Abstract
Introduction
Docetaxel is an established standard therapy after osimertinib and platinum-based doublet chemotherapy (Pt-doublet) for locally advanced or metastatic non-small cell lung cancer (NSCLC) with an epidermal growth factor receptor gene (EGFR) mutation. To facilitate future therapeutic developments in these patients after treatment with osimertinib and Pt-doublet, we estimated the outcomes of currently used post-treatment therapies.
Methods
Data of patients with NSCLC who received at least one medication after osimertinib and Pt-doublet between April 2008 and August 2021 were extracted from the Medical Data Vision claims database. The duration of treatment (DoT) (first treatment after osimertinib and Pt-doublet) and overall survival (OS) were estimated. The index date was the first day on which the medication was prescribed.
Results
In total, 731 patients (mean age 64 years) were screened. The most frequent post-treatments were docetaxel-based chemotherapy (30.2%), immune checkpoint inhibitor (ICI) alone or in combination (17.2%), first-/second-generation EGFR-tyrosine kinase inhibitors (16.7%), osimertinib (16.3%), and Pt-doublet (5.2%). The median DoT and OS (95% confidence interval) of all post-treatments were 3.5 (3.27, 3.77) and 10.3 (9.3, 12.1) months, respectively, reflecting the median DoT (3.8 months) and OS (10.0 months) of docetaxel-based chemotherapy. Among all post-treatment regimens, ICIs resulted numerically the shortest [2.77 (2.33, 3.00) months] and osimertinib the longest [4.40 (3.47, 5.67) months] median DoT. The median OS was shortest in patients post-treated with ICIs [7.07 (5.40, 9.90) months] and longest in patients rechallenged with Pt-doublet (12.27 months), followed by patients post-treated with osimertinib (11.70 months). In a subset analysis of patients who received first-line osimertinib and second-line Pt-doublet as well as Pt-doublet immediately after osimertinib, those post-treated with ICIs had the shortest median DoT.
Conclusion
Given the limited real-world efficacy on EGFR-mutant NSCLC resistant to osimertinib and platinum-based chemotherapy, the development of more highly potent post-treatment therapies is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
We performed an investigational study of real-world data to clarify the first post-treatments administered after osimertinib and platinum-based doublet chemotherapy and to estimate the effectiveness of these post-treatments, with the aim of providing insights into innovative personalized treatment development. |
What was learned from the study? |
The most frequent post-treatment was docetaxel-based chemotherapy (alone and in combination with ramucirumab). |
The median duration of treatment and overall survival (95% confidence interval) of all post-treatments were 3.5 (3.27, 3.77) and 10.3 (9.3, 12.1) months, respectively. |
The median duration of treatment of immune checkpoint inhibitors and first-/second-generation epidermal growth factor receptor–tyrosine kinase inhibitors were numerically shorter than those of all other post-treatments. |
Introduction
Lung cancer is one of the most commonly diagnosed cancers and the leading cause of cancer-related deaths worldwide [1, 2]. Non-small cell lung cancer (NSCLC), which accounts for 82% of lung cancers, has a very poor prognosis in its advanced stage [3]. The efficacy of cytotoxic systemic chemotherapy is limited, and the expected increase in overall survival (OS) with such treatment is less than 1 year with a high possibility of toxicity [4].
Epidermal growth factor receptor gene (EGFR) mutations are commonly detected in adenocarcinoma, with higher rates among Asians (47%) than among Europeans (15%) or North Americans (22%) [5]. Various studies have demonstrated significant efficacy of first- and second-generation EGFR–tyrosine kinase inhibitors (EGFR-TKIs) (gefitinib, erlotinib, and afatinib) with a survival benefit and tumor responses in patients with NSCLC harboring EGFR mutations [6,7,8,9,10,11]. However, relapse often occurred within 1 year, mainly related to the acquisition of resistance to EGFR-TKIs and limited distribution of the drugs to central nervous system metastases [12,13,14].
Osimertinib is a third-generation irreversible EGFR-TKI that selectively inhibits EGFR-TKI-sensitizing and EGFR T790M resistance mutations. It also has significant central nervous system activity [14]. The randomized double-blind phase 3 FLAURA study demonstrated superior efficacy of osimertinib over first-generation EGFR-TKIs with a similar safety profile and lower rates of serious adverse events [15]. Additionally, an OS benefit was shown in patients treated with osimertinib [16]. These results were consistent in the Japanese subset of the FLAURA study [17].
Based on these results, the third-generation EGFR-TKI osimertinib was approved in August 2018 as a first-line treatment for patients with NSCLC harboring an active EGFR mutation. The Japanese Lung Cancer Society guidelines recommend osimertinib as standard therapy for first-line treatment of NSCLC with an EGFR mutation in patients with a performance status of 0–1 and second-line treatment for relapse or resistant NSCLC treated with first- and second-generation EGFR-TKI with a T790M EGFR mutation [18, 19].
For second-line treatment of EGFR-mutated NSCLC, the Japanese Lung Cancer Society guidelines recommend platinum-based doublet chemotherapy (Pt-doublet), which consists of a platinum-containing drug plus third-generation cytotoxic agents with or without bevacizumab [18, 19]. The efficacy profiles of second Pt-doublet after treatment with a first- or second-generation EGFR-TKI were shown in a comparator arm of the AURA3 phase 3 study of second-line osimertinib versus carboplatin + pemetrexed in patients with T790M-positive advanced NSCLC who had disease progression after first-line EGFR-TKI (first- or second-generation) therapy [20, 21]. The median progression-free survival (PFS), time to first subsequent therapy, and OS [95% confidence interval (CI)] were 4.4 (4.2, 5.6) months [20], 6.0 (5.2, 6.9) months [20], and 22.5 (20.2, 28.8) months [21], respectively. The OS benefit is thought to reflect the high crossover rate; namely, 73% of patients received osimertinib after carboplatin + pemetrexed because the AURA3 study allowed crossover.
Docetaxel monotherapy or docetaxel in combination with ramucirumab is the standard therapy after osimertinib and Pt-doublet for locally advanced or metastatic NSCLC with an EGFR mutation [19]. To provide information for the development of future innovative post-treatments for patients in this line, we investigated the real-world first subsequent therapies and their outcomes after osimertinib and Pt-doublet in patients with NSCLC harboring EGFR mutations from a Japanese claims database.
Methods
Study Design
This retrospective cohort study was performed using a large-scale claims database called the Medical Data Vision (MDV) database, which is constructed from medical data (Diagnosis Procedure Combination data and receipt data) of anonymized patients from hospitals. The MDV database contains the administrative data of more than 460 hospitals that report the status and treatment of more than 40 million patients (including a substantial number of these patients aged > 65 years) in Japan to the end of 2022. The database is maintained by Medical Data Vision Co., Ltd. (Tokyo, Japan) (Fig. 1).
We extracted the medical data of patients with NSCLC who received at least one medication after osimertinib and Pt-doublet between April 2008 and August 2021 (study period). The primary objectives were to explore the first subsequent anticancer treatment after osimertinib and Pt-doublet in these screened patients and to estimate their duration of treatment (DoT). The secondary objective was to estimate the OS after initiating those therapies. The index date was the first prescription day of the first medication administered after osimertinib and Pt-doublet.
Institutional review board approval and patient informed consent were not required for this observational study because it used secondary data devoid of any patient-identifying information. Authors had full access to the de-identified data, through a license from Japan Medical Data Vision Co., Ltd.
Patients
Patients were screened from the MDV database according to the following inclusion criteria: (1) diagnosed with NSCLC, (2) aged ≥ 18 years at the first diagnosis of NSCLC (International Classification of Diseases 10th Revision code C34.9), (3) received osimertinib, (4) received Pt-doublet, and (5) received at least one medication after osimertinib and Pt-doublet during the study period (Fig. 1). Patients who received osimertinib or any anticancer chemotherapy before the first diagnosis were excluded. The full analysis set (FAS) consisted of all patients selected from the database in accordance with these criteria, in which patients received first-, second, and third-line or later osimertinib treatments.
The FAS included several subtypes of patients: those who received first-, second-, and third-line osimertinib and those who received Pt-doublet (1) immediately after osimertinib or (2) after another therapy following osimertinib. The eligible cohort comprised patients who received other EGFR-TKIs before osimertinib.
Data Collection and Analysis
We collected the patients’ demographics data from the MDV database on the initial prescribing day of the first anticancer treatment administered after osimertinib and Pt-doublet (index date) and the patients’ clinical characteristics, as well as descriptions of treatment patterns including drugs or regimens and their prescribing dates after the index date. Patients could be followed up to the last contact date available within the database.
DoT (Duration of First Subsequent Anticancer Treatment)
The DoT was defined as follows. The starting point of the DoT was the index date. The end date of the DoT for an oral drug was the prescription days after the last prescription date, and that for an injectable drug was the last date of the dosing cycle described in the package insert. When multiple drugs were prescribed, the latest administration date was defined as the end date of the DoT. If this date was 60 days after the last dosing of the previous treatment, the medication was considered to have shifted to a new therapy. When administration was not completed by the cut-off date, the data were censored and the cut-off date (31 August 2021) was the date of censoring. This study applied DoT to assess the effectiveness of treatments. The selection of this outcome is supported by previous studies in which the DoT was a surrogate endpoint to evaluate the efficacy of drugs in patients with NSCLC [22].
Overall Survival After First Subsequent Anticancer Treatment
OS was defined as the time from the index date to the date of death. Censored data in the OS analyses were any data without a record of the exact time of death during the study period. The date of censoring was either the last available date on which the patient was known to be alive in the database or the study completion date (cut-off date, 31 August 2021).
Subset Analysis
The median DoT and OS were also analyzed in two patient subsets using the same definition for estimation of DoT and OS: (1) patients who received Pt-doublet immediately after osimertinib and (2) patients who received osimertinib as first-line treatment and Pt-doublet as second-line treatment. Subgroup (1) contained subgroup (2).
Statistical Analysis
The number and percentage of patients as well as their descriptive statistics (mean ± standard deviation) were determined as continuous variables. Frequencies in categorical data including numbers and percentages were calculated as categorical variables.
We used the Kaplan–Meier method to estimate the DoT and OS, and we drew Kaplan–Meier curves to visualize the DoT and OS. The median DoT and 95% CI were then calculated.
Data extraction from the database was performed using Amazon Redshift and SQL. All other analyses were conducted using R version 4.1.1 ® Core Team (2021).
Results
Study Population
The flow of patients in this study is shown in Fig. 1. Overall, 731 patients and their data were screened and analyzed. Totals of 378, 232, and 121 patients received first-, second-, and third-line osimertinib treatment, respectively. The median DoT (standard deviation) of osimertinib starting from the first to last day of osimertinib prescription was 6.5 (7.92), 8.95 (8.81), and 4.25 (7.17) months in the first-, second-, and third-line subgroups, respectively. The patients’ mean age was 64 ± 10.21 years, and 63% of patients were female (Table 1).
Treatment Trends
Table 2 shows the post-treatment trends after osimertinib and Pt-doublet. The most frequently used medication for the first subsequent treatment was docetaxel-based chemotherapy (n = 221, 30.2%). Immune checkpoint inhibitors (ICIs) alone or in combination were used in 126 (17.2%) patients. EGFR-TKI rechallenge was performed in 241 patients, who received a first-/second-generation EGFR-TKI (n = 122, 16.7%) and osimertinib (n = 119, 16.3%). The footnote of Table 2 indicates the various drugs used in this post-treatment line.
DoT
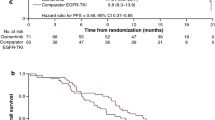
The median DoT (95% CI) of all the first subsequent therapies combined was 3.5 (3.27, 3.77) months (Fig. 2a). Figure 2b shows the DoT by regimen. The median DoT (95% CI) of the docetaxel regimen was 3.77 (3.50, 4.20) months. Figures 2c and 5c show the DoT and OS of DOC and DOC/ramucirumab separately. The median DoT of osimertinib rechallenge was numerically the longest among the various regimens at 4.40 (3.47, 5.67) months. The median DoT of the first- and second-generation EGFR-TKIs was 3.00 (2.23, 4.60) months. The median DoT (95% CI) of the ICIs was the shortest at 2.77 (2.33, 3.00) months. The number of censored patients and rate of censoring due to cut-off for DOC/DOC + RAM, ICI mono/Comb, EFGR-others, EGFR-Osi, and Platinum Doublet are 52 (24%), 21 (17%), 36 (30%), 33 (28%), and 7 (18%), respectively. The median follow-up time from index was 4.68 months (range 1 day–43.8 months).
Kaplan–Meier estimates of duration of all first post-treatments: a combined, b by regimen, and c by DOC and DOC/ramucirumab separated after osimertinib and Pt-doublet. The starting point of the DoT was the initial dosing date of the first post-treatment. The end date of the DoT for an oral drug was the prescription days after the last prescription date, and that for an injectable drug was the last date of the dosing cycle described in the package insert. When multiple drugs were prescribed, their latest administration date was used as the end date. CI confidence interval, DOC docetaxel, RAM ramucirumab, ICI immune checkpoint inhibitor, mono/Combi monotherapy/combination therapy, 1st, 2nd-G EGFR-TKI first-, second-generation epidermal growth factor receptor-tyrosine kinase inhibitor, mDoT median duration of treatment, Pt-doublet platinum-based doublet chemotherapy
Figure 3a shows the results of the subset analysis (DoT of patients who received Pt-doublet immediately after osimertinib, regardless of the line of osimertinib). The median DoT (95% CI) was 3.42 (2.80, 3.77) months, which was similar to that of the FAS [3.5 (3.27, 3.77) months] (Fig. 2a). Rechallenge of Pt-doublet and osimertinib had a longer DoT than the other regimens in the subset, although a few patients received Pt-doublet (n = 16) (Fig. 3b).
Duration of all first post-treatments: a combined and b by regimen in subset treated with Pt-doublet immediately after osimertinib. CI confidence interval, DOC docetaxel, RAM ramucirumab, ICI immune checkpoint inhibitor, mono/Combi monotherapy/combination therapy, 1st, 2nd-G EGFR-TKI first-, second-generation epidermal growth factor receptor-tyrosine kinase inhibitor, mDoT median duration of treatment, NA not applicable, Pt-doublet platinum-based doublet chemotherapy
In the subset of patients who received first-line osimertinib and second-line Pt-doublet, the median DoT of the first therapy administered after osimertinib and Pt-doublet was shorter than that in the FAS (Fig. 4a). The median DoT of ICIs was 1.63 (0.93, 3.30), which was shorter than that of the other regimens.
Duration of all first post-treatments: a combined and b by regimen in subset treated with first-line osimertinib and second-line Pt-doublet. CI confidence interval, DOC docetaxel, RAM ramucirumab, ICI immune checkpoint inhibitor, mono/Combi monotherapy/combination therapy, 1st, 2nd-G EGFR-TKI first-, second-generation epidermal growth factor receptor-tyrosine kinase inhibitor, mDoT median duration of treatment; NA not applicable, Pt-doublet platinum-based doublet chemotherapy
Kaplan–Meier curves of the DoT in the osimertinib-treated subgroup indicated a higher probability of remaining on treatment than that in the subgroup treated with ICI-based therapy at most time points in the FAS and in both subsets of patients treated with Pt-doublet immediately after osimertinib, and first-line osimertinib and second-line Pt-doublet (Figs. 2b, 3b, 4b).
Overall Survival
The median (95% CI) OS after the initial post-therapy dosing was 10.3 (9.3, 12.1) months in the FAS (Fig. 5a). The median OS in ICI monotherapy and combination regimens tended to be shorter than that in the other regimens (Fig. 5b, c).
Overall survival (OS) of all first post-treatments: a combined, b by regimen, and c by DOC and DOC/ramucirumab separated after osimertinib and Pt-doublet. OS was defined as the time from initial dosing of the first post-treatment to the date of death. Censored data were any data without a record of the exact time of death during the study period. The censored date was either the last available date on which the patient was known to be alive in the database or the study completion date (cut-off date) of 31 August 2021. CI confidence interval, DOC docetaxel, RAM ramucirumab, ICI immune checkpoint inhibitor, mono/Combi monotherapy/combination therapy, 1st, 2nd-G EGFR-TKI first-, second-generation epidermal growth factor receptor-tyrosine kinase inhibitor, MST median survival time, NA not applicable, Pt-doublet platinum-based doublet chemotherapy
Similar median OS times (10.47 and 10.3 months) were observed in the subset of patients receiving Pt-doublet immediately after osimertinib and in the FAS, respectively (Figs. 3a, 6a). The median OS for osimertinib treatment (17.93 months) was numerically the longest and that of ICI monotherapy/combination therapy (7.73 months) was the shortest (Fig. 6b).
Overall survival of all first post-treatments: a combined and b by regimen in subset treated with Pt-doublet immediately after osimertinib. CI confidence interval, DOC docetaxel, RAM ramucirumab, ICI immune checkpoint inhibitor, mono/Combi monotherapy/combination therapy, 1st, 2nd-G EGFR-TKI first-, second-generation epidermal growth factor receptor-tyrosine kinase inhibitor, MST median survival time, NA not applicable, Pt-doublet platinum-based doublet chemotherapy
The median OS was 10.93 months in the subset of patients treated with first-line osimertinib and second-line Pt-doublet (Fig. 7a). In the osimertinib and Pt-doublet subgroups, the OS rate did not reach 50% during the study period and the median OS was not estimated (Fig. 7b).
Overall survival of all first post-treatments: a combined and b by regimen after first-line osimertinib and second-line Pt-doublet. CI confidence interval, DOC docetaxel, RAM ramucirumab, ICI immune checkpoint inhibitor, mono/Combi monotherapy/combination therapy, 1st, 2nd-G EGFR-TKI first-, second-generation epidermal growth factor receptor-tyrosine kinase inhibitor, MST median survival time, NA not applicable, Pt-doublet platinum-based doublet chemotherapy
The OS rate in the osimertinib-treatment subgroup was higher than that in the ICI-based treatment subgroup at most time points for 18 months in the FAS and subset analyses (Figs. 5b, 6b, 7b).
Discussion
This study investigated the pattern of subsequent treatment after osimertinib and Pt-doublet in a real-world setting. To the best of our knowledge, few studies have focused on the median DoT and OS in patients (with NSCLC resistance to osimertinib and platinum-based chemotherapy) who received at least one medication. The median DoT of the post-treatment was 2–7 months in all subsets and subgroups, which suggests that the effectiveness of currently available treatments might be limited.
The most frequent post-treatment in this study was docetaxel-based chemotherapy in the main analysis and the two subsets. Based on the REVEL study [23], combination therapy with docetaxel plus ramucirumab has been approved in several countries, including Japan, for the treatment of advanced or relapsed NSCLC in patients with tumors that are refractory to platinum-based chemotherapy. The current study showed that docetaxel with ramucirumab is used more often in real-world settings than is docetaxel monotherapy, potentially because of its better effectiveness as shown by the REVEL study.
Unexpectedly, rechallenge of osimertinib was common in the main and subgroup analyses. The OS and DoT of osimertinib were numerically longer than those of the other regimens. The study might have extracted those who withdrew from first-line osimertinib because of adverse events rather than weak effectiveness. In addition, multiple case studies have shown successful osimertinib rechallenge in patients with NSCLC [24,25,26]. In such cases, rechallenge use of osimertinib may have high effectiveness. However, it was difficult to clinically interpret the results of OS because of the rapid drop in the number of patients at risk secondary to censoring, and because of the insufficient follow-up period.
The median OS in the main analysis after the initial dosing of the post-therapy was 7–12 months. This result is consistent with previous clinical trials of advanced NSCLC [27]. The median OS of osimertinib in the main analysis was relatively long (11.7 months) compared with that of other regimens. The median OS was approximately 10 months, which was similar to that of the main analysis results and consistent with previously published results.
A previous study suggested that the similarity of real-world data to their clinical trial analogues depended on the drug class [28]. In this study, ICIs may have had prolonged effectiveness after stopping the treatment [29]; therefore, the PFS might have been longer than the end of treatment in the claims database. EGFR-TKIs are often used beyond progressive disease in patients with NSCLC [30], and the PFS may therefore be shorter than the DoT. Regimen-based considerations are required to interpret the regimen-based DoTs in the current study.
Regarding the background characteristics of the eligible cohort in this study, the median DoT of the initial treatment (first, second, and third lines) with osimertinib in Fig. 1 estimated without considering censoring was markedly shorter (4.25–8.95 months) than that in previous clinical trials [15, 16, 20]. This might explain why many patients experienced only a short follow-up period and were classified as censored cases. Osimertinib was approved for first-line NSCLC in August 2018, and the cut-off date of the present study was the end of August 2021, which means that many average follow-up periods in the dataset may be short. In addition, there was a possibility that the selection criteria of this study mainly screened patients who withdrew from initial treatment with osimertinib because of adverse events or a lack of usefulness of the treatment.
Considering the results, innovative treatments under development for the post-osimertinib and Pt-doublet setting, such as amivantamab, EGFR–mesenchymal epithelial transition factor bispecific antibody, and treatment combined with lazertinib (a third-generation EGFR-TKI) [31] and patritumab deruxtecan (human EGFR 3-directed monoclonal antibody–drug conjugate) [32], may be able to provide better treatment choices in this line of therapy.
This study had some limitations. Patient information including stage at index date or metastatic site information are missing due to the database characteristics that make it difficult to retrieve stage at index date information in all the patients and impossible to retrieve metastatic site information because it was not available. No statistical testing was conducted to compare the DoT or OS between regimens in this study; therefore, the results cannot be statistically interpreted. The analysis did not capture data on treatments received by patients in other hospitals, although that would be unlikely in this clinical setting. The DoT was estimated based on the standard regimen, but it may not necessarily reflect regimens actually used because this claims database does not contain a clinical assessment of the patients. Treatment failure was determined using an algorithm based on medication changes, although the reasons for medication changes are not recorded in the database. Because the data do not include each patient’s entire history, patients may have been diagnosed with NSCLC before the start of the time frame examined in the present study. The adherence of patients to medications is unknown. The diagnosis recorded in the database was for the reimbursement setting and may be different from clinical practice. Not all cases of death were recorded once the patients had left the hospital. Despite these limitations, this study extends our knowledge of EGFR mutation-positive NSCLC treatments, for which no recommended care exists in local guidelines. Therefore, these study findings from real-world data might be useful to design clinical trials and construct a synthetic control arm for single-arm trials of innovative personalized treatments [33, 34].
Conclusions
This database analysis using MDV data demonstrated that various treatments were used in patients with stage 4 NSCLC harboring an activating EGFR mutation after treatment with osimertinib and Pt-doublet. The median DoT of the post-treatment was approximately 2–7 months in all subsets and subgroups, which suggests that the effectiveness of currently available treatments may not be high enough. Further development of effective post-therapy is required.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
The Editorial Board of Cancer Statistics in Japan. Cancer Statistics in Japan 2022. Tokyo: Foundation for Promotion of Cancer Research (FPCR); 2022. https://ganjoho.jp/public/qa_links/report/statistics/pdf/cancer_statistics_2022.pdf. Accessed 21 Apr 2023.
American Society of Clinical Oncology (ASCO). Lung cancer: Non-small cell: Statistics. Alexandria (VA): American Society of Clinical Oncology (ASCO); 2022. Available from: https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics. Accessed 21 Apr 2023.
Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N Engl J Med. 2002;346:92–8.
Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5:2892–911.
Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8.
Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8.
Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46.
Zhou C, Wu Y-L, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42.
Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–22.
Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–51.
Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92.
Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26.
Lazzari C, Gregorc V, Karachaliou N, Rosell R, Santarpia M. Mechanisms of resistance to osimertinib. J Thorac Dis. 2020;12:2851–8.
Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25.
Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50.
Ohe Y, Imamura F, Nogami N, et al. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol. 2019;49:29–36.
Akamatsu H, Ninomiya K, Kenmotsu H, et al. The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV. Int J Clin Oncol. 2019;24:731–70.
The Japan Lung Cancer Society. The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV (2022) (in Japanese). Tokyo: The Japan Lung Cancer Society; 2022. https://www.haigan.gr.jp/guideline/2022/index.html. Accessed 21 Apr 2023.
Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–40.
Papadimitrakopoulou VA, Mok TS, Han J-Y, et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol. 2020;31:1536–44.
Yang G, Ma D, Xu H, et al. Treatment duration as a surrogate endpoint to evaluate the efficacy of crizotinib in sequential therapy for patients with advanced ALK-positive non-small cell lung cancer: a retrospective, real-world study. Cancer Med. 2019;8:5823–30.
Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–73.
Cortellini A, Leonetti A, Catino A, et al. Osimertinib beyond disease progression in T790M EGFR-positive NSCLC patients: a multicenter study of clinicians’ attitudes. Clin Transl Oncol. 2020;22:844–51.
Sekine A, Satoh H, Ikeda S, et al. Rapid effect of osimertinib re-challenge on brain metastases developing during salvage cytotoxic chemotherapy after osimertinib treatment failure: a case report. Mol Clin Oncol. 2019;10:451–3.
Bickert C, Kahnert K, Kauffmann-Guerrero D, et al. Osimertinib rechallenge under steroid protection following osimertinib-induced pneumonitis: three case studies. Ther Adv Med Oncol. 2021;13:17588359211018028.
Yang C-J, Hung J-Y, Tsai M-J, et al. The salvage therapy in lung adenocarcinoma initially harbored susceptible EGFR mutation and acquired resistance occurred to the first-line gefitinib and second-line cytotoxic chemotherapy. BMC Pharmacol Toxicol. 2017;18:21.
Mhatre SK, Machado RJM, Ton TGN, et al. Real-world progression-free survival as an endpoint in advanced non-small-cell lung cancer: replicating atezolizumab and docetaxel arms of the OAK trial using data derived from electronic health records. medRxiv [Preprint]. 2022 [posted 2 May 2022]. https://www.medrxiv.org/content/10.1101/2022.05.02.22274571v1. https://doi.org/10.1101/2022.05.02.22274571. Accessed 21 Apr 2023.
Yin J, Song Y, Tang J, Zhang B. What is the optimal duration of immune checkpoint inhibitors in malignant tumors? Front Immunol. 2022;13: 983581.
Di Noia V, D’Aveni A, D’Argento E, et al. Treating disease progression with osimertinib in EGFR-mutated non-small cell lung cancer: novel targeted agents and combination strategies. ESMO Open. 2021;6: 100280.
Petrini I, Giaccone G. Amivantamab in the treatment of metastatic NSCLC: patient selection and special considerations. Onco Targets Ther. 2022;15:1197–210.
Jänne PA, Baik C, Su WC, et al. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated non-small cell lung cancer. Cancer Discov. 2022;12:74–89.
Thorlund K, Dron L, Park JJH, Mills EJ. Synthetic and external controls in clinical trials—a primer for researchers. Clin Epidemiol. 2020;12:457–67.
Popat S, Liu SV, Scheuer N, et al. Addressing challenges with real-world synthetic control arms to demonstrate the comparative effectiveness of pralsetinib in non-small cell lung cancer. Nat Commun. 2022;13:3500.
Acknowledgements
Funding
Research, data collection, data analysis, medical writing support, and the journal’s Rapid Service and Open Access Fees were funded by Janssen Pharmaceutical K.K., the study sponsor.
Medical Writing, Editorial, and Other Assistance
Editorial and medical writing support was provided by ASCA Corporation and funded by Janssen Pharmaceutical K.K.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Hidetoshi Hayashi contributed to the study conceptualization, data interpretation, and reviewing and editing of the manuscript. Makoto Nishio contributed to the study conceptualization, data interpretation, and reviewing and editing of the manuscript. Michiko Takahashi contributed to the study conceptualization, data interpretation, and reviewing and editing of the manuscript. Hiroaki Tsuchiya contributed to the data curation, formal data analysis, data validation, study methodology, and reviewing and editing of the manuscript. Mami Kasahara-Kiritani contributed to the study conceptualization, study methodology, data curation, manuscript writing, data interpretation, and reviewing and editing of the manuscript.
Disclosures
Hidetoshi Hayashi has served as a speaker for Ono Pharmaceutical, Bristol-Myers Squibb, Chugai, AstraZeneca, Eli Lilly, and Pfizer and has received research funding from AstraZeneca and Chugai. Makoto Nishio has served as a speaker for Chugai, AstraZeneca, MSD, Ono Pharmaceutical, Takeda, Eli Lilly, Novartis, Pfizer, and Bristol-Myers Squibb. Michiko Takahashi, Hiroaki Tsuchiya, and Mami Kasahara-Kiritani are employees of Janssen Pharmaceutical K.K.
Compliance with Ethics Guidelines
Institutional review board approval and patient informed consent were not required for this observational study because it used secondary data devoid of any patient-identifying information. Authors had full access to the de-identified data, through a license from Japan Medical Data Vision Co., Ltd.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available because they were obtained from a commercial database and Janssen Pharmaceutical K.K. purchased the data. However, the data are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hayashi, H., Nishio, M., Takahashi, M. et al. Real-World Data About Treatment Outcomes for Patients with EGFR-Mutated NSCLC Resistance to Osimertinib and Platinum-Based Chemotherapy. Adv Ther 40, 4545–4560 (2023). https://doi.org/10.1007/s12325-023-02616-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02616-9