Abstract
Introduction
Long-term corticosteroid use in immune-mediated diseases is associated with increased risk of adverse events (AEs) and worsened health-related quality of life (HRQoL). Previous studies report chronic high-dose corticosteroid therapy results in higher rates of healthcare resource use and AE-related medical costs. Recent studies suggest Acthar® Gel (repository corticotropin injection) is an effective steroid-sparing therapy for sarcoidosis. This study compares the corticosteroid-sparing effect between Acthar Gel and comparators and evaluates the impact of Acthar Gel adherence on reduction of corticosteroid burden.
Methods
A retrospective analysis of a large administrative pharmacy and medical claims database (Symphony Health Solutions) was conducted. Patients were included with confirmed ICD-9/10 diagnosis for sarcoidosis in the study period (2014–2020), followed by ≥ 2 Acthar Gel claims or comparators (janus kinase inhibitor (JAKi)/rituximab), ≥ 18 years old, with 12 months coverage pre/post index. Outcomes were compared as change from baseline. Acthar Gel adherence was determined by proportion of days covered in the follow-up period.
Results
The Acthar Gel (n = 735) and comparator (n = 626) cohorts were mostly female (68–72%) between 55 and 58 years old. Compared to the comparator cohort at baseline, Acthar Gel patients had greater any corticosteroid use (80% vs. 56%, p < 0.001), extended use (61% vs. 32%, p < 0.001), and mean average daily dose (6.72 vs. 3.03, p < 0.001). After treatment, Acthar Gel patients had greater reduction from baseline in any corticosteroid use (– 9.0% vs. – 3.2%) and extended use (– 10.0% vs. – 3.0%). In the Acthar Gel adherence cohorts, patients with above average adherence had greater reduction in both measures (– 11.2% vs. – 6.1%; – 11.6% vs. – 7.6%, respectively) than patients with below average adherence. Acthar Gel patients had greater reduction of extended use at all dose levels.
Conclusion
Acthar Gel is associated with reductions in corticosteroid use compared to alternatives. Better adherence is associated with greater reduction in corticosteroid exposure.
Key Summary Points
Plain Language Summary
Patients who use corticosteroids long term for advanced sarcoidosis often suffer from negative health effects. This project aimed to evaluate whether Acthar® Gel (repository corticotropin injection) use led to reduced corticosteroid use and whether higher adherence to Acthar Gel led to further reduction in corticosteroid use. Pharmacy and medical claims data were used to identify patients who fit certain criteria: the Acthar Gel cohort included patients with sarcoidosis who used Acthar Gel and the comparator cohort included patients with sarcoidosis who used janus kinase (JAK) inhibitors or rituximab. The Acthar Gel cohort was split into high adherence and low adherence. The Acthar Gel cohort was found to have higher corticosteroid use than the comparator group in the baseline period before initiating Acthar Gel or a comparator therapy. After initiating treatment, Acthar Gel patients had a larger reduction in corticosteroid use according to a variety of metrics including number of corticosteroid fills and extended use fills. Furthermore, when comparing those with high Acthar Gel adherence and those with low Acthar Gel adherence, the patients with above average adherence had a larger reduction in the number of corticosteroid fills and extended use fills than patients with below average adherence to Acthar Gel. Patients who use Acthar Gel and more regularly tended to use corticosteroids less, which may allow them to avoid the negative health effects from long-term, high-dosage corticosteroid use. This finding may help providers and health plans evaluate situations in which Acthar Gel treatment may be beneficial to improve patient outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
First line treatment for symptomatic sarcoidosis is corticosteroids, but long-term use has been shown to lead to significantly lower health-related quality of life |
Acthar® Gel (repository corticotropin injection) is included in the European Respiratory Society and the US Sarcoidosis Delphi Expert Panel Consensus Statement guidelines for the treatment of sarcoidosis on a case-by-case basis and may have a steroid-sparing effect for sarcoidosis |
This study compares the reduction of steroid burden between Acthar Gel and other comparator therapies and assesses the impact of Acthar Gel adherence on steroid burden to demonstrate steroid-sparing effects and benefits of higher adherence after treatment with Acthar Gel |
What was learned from the study? |
The Acthar Gel cohort saw greater reduction in steroid use after starting Acthar Gel treatment compared to the comparator cohort and further burden reduction with above average Acthar Gel adherence, particularly with the proportion of patients using corticosteroids and among medium and high dosages |
Treatment with Acthar Gel demonstrated a steroid-sparing effect, with greater effects at higher levels of Acthar Gel adherence |
Introduction
Sarcoidosis is an inflammatory systemic granulomatous disease that impacts mostly young and middle-aged adults [1,2,3,4]. The disease is more common among patients of African-American or Scandinavian descent [5]. United States estimated overall incidence and prevalence of sarcoidosis are 8 and 60 cases per 100,000 persons, respectively. [6] In the US, > 25,000 patients annually are diagnosed with sarcoidosis, and 185,000 seek medical care for the disease. [6].
Sarcoidosis affects the lungs in 90% of patients and can also affect many extrapulmonary organs (eyes, joints, skin, liver, spleen, lymph nodes, salivary glands, heart, and nervous system) [2]. Up to half of patients experience pulmonary symptoms such as dry cough and chest pains—furthermore, patients with sarcoidosis often present with constitutional symptoms in addition to pulmonary symptoms, such as fatigue, malaise, and weight loss [2]. As sarcoidosis is a multi-organ disorder, patients may present with drastically different symptoms, and the clinical picture of the disease can vary greatly, dependent on factors such as ethnicity, illness duration, and organ involvement. Although most patients with sarcoidosis experience spontaneous remission, 10–30% have a chronic and progressive form of the disease that may result in severe organ impairment—treatment is often only instituted in the case of organ impairment threatening organ function. [5, 7]
Unfortunately, there is no cure for sarcoidosis. Although increased rates of complications such as weight gain and hypertension are associated with its use, corticosteroids (CS) are the most commonly used first-line therapy to treat sarcoidosis [8]. Following first-line treatment with corticosteroids, patients may receive a variety of immunosuppressive medications such as hydroxychloroquine and methotrexate, with methotrexate being a commonly used therapy among second-line treatments [6]. For a small proportion of patients for whom first- and second-line therapy was not effective, third-line therapies are required, including biologics (e.g., TNF-alpha inhibitors, interleukin inhibitors, etc.) such as infliximab and adalimumab. If disease progression continues or the patient relapses and other therapies have been ineffective, treatments including rituximab, janus kinase (JAK) inhibitors, and Acthar® Gel (repository corticotropin injection) are therapies considered for case-by-case use [9].
Acthar Gel, a naturally sourced complex mixture of adrenocorticotropic hormone analogs and other pituitary peptides, is approved for use by the Food and Drug Administration (FDA) for the treatment of patients with serious and rare conditions, including sarcoidosis, and is only commercially available in the US [10]. The European Respiratory Society guidelines list Acthar Gel among the various anti-inflammatory treatments for pulmonary sarcoidosis, noting that Acthar Gel can be used on a case-by-case basis when other therapies are ineffective or not tolerated [9]. In addition, these guidelines support existing US expert panel consensus on the use of Acthar Gel on a case-by-case basis for patients with advanced sarcoidosis that is unresponsive to third-line therapies [11]. Acthar Gel acts through multiple mechanisms of action, including anti-inflammatory and immunomodulatory pathways, to relieve symptoms and induce remission [12].
Recent studies have shown that Acthar Gel may be an effective steroid-sparing therapy for sarcoidosis [13,14,15]. As mentioned above, corticosteroids are the most commonly used first-line treatment for sarcoidosis. However, long-term high-dose use of corticosteroids can lead to serious adverse effects such as corticosteroid-induced osteoporosis and is linked to higher health care utilization costs [16,17,18,19,20].
This study aimed to build upon the growing body of literature on sarcoidosis treatments, by comparing clinical outcome differences from baseline between patients with Acthar Gel use and other comparator treatment use, and between patients with above and below average Acthar Gel adherence. As, similar to Acthar Gel, JAK inhibitors and rituximab are only referenced for case-by-case use in the instance of disease that has been unresponsive to prior therapies such as infliximab or methotrexate, these therapies were selected as comparators. We hypothesized that patients with Acthar Gel use would see greater corticosteroid burden reduction after treatment initiation compared to other comparator options and that, among those with Acthar Gel use, the higher adherence cohort would have further reduced corticosteroid use.
Methods
Data Source
This study used a de-identified open-source claims database (Symphony Health IDV) that links data from pharmacy point-of-service systems, payer adjudication services (clearing houses), and direct prescription, medical, and hospital feeds. Permission to use these data was granted to the authors by Symphony Clinical Research, the proprietor of Symphony Health IDV. These data contain approximately 168 million longitudinally tracked patients with prescription and medical claims in any recent year of the database. Patients in the database are representative of the US population age and gender mix with claims from diverse payers including basic Medicaid, Managed Medicaid, Medicare, assistance programs, commercial, and cash payers. Because this database does not contain eligibility records, not all claims for a given patient may be captured in the data. For approved claims, the data contain each prescription’s dispensed and payment details making it possible to assess patient characteristics of patients who were approved for use of Acthar Gel.
Sample Selection
Two samples were selected: a cohort for those who received Acthar Gel and a cohort for those who received comparator sarcoidosis treatments. Comparator therapies included oral JAK inhibitors and rituximab, which, like Acthar Gel, were outlined in the European Respiratory Society treatment guidelines for sarcoidosis for use on a case-by-case basis in instances when prior therapies were ineffective [9]. To be eligible for the study, patients in both cohorts were required to have a confirmed sarcoidosis diagnosis during the period of January 1, 2014, through December 31, 2020. A confirmed diagnosis was defined as either one inpatient claim or two outpatient claims on separate days for sarcoidosis using ICD-9-CM/ICD-10-CM diagnosis codes (ICD-9-CM: 135.xx; ICD-10-CM: D86.0, D86.2, D86.83, D86.85, D86.86, D86.87, D86.9).
Patients in the Acthar Gel cohort were further required to have at least one submitted prescription or medical claim for corticosteroids, disease-modifying anti-rheumatic drugs (DMARDS), or immunosuppressive drugs in addition to at least one Acthar Gel claim between 2014 and 2020. The index date for this cohort was defined as their first Acthar Gel administration date after a confirmed sarcoidosis diagnosis—thus, this cohort was required to have an Acthar Gel claim after a sarcoidosis diagnosis. In addition, patients were required to be 18 years or older at the time of the index date and have at least 12 months of eligibility prior to the index date and at least 24 months of eligibility following and including the index date. Patients in the comparator control cohort were required to have no history of claims for Acthar Gel. To ensure that they met the criteria for comparator treatment, they were required to have at least one rituximab or JAK inhibitor claim at any point in the 2014 to 2020 timeframe. Additionally, they were required to be 18 years or older at the time of their first rituximab or JAK inhibitor claim following their confirmed sarcoidosis diagnosis (“index date”) and to have at least 12 months of eligibility prior to the index date and at least 24 months of eligibility following and including the index date.
The Acthar Gel cohort was also divided into two mutually exclusive subgroups based on adherence levels. Adherence was calculated using proportion of days covered (PDC). Patients were classified as adherent if they had a calculated PDC of Acthar Gel in the 1-year follow-up period greater than the mean PDC for Acthar Gel among the overall sample in that period. Those with a calculated PDC higher than the average were classified as above average adherence, and the remaining patients were classified as below average adherence. The final sample for the Acthar Gel cohort was additionally restricted to patients with two or more Acthar Gel claims in the follow-up period.
Statistical Analyses
Outcomes were assessed between the baseline and follow-up periods as a difference from baseline for each clinical measure. Differences were compared among patients with above average adherence to Acthar Gel and those with below average adherence, as well as among those with any use of Acthar Gel and those with use of other comparator treatments. The differences from baseline for binary outcomes were calculated as the proportion of a cohort with an outcome of interest in the follow-up period minus the proportion of a cohort with an outcome of interest in the baseline period. Continuous outcome differences from baseline were calculated as the follow-up value of the continuous outcome minus the baseline value on a per-patient level, subsequently calculating the mean of patient-level differences by cohort. Pairwise comparisons of differences from baseline between cohorts were assessed using chi-square tests for binary variables and two-sample t-tests for continuous variables. Statistical analyses were performed using SAS Enterprise Guide version 7.15 (SAS Institute Inc., Cary, NC).
Study Measures
Demographic and clinical characteristics relevant to sarcoidosis were evaluated for all cohorts. Patient demographics included patient age at the time of index date, gender, US census region, the year in which their index date occurred, and insurance plan type (e.g., Medicare, Commercial, or Other/Unspecified). Clinical characteristics evaluated in the baseline period included patients’ Charlson Comorbidity Index (CCI, a composite measure of the patient’s health status), select comorbidities, and sarcoidosis care metrics.
In both the baseline and follow-up periods, the study assessed additional clinical characteristics such as treatments with other common sarcoidosis therapies, the incidence of other comorbid conditions commonly observed in patients with sarcoidosis including cardiovascular/circulatory, mental health, musculoskeletal, and respiratory conditions, and sarcoidosis care metrics such as blood testing, chest x-rays, and lung function testing. To compare the use of prescription medication in all cohorts, the average number of prescriptions and total number of unique drugs used were assessed. The study also analyzed patients’ CS and DMARD use in a variety of ways, such as the average number of prescriptions per patient and distribution of patients among CS dose levels (low, ≤ 7.5 mg/day; medium, > 7.5 and ≤ 15 mg/day; high, > 15 mg/day).
Compliance with Ethics Guidelines
Because of the nature of the retrospective study design using previously collected, de-identified data, Institutional Review Board approval was not necessary for this study.
Results
Sample Selection
For the Acthar Gel cohort, there were 1495 patients with a confirmed sarcoidosis diagnosis any time between 2014 and 2020. Of those patients, 1412 had a submitted prescription or medical claim for corticosteroids, DMARDs, or immunosuppressive drugs. Among those patients, 1306 had a submitted prescription medical claim for Acthar Gel as well; 1181 patients had a confirmed sarcoidosis diagnosis prior to the index date, and all but 1 of those patients were 18 years or older at the index date. Eight hundred seventy-one of the adults had 12 months of eligibility prior to the index date and 24 months of eligibility following the index date. Finally, 735 patients had two or more submitted claims for Acthar Gel (Table 1).
For the comparator treatment patients, of the 314,432 patients in the Symphony Health IDV database with a confirmed sarcoidosis diagnosis and no history of Acthar Gel claims, 1363 patients had a claim for rituximab or a JAK inhibitor. Among those patients, 1155 had a claim with a confirmed sarcoidosis diagnosis prior to their index date, and 1150 of those patients were an adult at the index date. Finally, 626 of the 1150 patients had sufficient months of eligibility prior to and after the index date (Table 2).
Baseline Characteristics
Patients within the other comparator treatment cohort were older than the Acthar Gel use cohort (mean age of 57.7 vs. 54.7, p < 0.001) and had a higher proportion of female patients (72.4% vs. 67.8%). More patients with comparator treatment use were on a commercial health plan than patients with Acthar Gel use (49.0% vs. 2.9%, p < 0.001). Patients with other comparator treatment use had a higher mean CCI (mean [standard deviation (SD)]: 2.31 [1.89] vs. 1.31 [1.59], p < 0.001) and a greater share of patients with blood testing (77.3% vs. 48.8%, p < 0.001) and chest x-rays (20.6% vs. 6.3%, p < 0.001). Patients with other comparator treatment use also had higher rates of cardiovascular (66.5% vs. 57.7%, p < 0.001), mental health (27.2% vs. 19.2%, p < 0.001), and other (54.5% vs. 49.0%, p = 0.043) comorbid conditions, while patients with Acthar Gel use had higher rates of respiratory comorbid conditions (61.8% vs. 56.7%, p = 0.058) (Table 3). Patients with other comparator treatment use had less corticosteroid use, with 55.9% of the cohort having at least one CS prescription compared to 79.6% (p < 0.001) of the Acthar Gel use cohort having at least one CS prescription. More patients in the Acthar Gel use cohort took anti-asthmatics (60.4% vs. 32.4%, p < 0.001) and antidepressants (38.6% vs. 32.4%, p = 0.017) than the other comparator use cohort (Table 4).
During the 12-month baseline period, patients in the above average and below average Acthar Gel cohorts were generally comparable in terms of age, gender, race/ethnicity, region, insurance plan type, and year of index date. Patients in the below average adherence cohort had higher mean CCI scores (1.44 vs. 1.22, p = 0.064) than patients with above average Acthar Gel adherence. Additionally, patients with below average Acthar Gel adherence had higher rates of lung function testing (43.0% vs. 32.8%, p = 0.005) than patients with above average Acthar Gel adherence (Table 3). Patients with below average Acthar Gel adherence also had lower rates of medium (> 7.5 and ≤ 15 mg/day) dose intermittent CS use (10.0% vs. 5.1%, p = 0.015) (Table 4).
Difference from Baseline
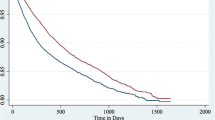
Patients with Acthar Gel treatment experienced greater reductions in CS use compared to patients with other comparator treatments. A greater proportion of patients in the Acthar Gel cohort had reduced overall CS use in the follow-up period (– 9.0% vs. – 3.2%) after treatment. Although the Acthar Gel cohort had an increase in intermittent use overall (1.0% vs. – 0.2%, p = 0.006), and at the low (0.8% vs. – 1.0%, p < 0.001) and medium doses (0.7% vs. – 1.8%, p = 0.006), extended CS use decreased by 10% after treatment, compared to 3% in the comparator cohort. Acthar Gel patients saw reductions at the extended CS medium (– 7.9% vs. – 1.4%, p < 0.001) and high doses (– 9.4% vs. – 2.4%, p < 0.001), with a significantly lower reduction at the low dose (– 0.8% vs. – 2.6%, p = 0.011), compared to the comparator cohort. In addition, Acthar Gel patients had a significant reduction in maximum CS dose (– 1.75 mg vs. 0.50 mg, p = 0.017) and average daily dose (– 1.75 mg vs – 0.02 mg, p < 0.001) compared to other comparator patients (Fig. 1).
Within the Acthar Gel cohort, comparing the above average and below average adherence cohorts demonstrates a clear trend of greater CS burden reduction with above average adherence. A greater proportion of patients in the above average cohort reduced CS use after treatment (– 11.2% vs. – 6.1%), with a significant difference at the intermittent medium dose (– 0.5% vs. 2.2%, p = 0.04). A greater proportion of patients in the above average cohort reduced extended CS use after treatment, (– 11.6% vs. – 7.6%), across low (– 1.2% vs – 0.3%), medium (– 9.5% vs. – 5.7%) and high doses (– 10.0% vs. – 8.6%). In addition, the above average cohort had greater reduction in extended use maximum CS dose (– 2.05 mg vs. – 1.35 mg) and average daily dose (– 1.98 mg vs. – 1.43 mg) (Fig. 2).
Patients with Acthar Gel treatment experienced a greater reduction from baseline in the percentage of patients with antineoplastic prescription use (– 2.3% vs. 0.3%) compared to patients with other comparator treatments (Fig. 3). The above average Acthar Gel adherence cohort experienced greater reductions in other drug use of interest compared to the below average Acthar Gel adherence cohort. Patients with above average adherence had a greater reduction in the percentage of patients with antineoplastic prescription use (– 4.5% vs. 0.3%), immunosuppressive use (– 1.2% vs. 0.3%), and other non-biologic DMARD prescription use (0.0% vs. 1.9%) (Fig. 4).
Discussion
This study was one of the first of its kind to assess the effect of Acthar Gel use, on both the intensive and the extensive margin, on clinical outcomes in a real-world setting. To control for the numerous underlying differences in patient demographics and clinical outcomes in the baseline period between the Acthar Gel and comparator control cohorts and the above and below average Acthar Gel adherence cohorts, differences from baseline metrics were reported for each clinical outcome of interest. In addition to reporting the outcomes with statistically significant differences in differences from baseline across cohorts, this study also reports some outcomes with large but statistically insignificant differences in differences from baseline.
At baseline, the above average Acthar Gel adherence cohort was comparable to the below average cohort across many demographic characteristics but had higher rates of CS use than the below average cohort. The below average cohort had higher rates of some select comorbidities than the above average cohort, such as mild liver disease. Compared to the comparator control cohort, the Acthar Gel cohort was slightly younger and had a smaller proportion of female patients. The comparator control cohort included a larger proportion of patients on a commercial insurance plan than the Acthar Gel cohort. However, this comparison may be misleading as 43.9% of patients in the Acthar Gel cohort were observed to be on an unknown insurance plan, which may include a large proportion of patients who are commercially insured. The Acthar Gel cohort also had lower rates of comorbid conditions, with the exception of respiratory comorbidities. In addition, the Acthar Gel cohort had substantially higher rates of CS use at baseline than the comparator control cohort. These differences in patient demographic and clinical characteristics at baseline across the cohorts of interest underscore the importance of using differences from baseline to evaluate the effect of Acthar Gel use on outcomes of interest.
As stated above, the various comparator cohorts were observed to have substantial disparities in baseline characteristics. To control for these disparities, differences from baseline for each outcome of interest were evaluated for each cohort, and these differences from baseline were then compared across cohorts. As this method thus controls for differences in baseline characteristics, the differences in differences that are observed across cohorts can be reasonably attributed to the exposure of interest, whether that exposure is utilization of Acthar Gel or adherence to Acthar Gel. This comparison of differences from baseline showed that the Acthar Gel cohort had beneficial reductions compared to the comparator control cohort for several CS use measures, such as the proportion of patients with any CS use, the proportion of patients with extended CS use, and the average daily dosage of CS. In addition, this comparison showed that the above average Acthar Gel adherence cohort had beneficial reductions from baseline in CS use compared to the below average adherence cohort, including the proportion of patients with any CS use, the proportion of patients with extended CS use, and the proportion of patients with medium and high dose CS. Taken together, these findings imply that use of Acthar Gel has a substantial steroid-sparing effect compared to similar later-line treatments for sarcoidosis and that this steroid-sparing effect is magnified with better adherence to Acthar Gel. Because long-term corticosteroid use can lead to serious adverse events and worse health outcomes, a reduction in steroid burden due to Acthar Gel use may mean that patients can enjoy improved quality of life measures for fatigue, daily activities, and general satisfaction [16,17,18,19,20].
This study assessed the effect of Acthar Gel use on several other clinical outcomes in addition to CS use measures. The above average adherence cohort had beneficial reductions from baseline compared to below average adherence patients in rates of other drug use, specifically use of antineoplastic agents, immunosuppressives, and other non-biologic DMARDs. These findings imply that there are additional benefits of maintaining high adherence to Acthar Gel beyond the steroid-sparing effect of high adherence.
This study is subject to several limitations inherent to the data source and claims studies more generally. First, as this is a claims study, this is a retrospective and not a prospective analysis. Therefore, this study is subject to the usual limitations of retrospective studies compared to prospective analyses, such as increased risk of unobserved confounding variables. In addition, because the Symphony IDV is an open-source database linking patient claims from diverse data feeds, the database does not contain complete eligibility records for patients making it possible that not all claims for a given patient may be captured in the data. Furthermore, many measures assessed for this project are subject to the usual drawbacks of claims data resulting from errors in the entries of claims—for example, the identification of diagnoses of interest relies on ICD codes, which may be listed on a claim erroneously. Additionally, claims data are inherently left-censored, which means that there is no way to discern with absolute certainty whether patients in the control cohort have no use of Acthar Gel, as it is impossible to observe any potential use of Acthar Gel prior to their claims history that is included in the data.
The adherence analysis is also subject to limitations presented by the use of claims data. The primary limitation is that there is no visibility into how a patient utilizes Acthar Gel after a claim has been filled—one cannot tell whether a patient uses the full supply of Acthar Gel as recommended upon dispensing or fails to use Acthar Gel as recommended. Therefore, assumptions must be made that patients utilize the full quantity of any drug dispensed and do not stop use early. Another limitation of the adherence analysis is that Acthar Gel dosage is dependent on the weight of the patient, and, as weight information is not available in Symphony IDV, there is no way to tell how many true days of supply are dispensed based on the quantity of Acthar Gel dispensed. Therefore, one must assume that the days of supply associated with a particular quantity of Acthar Gel dispensed is the same for all patients.
The observed reduction in CS dosage is subject to limitations that have been observed in prior research. Some prior studies have indicated that patients with sarcoidosis using high doses of CS can occasionally taper down to a lower dose while still maintaining control of disease [21]. Therefore, some of the reduction in CS dosage observed among high-dose patients may be attributable to natural tapering that is indistinguishable from reduction in CS dosage due to use of Acthar Gel.
Conclusions
This study is one of the first to utilize real-world administrative claims data to estimate the impact of Acthar Gel adherence on clinical outcomes among patients with sarcoidosis. In addition, this study estimates the impact of Acthar Gel use on clinical outcomes compared to other later-line treatments for sarcoidosis. The primary finding of this study is that Acthar Gel has a steroid-sparing effect compared to other later-line sarcoidosis treatments and that this steroid-sparing effect is magnified with better adherence to Acthar Gel. This finding may help providers and health plans evaluate situations in which Acthar Gel treatment may be beneficial to improve quality of care and patient outcomes, particularly among patients who are unable to tolerate corticosteroids. Furthermore, this finding emphasizes the importance of adherence to Acthar Gel in maximizing potential benefits to patient outcomes. Further investigation, such as a prospective study, would aid in demonstrating the efficacy and steroid-sparing effect of Acthar Gel. These results underline the importance of continued access to alternative therapies in the population of patients with sarcoidosis.
References
Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–55.
Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155–67.
Soto-Gomez N, Peters JI, Nambiar AM. Diagnosis and management of sarcoidosis. Am Fam Physician. 2016;93:840–8.
Wu JJ, Schiff KR. Sarcoidosis. Am Fam Physician. 2004;70:312–22.
Jain R, Yadav D, Puranik N, Guleria R, Jin JO. Sarcoidosis: causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9(4):1081.
Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis based on health care use. Ann Am Thorac Soc. 2016;13:1244–52.
Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–65.
Khan NA, Donatelli CV, Tonelli AR, et al. Toxicity risk from glucocorticoids in sarcoidosis patients. Respir Med. 2017;132:9–14.
Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021;58:2004079.
Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
Ross AP, Ben-Zacharia A, Harris C, et al. Multiple sclerosis, relapses, and the mechanism of action of adrenocorticotropic hormone. Front Neurol. 2013;4:21.
Baughman RP, Barney JB, O’Hare L, Lower EE. A retrospective pilot study examining the use of Acthar gel in sarcoidosis patients. Respir Med. 2016;110:66–72.
Baughman RP, Sweiss N, Keijsers R, et al. Repository corticotropin for chronic pulmonary sarcoidosis. Lung. 2017;195(3):313–22.
Chopra I, Qin Y, Kranyak J, et al. Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: retrospective analysis of medical records. Therap Adv Respir Dis. 2019;13:175346661988812.
Yasir M, Goyal A, Sonthalia S. Corticosteroid adverse effects. 2022 Jul 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001;33(4):289–94.
Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204 (Crossref. PubMed).
Rice JB, White AG, Johnson M, et al. Quantitative characterization of the relationship between levels of extended corticosteroid use and related adverse events in a US population. Curr Med Res Opin. 2018;34:1519–27 (Crossref. PubMed).
Rice JB, White AG, Johnson M, et al. Healthcare resource use and cost associated with varying dosages of extended corticosteroid exposure in a US population. J Med Econ. 2018;21:846–52 (Crossref. PubMed).
Moller DR. Negative clinical trials in sarcoidosis: failed therapies or flawed study design? Eur Respir J. 2014;44(5):1123–6. https://doi.org/10.1183/09031936.00156314.
Author Contribution
All authors participated in the design of the study. All authors participated in the collection of the data and all authors were involved in the analyses and interpretation of the study. All authors participated in the development of this manuscript and in its critical review, with important intellectual contributions. All authors had full access to the data and gave approval before submission. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The work described was carried out in accordance with the recommendations of the International Committee of Medical Journal Editors for conduct, reporting, editing, and publication of scholarly work in medical journals.
Funding
Sponsorship of this study and article processing charges, including the journal’s Rapid Service and Open Access Fees, were funded by Mallinckrodt Pharmaceuticals (Bridgewater, NJ).
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available because they are proprietary administrative health claims data owned by Symphony Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Kyle Hayes, John Niewoehner, and George J. Wan are employees of Mallinckrodt Pharmaceuticals, which provided research funding to Analysis Group (employer of J. Bradford Rice, Nathaniel Downes, Ella Hagopian, and Izzy Ma).
Ethical Approval
This article is based on previously collected, de-identified data and did not explicitly precipitate human or animal subjects.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hayes, K., Niewoehner, J., Rice, J.B. et al. Corticosteroid Use and Adherence in Patients Treated with Acthar Gel for Advanced Sarcoidosis. Adv Ther 40, 4999–5015 (2023). https://doi.org/10.1007/s12325-023-02630-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02630-x