-fatal myocardial infarction, non-fatal stroke, and death from any cause, relative to leuprolide [3].
Men with CV risk factors are often on multiple medications to manage their CV disease. Although there are no specific drug–drug interactions between relugolix and CV medications, it is important to confirm that the concomitant use of CV medications does not impact the efficacy/safety of relugolix. Herein, we characterize the impact of concomitant CV therapies on efficacy and safety in the HERO study.
Methods
The HERO study was designed to evaluate relugolix in men with advanced prostate cancer (APC), and details of design have been previously published [3]. Briefly, there were 934 men randomized in a 2:1 ratio (930 treated) to be given 120 mg orally once-daily relugolix after a single 360 mg loading dose or leuprolide injections every 12 weeks for 48 weeks.
Eligibility requirements required patients to be at least 18 years of age and to have had histologically or cytologically confirmed adenocarcinoma of the prostate that required at least 1 year of continuous androgen deprivation therapy. To be eligible for the HERO study, patients were also required to have one of three clinical disease presentations: evidence of biochemical (prostate specific antigen) or clinical relapse following local primary intervention with curative intent, newly diagnosed hormone-sensitive metastatic disease, or advanced localized disease unlikely to be cured by local primary intervention with curative intent. Exclusion criteria included MACE within 6 months before trial initiation. The study was conducted in accordance with the provisions of the Declaration of Helsinki. All patients provided informed consent and the trial was approved by the institutional review board or independent ethics committee at each center.
Subgroups included in this post hoc analysis included men who received antihypertensives, antithrombotics, or lipid-modifying therapies (LMAs), as well as the most common drug classes (> 10%) within these categories [angiotensin receptor blockers, calcium channel blockers, beta blockers, cyclooxygenase (COX) inhibitors, (adenosine diphosphate) ADP receptor inhibitors, and statins], and single most common agent within each class (losartan, amlodipine, metoprolol, acetylsalicylic acid, clopidogrel, and simvastatin).
Assessments included sustained testosterone suppression to castrate levels (< 50 ng/dL) through 48 weeks (primary endpoint in HERO) and safety parameters (AEs). Rates for sustained testosterone suppression to castrate levels were estimated for each treatment group using the Kaplan–Meier method. In this post hoc analysis, no formal statistical hypothesis and/or comparison was conducted. All analyses were descriptive and conducted in a modified intent to treat (mITT) population, which included all randomized patients who took at least one dose of study treatment.
The trial was approved by the institutional review board or independent ethics committee at each center and was conducted in accordance with the requirements of the regulatory authorities of each country and with the provisions of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonisation. All patients provided written informed consent.
Results
Patients
Of the 930 men treated in HERO, antihypertensives, antithrombotics, and LMAs were utilized in both treatment arms by 52.7%, 39.1%, and 39.6%, respectively. The number of men taking at least one CV concomitant medication was 73.3% and 81.8% in the relugolix and leuprolide groups, respectively.
Efficacy
Figure 1 shows the results for sustained castration rates from day 29 through week 48 for the subgroups of patients who received concomitant antihypertensives, antithrombotics, and LMAs. Point estimates for sustained castration rates in each subgroup analyzed were consistent with the estimates in the overall population for relugolix and leuprolide observed (Fig. 1). Sustained castration rates for the subgroups of men who received antihypertensives (relugolix 96.2% vs leuprolide 87.9%), antithrombotics (97.3% vs 91.1%), and LMAs (97.9% vs 89.4%) were generally similar to the overall HERO trial mITT population (96.7% vs 88.8%). In addition, sustained castration rates were consistent for men taking the most common drug classes (Fig. 1) and individual agents in each class (i.e., losartan [n = 103]: relugolix, 95.4% vs leuprolide, 80.6%; amlodipine [n = 229]: 97.2% vs 85.5%; metoprolol [n = 88]: 95.7% vs 86.9%; acetylsalicylic acid [n = 259]: 97.0% vs 92.1%; clopidogrel [n = 43]: 96.4% vs 86.7%; simvastatin [n = 78]: 98.0% vs 87.3%).
Safety
Types of AEs were generally similar among men who received antihypertensives, antithrombotics, and LMAs during the trial relative to those in the overall population, with some exceptions in incidence (Table 1). Men experiencing at least one AE ranged from 93% to 96% and men experiencing a grade ≥ 3 AE ranged from 21% to 29% in the top-level subgroups. In the relugolix group, the proportion of men who had an AE leading to treatment withdrawal or interruption was similar or slightly higher in the subgroups relative to the overall population. In all top-level subgroups analyzed, hot flash remained the most common AE for both treatment groups, ranging from 49% to 59% (overall population: relugolix 54%; leuprolide 52%). Similar to the overall population, fatigue, constipation, diarrhea, arthralgia, and hypertension were the next most common AEs (> 10% in at least one subgroup).
There were several notable differences in the safety results among the subgroups. For example, fatal AEs were numerically higher for men receiving antithrombotics (relugolix 2.6%; leuprolide 5.8%) and lipid-modifying agents (relugolix 1.6%; leuprolide 4.8%) than the overall population (relugolix 1.1%; leuprolide 2.9%). The frequencies of fatal AEs in these subgroups were numerically higher in the leuprolide group than the relugolix group. In addition, grade ≥ 3 AEs were increased in most of the subgroups relative to the overall population (relugolix 18.0%; leuprolide 20.5%), most notably in the antithrombotics subgroup (relugolix 28.6%; leuprolide 29.2%).
Discussion
Relugolix demonstrated suppression of testosterone to castrate levels in 96.7% of men, which was superior to leuprolide (88.8%), and a 54% risk reduction in MACE relative to leuprolide in the phase 3 HERO study [3]. Over 90% of men in this trial had at least one cardiovascular risk factor (i.e., lifestyle risk factors, comorbidities, and history of MACE) and most men were on at least one CV concomitant medication. Given that men with APC frequently are on multiple medications to manage their CV disease, it is important to investigate the impact of concomitant CV medications on efficacy/safety of relugolix.
In this subgroup analysis of men who received concomitant CV medications during the HERO study, we observed efficacy results and types of AEs that were generally similar to the results of the overall HERO population, with several notable exceptions in incidence of safety results in some subgroups. Point estimates for sustained castration rates and the types of AEs were consistent with overall population results. Numerically higher incidences were observed for men in both treatment groups for grade ≥ 3 AEs for antithrombotic subgroup and fatal AEs in men who received antithrombotic or lipid-modifying agents, potentially reflecting higher-risk patient subgroups. Of note, the frequency of fatal AEs in these subgroups were numerically higher in the leuprolide group compared to the relugolix group. There were fewer CV-related fatal events in the relugolix group (two patients with myocardial infarction) than in the leuprolide group (six patients: one with congestive heart failure, four with cardiorespiratory arrest or cardiopulmonary failure, and one with cerebral hemorrhage).
The results from this analysis are highly relevant in men with APC, which disproportionately affects older men who are at a higher risk of CV events [1, 2, 11]. Men with APC and multiple CV-related comorbidities will generally need concomitant CV-related medications. In this analysis, we show these medications do not appear to impact relugolix efficacy and are generally well tolerated. The main limitations of this analysis were that it is post hoc and that it did not explore implications for men in our study population who were on multiple concomitant medications. In addition, the lack of formal statistical comparisons is a significant limitation in this manuscript and so the results are only descriptive in nature and should be considered hypothesis generating.
Another limitation of this analysis is that only medical castration options were included in the HERO study. Guidelines recommend ADT with either LHRH agonists or GnRH antagonists or surgical castration (i.e., orchiectomy) in individuals with hormone-sensitive prostate cancer to achieve castrate levels of testosterone (< 50 ng/dL) [12, 13]. Although these treatments have never been directly compared in large randomized controlled trials, they are considered equivalent in cancer control [12, 13]. Medical castration has been shown to be associated with higher risks of several clinically relevant adverse effects compared with orchiectomy; however, ADT is generally preferred by patients over surgical castration [14,15,16]. Orchiectomy could be considered by individuals with CV risk.
Conclusions
In the HERO trial, relugolix suppressed testosterone with efficacy consistent with those of the overall population. Relugolix and leuprolide were generally well tolerated when given with concomitant CV agents, with some notable increases in grade ≥ 3 and fatal AEs.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–97. https://doi.org/10.1093/eurheartj/ehz766.
Leong DP, Fradet V, Shayegan B, et al. Cardiovascular risk in men with prostate cancer: insights from the RADICAL PC study. J Urol. 2020;203(6):1109–16. https://doi.org/10.1097/JU.0000000000000714.
Shore ND, Saad F, Cookson MS, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(23):2187–96. https://doi.org/10.1056/NEJMoa2004325.
Tisseverasinghe S, Tolba M, Saad F, Gravis G, Bahoric B, Niazi T. Should prostate cancer patients with history of cardiovascular events be preferentially treated with luteinizing hormone-releasing hormone antagonists? J Clin Oncol. 2022. https://doi.org/10.1200/JCO.22.00883.
Tsai HK, D’Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99(20):1516–24. https://doi.org/10.1093/jnci/djm168.
Conteduca V, Di Lorenzo G, Tartarone A, Aieta M. The cardiovascular risk of gonadotropin releasing hormone agonists in men with prostate cancer: an unresolved controversy. Crit Rev Oncol Hematol. 2013;86(1):42–51.
Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65(3):565–73. https://doi.org/10.1016/j.eururo.2013.10.032.
Margel D, Peer A, Ber Y, et al. Cardiovascular morbidity in a randomized trial comparing GnRH-agonist and GnRH-antagonist among patients with advanced prostate-cancer and pre-existing cardiovascular disease. J Urol. 2019;202(6):1199–1208. https://doi.org/10.1097/JU.0000000000000384.
Lopes RD, Higano CS, Slovin SF, et al. Cardiovascular safety of degarelix versus leuprolide in patients with prostate cancer: the primary results of the PRONOUNCE randomized trial. Circulation. 2021;144(16):1295–307. https://doi.org/10.1161/CIRCULATIONAHA.121.056810.
Scailteux LM, Vincendeau S, Balusson F, et al. Androgen deprivation therapy and cardiovascular risk: no meaningful difference between GnRH antagonist and agonists-a nationwide population-based cohort study based on 2010–2013 French Health Insurance data. Eur J Cancer. 2017;77:99–108.
Higano CS. Cardiovascular disease and androgen axis-targeted drugs for prostate cancer. N Engl J Med. 2020;382(23):2257–9.
Lowrance W, Dreicer R, Jarrard DF, et al. Updates to advanced prostate cancer: AUA/SUO guideline (2023). J Urol. 2023;209(6):1082–90.
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Prostate cancer. 2022. Version 2.2023. July 17, 2023. Available at NCCN.org. Accessed 19 July 2023.
Cassileth BR, Soloway MS, Vogelzang NJ, Schellhammer PS, Seidmon EJ, Hait HI, Kennealey GT. Patients’ choice of treatment in stage D prostate cancer. Urology. 1989;33(5 Suppl):57–62.
Garje R, Chennamadhavuni A, Mott SL, Chambers IM, Gellhaus P, Zakharia Y, Brown JA. Utilization and outcomes of surgical castration in comparison to medical castration in metastatic prostate cancer. Clin Genitourin Cancer. 2020;18(2):e157–66.
Sun M, Choueiri TK, Hamnvik OP, et al. Comparison of gonadotropin-releasing hormone agonists and orchiectomy: effects of androgen-deprivation therapy. JAMA Oncol. 2016;2(4):500–7.
Acknowledgements
With thanks to the men who participated in this study and their supporters, as well as all the investigators and site staff who made the HERO study possible.
Medical Writing, Editorial and Other Assistance
Medical writing assistance was provided by JD Cox of Mayville Medical Communications and was funded by Myovant Sciences, in partnership with Pfizer Inc.
List of Investigators
A list of investigators have been previously published (Shore ND, et al. N Engl J Med. 2020 Jun 4;382(23):2187–2196).
Funding
This study and its publication, including the journal’s Rapid Service and Open Access Fees, was funded by Myovant Sciences in partnership with Pfizer.
Author information
Authors and Affiliations
Contributions
Neal D. Shore, Bruce Brown, and Sophia Lu wrote the first draft of the manuscript, with professional medical writing assistance funded by Myovant Sciences, and all authors contributed to subsequent drafts. Neal D. Shore, Bryan A. Mehlhaff, Michael S. Cookson, Daniel R. Saltzstein, Ronald Tutrone, Bruce Brown, Sophia Lu, Mark Fallick, Sarah Hanson, and Fred Saad had full access to the data and assume responsibility for the completeness and accuracy of the data.
Corresponding author
Ethics declarations
Conflict of Interest
Neal D. Shore—Consulting or Advisory Role: Bayer, Janssen Scientific Affairs, Dendreon, Tolmar, Ferring, Medivation/Astellas, Amgen, Pfizer, AstraZeneca, Genentech, Myovant Sciences. Speakers’ Bureau: Janssen, Bayer, Dendreon. Bryan A. Mehlhaff—Honoraria, travel, and accommodation expenses, and acted as an advisor/consultant for Astellas, Amgen, Bayer, Janssen, and Pfizer; and has received grants/funding from Astellas, Bayer, Janssen, and Pfizer. Michael S. Cookson—Honoraria: Merck, Janssen Biotech, Bayer, Astellas Pharma, Myovant Sciences. Consulting or Advisory Role: Merck, Janssen Biotech, MDxHealth, Bayer, Astellas Pharma, Myovant Sciences, TesoRx Pharma, Genomic Health, Ferring Pharmaceuticals, Precision Biopsy. Daniel R. Saltzstein—No relevant conflicts. Ronald Tutrone—Advisory/Consulting role: Astellas, Pfizer, Myovant, Janssen, Biotechne, Nymox, Dendreon. Speaker: Biotechne, Astellas, Pfizer, Myovant. Bruce Brown; Sophia Lu—Employees of Myovant. Mark Fallick—Employed at Myovant at the time of research and manuscript development. Sarah Hanson—Employee of Pfizer. Fred Saad—Advisory roles for Astellas Pharma, AstraZeneca/MedImmune, Bayer, Janssen Oncology, and Sanofi; has received honoraria from AbbVie, Amgen, Astellas Pharma, AstraZeneca, Bayer, Janssen Oncology, and Sanofi; and has received research funding grants provided to the institution from Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb, Janssen Oncology, Pfizer, and Sanofi.
Ethical Approval
The trial was approved by the institutional review board or independent ethics committee at each center and was conducted in accordance with the requirements of the regulatory authorities of each country and with the provisions of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonisation. All patients provided written informed consent.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shore, N.D., Mehlhaff, B.A., Cookson, M.S. et al. Impact of Concomitant Cardiovascular Therapies on Efficacy and Safety of Relugolix vs Leuprolide: Subgroup Analysis from HERO Study in Advanced Prostate Cancer. Adv Ther 40, 4919–4927 (2023). https://doi.org/10.1007/s12325-023-02634-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02634-7