Abstract
Introduction
Pharmacological asthma management focuses on the use of inhaled corticosteroid (ICS)-containing therapies, which reduce airway inflammation and provide bronchoprotection, improving symptom control and reducing exacerbation risk. ICS underuse due to poor adherence is common, leading to poor clinical outcomes including increased risk of mortality. This article reviews efficacy versus systemic activity profiles for various adherence patterns and dosing regimens of fluticasone furoate (FF)-containing and budesonide (BUD)-containing asthma therapies in clinical trials and real-world studies.
Methods
We performed a structured literature review (1 January 2000–3 March 2022) and mathematical modelling analysis of FF-containing and BUD-containing regular daily dosing in patients with mild-to-severe asthma, as-needed BUD/formoterol (FOR) in mild asthma, and BUD/FOR maintenance and reliever therapy (MART) dosing in moderate-to-severe asthma, to assess efficacy (bronchoprotection) and systemic activity (cortisol suppression) profiles of dosing patterns of ICS use in multiple adherence scenarios.
Results
A total of 22 manuscripts were included in full-text review and 18 in the model simulations. Focusing on FF-containing or BUD-containing treatments at comparable adherence rates, regular daily FF or FF/vilanterol (VI) dosing provided more prolonged bronchoprotection and fewer systemic effects than daily BUD, daily BUD/FOR, or BUD/FOR MART dosing, especially in low adherence scenarios. In model simulations and the real-world setting, FF/VI generally provided longer bronchoprotection, lower systemic activity, and greater clinical benefits over BUD/FOR as well as consistently higher adherence.
Conclusion
In this literature review and modelling analysis, FF/VI was found to show clinical advantages on asthma control over BUD/FOR. These findings have implications for helping clinicians select the most suitable inhaled therapy for their patients with asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Poor adherence to inhaled corticosteroid (ICS)-containing therapies is a common cause of poor asthma control and exacerbations. |
This structured literature review and mathematical modelling analysis explored efficacy (bronchoprotection) and systemic activity (cortisol suppression) profiles of daily fluticasone furoate (FF), FF/vilanterol (VI), and budesonide (BUD) in mild and moderate-to-severe asthma, and as-needed BUD/formoterol (FOR) in mild asthma, across a range of adherence scenarios. |
What was learned from the study? |
As-needed BUD/FOR use in mild asthma could leave patients without bronchoprotection most of the time. |
At comparable adherence rates for patients with moderate-to-severe asthma, FF/VI provided more a more favourable bronchoprotection profile and fewer systemic effects than BUD/FOR regular dosing and maintenance and reliever therapy. |
These findings may help clinicians select the most suitable therapy for asthma management and in advising patients. |
Introduction
The characteristic pathophysiological features of asthma include airway inflammation, airway hyperresponsiveness, and variable airflow limitation [1]. Inhaled corticosteroids (ICS) have been the cornerstone of asthma treatment for decades, as these drugs reduce airway inflammation and provide bronchoprotection, thereby improving symptom control and reducing the risk of exacerbations [1]. The underuse of ICS therapies due to inadequate adherence is common, leading to poor clinical outcomes [2]. Real-world studies have demonstrated that reduced adherence to ICS is associated with worse asthma outcomes [2, 3], including an increased risk of mortality [3]. To ensure that asthma is managed effectively, ICS dosing regimens that control the underlying inflammation without causing significant side effects are required [1].
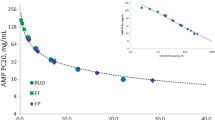
A therapeutic index (TI [systemic activity/airway potency ratio]) measures drug efficacy relative to adverse effects. We have previously compared the TI of budesonide (BUD), fluticasone furoate (FF), and fluticasone propionate (FP) in a randomized controlled trial (RCT) assessing bronchoprotection and cortisol suppression [4] as measures of airway efficacy and unwanted effects, respectively [4,5,6]. In this trial, adenosine-5′-monophosphate (AMP) challenges were performed, which is an established method for evaluating the bronchoprotective effects of ICS in asthma clinical studies [7,8,9]. The results showed that FF reduced airway hyperresponsiveness with less systemic activity and had the widest TI compared with FP or BUD [4]. In this AMP challenge model, the potency of FF was greater than both FP and BUD [4].
Using these data, we subsequently conduced a structured literature review and modelling analysis of efficacy versus systemic activity for various dosing regimens of two ICS-containing therapies, BUD and FP [10]. The review demonstrated that regular daily dosing with BUD or FP had higher airway efficacy and lower systemic activity (i.e. a higher TI) compared with symptom-driven, as-needed BUD/formoterol (FOR) dosing in mild asthma, and BUD/FOR maintenance and reliever therapy (MART) in moderate and moderate-to-severe asthma [10].
In the current study, we performed a structured literature review and modelling analysis to assess the airway efficacy and systemic safety of dosing regimens containing the short-acting ICS BUD, and the long-acting ICS FF. The literature review served to identify different regimens of FF, FF/vilanterol (VI), BUD, and BUD/FOR reported in RCTs and in real-world studies in order to apply them to the mathematical modelling analysis. We investigated efficacy (bronchoprotection) versus systemic activity (cortisol suppression) profiles across various levels of adherence for regular daily dosing with once-daily (OD) FF-containing and twice-daily (BID) BUD-containing asthma therapies in mild and moderate-to-severe asthma. As-needed BUD/FOR in patients with mild asthma and BUD/FOR MART in moderate-to-severe asthma was also studied.
Methods
Literature Review Search Strategy and Selection Criteria
This study comprised two separate literature searches. Firstly, a PubMed and Cochrane library search of manuscripts published between 1 January 2000 and 27 April 2021 was carried out, per the criteria used by Singh et al. [10], and adjusted to also include FF-containing regimens; this is further described below. Secondly, the above search was repeated to include FF manuscripts published later, between 27 April 2021 and 3 March 2022; the additional search period was incorporated to account for recent FF publications further to our previous publication [10]. As per structured literature search methodology, one reviewer screened all titles and abstracts of potential interest for full-text review and data extraction (including real-world studies); 20% of these articles were chosen by a random sequence generator for review by a second reviewer, and in the event of any disagreement over inclusion, both reviewers discussed further to reach a consensus.
Inclusion/exclusion criteria of the first literature search screen were published previously [10]. Complete inclusion/exclusion criteria for the additional FF search in the present study are detailed in Table 1. Briefly, publications were included at initial title screening if they met pre-defined criteria, namely an adult/adolescent population (≥ 12 years old); regular (OD/BID), as-needed, and/or MART ICS dosing; mild and/or moderate or moderate-to-severe persistent asthma; and appropriate study design (RCT, real-world data [RWD] or observational trials, meta-analysis or systematic literature review). Publications with titles describing studies of patients with moderate-to-severe asthma were also included for further review and analysis. All studies included FF, BUD, and/or FF-containing and BUD-containing ICS/long-acting beta agonist (LABA) therapies. Key exclusions were paediatric studies, studies including ICS/long-acting muscarinic antagonist or with no ICS therapies, and studies of patients with comorbid or controlled asthma. Articles agreed by the reviewers for inclusion (from the 20% of articles screened by two reviewers and the remaining 80% of articles screened by one reviewer) were subjected to full-text review. Following full-text review, data were extracted from studies deemed suitable for inclusion in the airway efficacy, systemic activity, and adherence simulations.
Data extracted included ICS dose (dose range; total, median and mean daily dosage) and dosage schedule (such as the number of inhalations per day, day’s use of ICS), while details on inhaler type were not collected, as described previously [10].
Airway Efficacy and Systemic Activity by ICS Dose and Adherence Simulations
The methodology for mild and moderate-to-severe asthma simulations was based upon that previously reported by Singh et al. [10], with different dosing and adherence simulations modelled using appropriate thresholds depending on asthma severity.
For mild asthma simulations, > 1 doubling dose (DD) and < 0.25 DD thresholds were used to assess the extent of airway efficacy (percentage of time bronchoprotection was provided or not, respectively). The former was calculated as the percentage of time during 28 days of treatment when there was bronchoprotection equating to a > 1.0 DD difference [10] from placebo in an AMP challenge test [7, 11]. The percentage of time below the 0.25 DD threshold means that no discernible bronchoprotection was provided with a treatment effect comparable to placebo [11]. Four different adherence simulations were considered (30%, 50%, 70% [10, 12,13,14,15,16,17,18], and 90% [19]).
For moderate-to-severe asthma simulations, > 2.0 DD and < 0.25 DD thresholds were used. A higher threshold for bronchoprotection (> 2.0 DD difference) assumed that higher ICS doses/bronchoprotective effects were required than the 1 DD threshold described above for mild asthma [10]; < 0.25 DD assumed no bronchoprotection. For BUD/FOR MART dosing, the same four different adherence simulations to the regular component of the MART regimen were considered (30%, 50%, 70%, and 90%) and combined with extra BUD/FOR reliever use. Simulation of BUD/FOR reliever usage was based on the number of reliever puffs/day reported in five BUD/FOR MART studies (range 0.59–1.08 as-needed puffs/day; mean 0.84 puffs/day) [20,21,22,23,24]. The generated pattern was further based on percentage of days with different BUD/FOR as-needed inhalation use (Supplementary Material Fig. S1), as reported by Stallberg et al. [23]. The applied pattern had a mean of 0.86 BUD/FOR reliever inhalations/day with no reliever use on 63% of days. Systemic activity was shown by the percentage of cortisol suppression, with 20% considered to be a reference point for a low suppression level [10, 25].
For the RWD simulations, the reported real-world adherence rates for FF/VI- and BUD/FOR-containing therapies (in publications identified by the literature search) [26,27,28,29,30,31] were considered alongside the extent of bronchoprotection and cortisol suppression (using > 2.0 DD and < 0.25 DD thresholds, respectively, for bronchoprotection and no bronchoprotection; and the 20% threshold for cortisol suppression) observed for the different ICS treatments.
This article is based on in silico modelling and simulation studies as well as previously conducted studies. It does not contain any new studies with human participants or animals performed by any of the authors.
Results
Literature Search and Inclusion of Studies in Model Simulations
The results of both literature (PubMed and Cochrane) searches were combined and duplicate articles removed, leaving 136 articles included in title and abstract screen (Fig. 1); 114 articles were excluded and 22 manuscripts were included in full-text review [4, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] . Four of these 22 manuscripts were discounted; three were meta-analyses or systematic literature reviews describing previously published data [37, 41, 43] and one had a small sample size (N = 15) [42]. A total of 18 studies were therefore considered for inclusion in the dose and adherence simulation models; six FF studies and 12 studies reporting data for BUD-containing regimens (n = 11 identified previously [10] and an additional publication of a BUD/FOR maintenance and reliever therapy (MART) regimen that was identified manually [20]) (Fig. 1).
After evaluation of the results obtained, individual studies reporting RCT data were not included in the modelling as all FF-containing studies reported adherence above 90%. The modelled data from these individual RCTs would therefore be near identical in time with bronchoprotection and cortisol suppression with values obtained from the 90% adherence simulations. In contrast, by selecting the six individual studies reporting RWD [26,27,28,29,30,31] for the modelling analyses, it was possible to simulate a wider range of real-world adherence scenarios—from 34.5% to 90.6% adherence—to complement the four different adherence simulations (30–90%) (Table 2).
ICS Efficacy on TI in Mild Asthma
Airway efficacy and systemic activity by dose and adherence in mild asthma simulations are shown in Fig. 2 and Supplementary Material Table S1.
Regular FF treatment demonstrated a better bronchoprotection profile than regular BUD treatment across the simulations for 30%, 50%, 70%, and 90% adherence. The percentage of time with no bronchoprotection (< 0.25 DD) increased significantly with suboptimal adherence to regular BUD use: this reached over 60% of time in patients with 30% adherence to regular BUD. For FF, the percentage of time with no bronchoprotection remained under 20% (i.e. ≤ 15%) for all adherence simulations.
For regular daily dosing with FF 100 µg OD and BUD 100 µg BID, systemic activity (cortisol suppression) was < 10%, (range 1.9–5.7% for FF, and 3.1–8.6% for BUD) in all adherence simulations. Systemic activity was slightly higher across respective adherence simulations for BUD 200 µg BID (range 6.0–15.8%). Cortisol suppression remained below the reference point of 20% for all regimens.
Across adherence simulations, regular ICS dosing regimens (apart from 30% adherence to BUD 100 µg BID) demonstrated greater bronchoprotection compared with BUD/FOR as-needed (28.1% of time with bronchoprotection > 1.0 DD). Systemic activity for BUD/FOR as needed (5.3%) was similar to the 90% adherence simulation for regular FF dosing (5.7%).
ICS Efficacy on TI in Moderate-to-Severe Asthma
Airway efficacy and systemic activity by ICS/LABA dose and adherence in the moderate-to-severe asthma simulations are shown in Fig. 3 and Supplementary Material Table S2. Plots for some of the model simulations are also shown in Fig. 4 for illustration.
Model predicted time course of ICS/LABA induced airway bronchoprotection for 28 days dosing in moderate-to-severe asthma. Dosing was regular daily maintenance with FF/VI 200/25 μg OD (left) and BUD/FOR 400/12 μg × 2 BID (middle), and MART with BUD/FOR 200/6 μg × 2 BID + PRN (right) with adherence scenario of 70% (top row) and 30% adherence (bottom row) to the regular daily maintenance component of the three dosing regimens. Horizontal dotted lines indicate thresholds for no treatment-related bronchoprotective effect defined as bronchoprotection less than an AMP PC20 of 16 mg/mL (0.25 DD) and a clinically significant bronchoprotective effect defined as bronchoprotection greater than an AMP PC20 of 54 mg/mL (2.0 DD). BID twice daily, BUD budesonide, DD doubling dose, FF fluticasone furoate, FOR formoterol, OD once daily, MART maintenance and reliever therapy, PRN as needed
Across each respective adherence simulation (30%, 50%, 70%, and 90%), regular OD dosing with FF/VI 200/25 µg demonstrated a better bronchoprotection profile than regular daily dosing with BUD/FOR regardless of dose in moderate-to-severe asthma. BUD/FOR MART dosing showed bronchoprotection results that were more similar to BUD/FOR than FF/VI dosing regimens across adherence simulations.
Considering time with > 1.0 DD bronchoprotection, FF/VI OD 100/25 µg demonstrated significantly more time with bronchoprotection at this threshold than BUD/FOR dosing regimens at all comparable adherence scenarios, regardless of regular or MART use (Supplementary Material Table S2). Across all adherence simulations, the time with no bronchoprotection for FF/VI did not exceed 20%, whereas for all BUD/FOR dosing regimens, this time exceeded 20% unless adherence was at least 70% (or 90% for BUD/FOR 200/6 µg BID [22% for 70% adherence with this dose]); at 30% adherence, the time with no bronchoprotection ranged from 47.8% to 60.1% with regular BUD/FOR regimens and from 38.8% to 40.8% with BUD/FOR MART dosing regimens.
Both modelled BUD/FOR MART doses had a better bronchoprotection profile than regular dosing with BUD/FOR at 30% adherence. Bronchoprotection > 2.0 DD was achieved for less than 70% of the time for any adherence simulation with regular daily dosing of 400 µg BUD, given either as BUD/FOR 200/6 µg BID or low-dose BUD/FOR MART.
Systemic activity was notably lower with regular daily FF/VI dosing (< 11% across all adherence simulations) than BUD/FOR dosing regimens, which were generally above 10% cortisol suppression. Both BUD/FOR MART dosing scenarios had cortisol suppression above 10%, even at 30% adherence, reaching 21.8% with low-dose MART, and 31.8% with medium-dose MART at 90% adherence. Regular BUD/FOR exceeded 20% cortisol suppression for 70% and 90% adherence to BUD/FOR 200/6 µg × 2 BID (800 µg daily) and for all adherence scenarios of BUD/FOR 400/12 µg × 2 BID (1600 µg daily). The latter reached a maximum of 43% at 90% adherence.
RWD Studies
Airway efficacy and systemic activity by dose and adherence in the RWD simulations are shown in Fig. 5 and Supplementary Material Table S3. All but one study compared both fixed dose combinations in the same patient populations, and all identified studies reported data from patients using ICS/LABA combinations, therapies which are not prescribed for mild asthma, and therefore by definition the patients in these studies were considered to have moderate-to-severe asthma. Modelling of the various adherence simulations to FF/VI and BUD/FOR using > 2.0 DD improvement showed that bronchoprotection increased with ICS dose. Adherence rates in these RWD studies ranged from 43% to 90.6% for FF/VI and 34.5–78.2% for BUD/FOR. In studies where both therapies were assessed, adherence (mean percentage of days covered) reported for FF/VI was higher by 5.3–10.8% compared with BUD/FOR [26, 28, 31]. More time with bronchoprotection (> 2.0 DD), less time with no bronchoprotection (< 0.25 DD), and lower cortisol suppression were shown for FF/VI compared with BUD/FOR. When adherence to FF was 90.6% (as reported in the RWD study by Dal Negro et al. [27]), bronchoprotection > 2.0 DD was 91.4%. Systemic activity was greater with all BUD/FOR doses compared with FF/VI.
Discussion
This modelling study investigated the relationship between ICS dosing regimens and treatment efficacy/safety for FF-containing OD and BUD-containing BID treatments in mild and moderate-to-severe asthma. Our simulations in mild asthma showed that regular daily FF dosing provided a better bronchoprotection profile than daily BUD dosing and had a better systemic safety profile at all adherence thresholds; the shortest bronchoprotection time was associated with as-needed BUD/FOR. Regular FF/VI OD also demonstrated a better bronchoprotection profile and lower systemic activity than BUD/FOR in moderate-to-severe asthma. Overall, RWD studies reported higher adherence rates for FF/VI than BUD/FOR, with modelling showing that FF/VI was associated with longer bronchoprotection and lower systemic activity than BUD/FOR.
In our previous RCT using AMP challenges, the long duration of action of FF, along with its high affinity for the glucocorticoid receptor [25], facilitated greater bronchoprotection and fewer systemic effects compared with BUD. The numerical difference between the AMP provocation dose producing a 20% fall in forced expiratory volume in 1 s (FEV1; PC20) and median effective dose values for FP and BUD was broadly in line with their relative glucocorticoid receptor binding affinities [4, 25]. Here, we show how these pharmacological properties translate into differences in TI in different scenarios of asthma severity and ICS dosing regimens. Importantly, the long duration of action of FF means that bronchoprotection can be maintained even with suboptimal adherence. In contrast, suboptimal adherence to regular daily BUD, or the use of as-needed BUD/FOR, can result in extended periods of no bronchoprotection, which can allow persistent inflammation, thereby increasing the risk of exacerbations [10]. Furthermore, regular daily FF dosing was associated with a lower level of cortisol suppression than regular daily BUD dosing. The comparable cortisol suppression (< 6%) for regular FF and as-needed BUD/FOR in mild asthma indicates that there should be minimal concerns about the risks of systemic adverse effects with low doses of the longer-acting ICS.
In moderate-to-severe asthma, the model simulations showed a more favourable bronchoprotection profile with regular daily dosing of FF/VI than with BUD/FOR (regular daily dosing or MART). The results were similar for FF/VI 200/25 µg and BUD/FOR 400/12 µg (1600 µg daily BUD), with around 90% of time of bronchoprotection provided with either regimen when adherence was 90%, although cortisol suppression was much lower for FF/VI. Higher ICS doses (within recommended dose range) reduce the underlying airway inflammation [47] and improve lung function [48] to a greater extent than lower doses. The level of adherence required to reach bronchoprotection (> 1.0 DD) > 70% of the time was ≥ 70% across both ICS analysed, which indicates that to achieve sustained bronchoprotection in moderate-to-severe asthma, it is important to give an adequate ICS dose and achieve good adherence to therapy.
Overall, our analysis showed that airway efficacy appeared to be broadly similar for regular daily dosing of BUD/FOR compared with the BUD/FOR MART regimens in moderate-to-severe asthma, although the total regular daily dose of BUD 1600 µg (and 90% adherence) provided the highest degree of bronchoprotection. The added clinical benefit of high adherence to regular daily ICS dosing when compared with as-needed dosing or MART was also the key finding of our previous analysis [10].
As seen in the mild asthma simulation, systemic activity in moderate-to-severe asthma was notably lower with FF/VI than with BUD/FOR therapy. Cortisol suppression was frequently high (≥ 20%) with BUD/FOR daily dosing (≥ 800 µg daily BUD) or BUD/FOR MART regimens across multiple adherence thresholds. The highest degree of bronchoprotection, provided by 90% adherence to BUD 1600 µg/day, comes at the expense of causing the highest level of cortisol suppression (43%). Given the OD and BID dosing difference between regular FF/VI and BUD/FOR, the number of inhalations required to achieve higher bronchoprotection for FF/VI was found to be lower compared with regular BUD/FOR. Compared with FF/VI, BUD/FOR MART required a significantly higher number of ICS/LABA inhalations (ranging from 3 to 7.25 times more inhalations). This suggests that once-daily FF/VI offers higher bronchoprotection and lower systemic activity with a less complex dosing regimen compared with MART, making FF/VI a seemingly easier regimen to adhere to as this is achieved with a fraction of required inhalations.
Our findings support a previous study [4] that has questioned the validity of the current approach to ICS dose equivalence stated in asthma treatment guidelines, because the higher efficacy of FF can be achieved with lower systemic activity compared with BUD. Our results show differences between ICS/LABA combinations that clinicians should be aware of, with the newer-generation ICS FF demonstrating clinical benefits beyond the practical benefit of OD dosing, attributable to the highest glucocorticoid receptor affinity and longest duration of action of any available ICS to date. The real-world evidence for FF/VI and regular BUD/FOR has consistently demonstrated an adherence benefit for FF/VI. Even though few studies to date have assessed the adherence to MART in the real world [49], it is unlikely that patients would be more adherent to the regular component of MART compared with regular ICS/LABA regimens. Furthermore, many patients on MART regimen request additional rescue inhalers, indicating both a perceived inadequate asthma control with this regimen and a misapplication of MART dosing in clinical practice [50, 51]. For as-needed BUD/FOR dosing, disparate clinical trial findings [52,53,54,55] have resulted in non-universal adoption by different asthma treatment guidelines and health authorities, meaning that this therapeutic option currently remains off-label for many countries [51, 56].
RWD studies provide the opportunity to evaluate effectiveness of ICS in clinical practice, monitor patient use of ICS over time [2], and examine the potential impact of different dosing regimens on adherence and associated asthma outcomes. Six RWD studies were included in the literature review/model simulation; two studies found that regular daily dosing of FF/VI improved lung function and asthma control, or reduced the likelihood of requiring hospital care for exacerbations, without compromising safety [27, 30]. These findings are supported by an analysis of data from phase 2/3 trials of FF in asthma, which demonstrated significant increases in FEV1 compared with placebo, without any evidence of cortisol suppression at 100 µg and 200 µg OD [57]. Four RWD studies demonstrated that the initiation of regular FF/VI OD dosing led to generally better treatment persistence or adherence than regular ICS/FOR dosing [26, 28, 29, 31]. Consequently, real-world evidence to date has shown that FF/VI provided benefits for lung function, asthma control, and reduced reliever use and risk of exacerbations compared with regular ICS/FOR [27, 29, 30]. This is an important finding as it is well recognized that poor adherence to ICS therapy can have a negative impact on asthma outcomes [12, 29, 31, 58, 59].
The RWD model simulation results were generally consistent with the mild and moderate-to-severe asthma simulations. The degree of bronchoprotection with FF/VI and BUD/FOR increased with dose and was maximized when adherence was high. As expected, systemic activity was notably higher with higher BUD doses compared with FF. A previous study showed that 3 weeks of regular daily BUD 1600 µg caused marked cortisol suppression [60]. Another study found the potential for systemic glucocorticoid potency of BUD to be 12.3 times greater than prednisone in patients with moderate-to-severe asthma not dependent on prednisone [61]. In contrast, 6 weeks of regular FF/VI 100/25 µg and 200/25 µg had no significant effect on cortisol [62]. The difference in cortisol suppression for FF and BUD is clinically important because it is well established that higher cortisol suppression increases the risk for systemic adverse events such as bone fractures, osteoporosis, and glaucoma [63]. Although these adverse events are mainly thought to be associated with oral corticosteroid (OCS) use, high doses of BUD have consistently been demonstrated to cause cortisol suppression in the range of maintenance OCS use [60, 61]. For example, Toogood et al. have shown that BUD doses ≥ 1840 µg daily carry a potential risk of systemic adverse effects comparable to that associated with ≥ 15 mg daily prednisone [61]. At these levels, dose-dependent relationships with bone mass attrition and occurrence of fractures are known to occur [61].
A potential limitation of this study was that we did not separate out a possible effect of LABA on bronchoprotection, though this is anticipated to be minimal since the bronchoprotection outcome was based on the AMP challenge test, which measures mast cell activity and neuronal signalling-regulated airway smooth muscle tone [9]. In addition, most current inhaled treatments contain ICS and LABA in the same device, not ICS alone. Therefore, separating the possible effect of both types of molecules would not be of much practical interest. A further limitation was the focus on FF/VI and BUD/FOR regimens rather than a wider range of ICS/LABA therapies. The number of identified published studies was also low. Most studies located from the literature search were RCTs reporting adherence over 90%, and therefore bronchoprotection and cortisol suppression are expected to be in line with the 90% adherence simulation reported.
A strength of our study was that the model simulations included a wide range of adherence scenarios and RWD, helping to provide a more complete picture of the impact of ICS dosing regimens and adherence on airway efficacy and safety in patients with mild or moderate-to-severe asthma. No RWD for FF in mild asthma or BUD/FOR MART in moderate-to-severe asthma were identified. To model BUD/FOR MART, accurate information on both maintenance and reliever usage patterns is needed. Our literature search identified several clinical trials reporting MART reliever puffs/day: only one trial reported a MART reliever use pattern [23], which we based our calculations on. Whilst adherence to MART was high owing to the controlled nature of clinical trials, it is expected that in the real world adherence would be suboptimal and thus we calculated the same adherence range used for regular daily therapy. MART reliever use was kept at the rate (0.86 puffs/day) reported in clinical trials. Since no studies have reported real-world adherence to MART maintenance and reliever use to date, it was not possible to ascertain how MART therapy is used in the real world, which is a weakness of the MART regimen.
Conclusions
At comparable adherence rates, we observed that FF/VI provided a more favourable and better bronchoprotection profile and fewer systemic effects than BUD/FOR regular dosing and MART in moderate-to-severe asthma. Likely as a result of the ultra-long duration of action of FF, this positive effect was more pronounced in low-adherence settings. As-needed BUD/FOR use in mild asthma could leave patients without bronchoprotection most of the time, a problem which can be avoided by using regular regimes containing BUD with good adherence or FF with any level of adherence.
Change history
07 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12325-023-02645-4
References
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (2022 update). 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf. Accessed Mar 2023.
Vervloet M, van Dijk L, Spreeuwenberg P, et al. The relationship between real-world inhaled corticosteroid adherence and asthma outcomes: a multilevel approach. J Allergy Clin Immunol Pract. 2020;8:626–34.
Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332–6.
Daley-Yates P, Brealey N, Thomas S, et al. Therapeutic index of inhaled corticosteroids in asthma: a dose-response comparison on airway hyperresponsiveness and adrenal axis suppression. Br J Clin Pharmacol. 2021;87:483–93.
Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100:1307–17.
Pandya D, Puttanna A, Balagopal V. Systemic effects of inhaled corticosteroids: an overview. Open Respir Med J. 2014;8:59–65.
Joos GF, O’Connor B, Anderson SD, et al. Indirect airway challenges. Eur Respir J. 2003;21:1050–68.
Phillips K, Oborne J, Harrison TW, Tattersfield AE. Use of sequential quadrupling dose regimens to study efficacy of inhaled corticosteroids in asthma. Thorax. 2004;59:21–5.
Singh D, Fairwood J, Murdoch R, et al. The reproducibility of adenosine monophosphate bronchial challenges in mild, steroid-naive asthmatics. Br J Clin Pharmacol. 2008;66:261–5.
Singh D, Garcia G, Maneechotesuwan K, et al. New versus old: the impact of changing patterns of inhaled corticosteroid prescribing and dosing regimens in asthma management. Adv Ther. 2022;39:1895–914.
Daley-Yates P, Aggarwal B, Lulic Z, Fulmali S, Cruz AA, Singh D. Pharmacology versus convenience: a benefit/risk analysis of regular maintenance versus infrequent or as-needed inhaled corticosteroid use in mild asthma. Adv Ther. 2022;39:706–26.
Bårnes CB, Ulrik CS. Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respir Care. 2015;60:455–68.
Bender B, Wamboldt FS, O’Connor SL, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol. 2000;85:416–21.
Cerveri I, Locatelli F, Zoia MC, Corsico A, Accordini S, de Marco R. International variations in asthma treatment compliance: the results of the European Community Respiratory Health Survey (ECRHS). Eur Respir J. 1999;14:288–94.
Eakin MN, Rand CS. Improving patient adherence with asthma self-management practices: what works? Ann Allergy Asthma Immunol. 2012;109:90–2.
Makela MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107:1481–90.
Mulgirigama A, Barnes N, Fletcher M, Pedersen S, Pizzichini E, Tsiligianni I. A review of the burden and management of mild asthma in adults—implications for clinical practice. Respir Med. 2019;152:97–104.
Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009.
National Institute for Health and Care Excellence. Scenario: acute exacerbation of asthma. 2023. https://cks.nice.org.uk/topics/asthma/management/acute-exacerbation-of-asthma/. Accessed Mar 2023.
Aubier M, Buhl R, Ekstrom T, et al. Comparison of two twice-daily doses of budesonide/formoterol maintenance and reliever therapy. Eur Respir J. 2010;36:524–30.
Bousquet J, Boulet LP, Peters MJ, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med. 2007;101:2437–46.
Kuna P, Peters MJ, Manjra AI, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61:725–36.
Stallberg B, Naya I, Ekelund J, Eckerwall G. Real-life use of budesonide/formoterol in clinical practice: a 12-month follow-up assessment in a multi-national study of asthma patients established on single-inhaler maintenance and reliever therapy. Int J Clin Pharmacol Ther. 2015;53:447–55.
Vogelmeier C, D’Urzo A, Pauwels R, et al. Budesonide/formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur Respir J. 2005;26:819–28.
Daley-Yates PT. Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br J Clin Pharmacol. 2015;80:372–80.
Averell CM, Stanford RH, Laliberte F, Wu JW, Germain G, Duh MS. Medication adherence in patients with asthma using once-daily versus twice-daily ICS/LABAs. J Asthma. 2021;58:102–11.
Dal Negro RW, Bonadiman L, Turco P. Fluticasone furoate/vilanterol 92/22 µg once a day: a 12-month study on outcomes in mild to moderate asthma. Ther Adv Respir Dis. 2018;12:1753466618789894.
Parimi M, Svedsater H, Ann Q, et al. Persistence and adherence to ICS/LABA drugs in UK patients with asthma: a retrospective new-user cohort study. Adv Ther. 2020;37:2916–31.
Sicras-Mainar A, Gomez Rodriguez B, Traseira-Lugilde S, Fernandez-Sanchez T, Velasco Garrido JL. Treatment persistence and exacerbations in patients with asthma initiating treatment with inhaled corticosteroids and beta-adrenergic agonists: retrospective cohort study. BMJ Open. 2022;12: e053964.
Woodcock A, Vestbo J, Bakerly ND, et al. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: an open-label, parallel group, randomised controlled trial. Lancet. 2017;390:2247–55.
Stanford RH, Averell C, Parker ED, Blauer-Peterson C, Reinsch TK, Buikema AR. Assessment of adherence and asthma medication radio for a once-daily and twice-daily inhaled corticosteroid/long-acting beta-agonist for asthma. J Allergy Clin Immunol Pract. 2019;7(1488–96): e7.
Bleecker ER, Bateman ED, Busse WW, et al. Once-daily fluticasone furoate is efficacious in patients with symptomatic asthma on low-dose inhaled corticosteroids. Ann Allergy Asthma Immunol. 2012;109(353–8):e4.
Busse WW, O’Byrne PM, Bleecker ER, et al. Safety and tolerability of the novel inhaled corticosteroid fluticasone furoate in combination with the beta2 agonist vilanterol administered once daily for 52 weeks in patients >=12 years old with asthma: a randomised trial. Thorax. 2013;68:513–20.
Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting beta(2)-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111–20.
Kempsford RD, Oliver A, Bal J, Tombs L, Quinn D. The efficacy of once-daily fluticasone furoate/vilanterol in asthma is comparable with morning or evening dosing. Respir Med. 2013;107:1873–80.
Lotvall J, Bleecker ER, Busse WW, et al. Efficacy and safety of fluticasone furoate 100 mug once-daily in patients with persistent asthma: a 24-week placebo and active-controlled randomised trial. Respir Med. 2014;108:41–9.
Rodrigo GJ, Plaza V. Once-daily fluticasone furoate and vilanterol for adolescents and adults with symptomatic asthma: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2016;116:565–70.
Shimizu Y, Shiobara T, Arai R, Chibana K, Takemasa A. Real-life effectiveness of fluticasone furoate/vilanterol after switching from fluticasone/salmeterol or budesonide/formoterol therapy in patients with symptomatic asthma: Relvar Ellipta for Real Asthma Control Study (RERACS study). J Thorac Dis. 2020;12:1877–83.
Woodcock A, Bateman ED, Busse WW, et al. Efficacy in asthma of once-daily treatment with fluticasone furoate: a randomized, placebo-controlled trial. Respir Res. 2011;12:132.
Woodcock A, Lotvall J, Busse WW, et al. Efficacy and safety of fluticasone furoate 100 mug and 200 mug once daily in the treatment of moderate-severe asthma in adults and adolescents: a 24-week randomised study. BMC Pulm Med. 2014;14:113.
Tong X, Liu T, Li Z, Liu S, Fan H. Is it really feasible to use budesonide-formoterol as needed for mild persistent asthma? A systematic review and meta-analysis. Front Pharmacol. 2021;12: 644629.
Hozawa S, Terada M, Haruta Y, Hozawa M. Comparison of early effects of budesonide/formoterol maintenance and reliever therapy with fluticasone furoate/vilanterol for asthma patients requiring step-up from inhaled corticosteroid monotherapy. Pulm Pharmacol Ther. 2016;37:15–23.
Svedsater H, Stynes G, Wex J, et al. Once-daily fluticasone furoate/vilanterol versus twice daily combination therapies in asthma-mixed treatment comparisons of clinical efficacy. Asthma Res Pract. 2016;2:4.
Bateman ED, Bleecker ER, Lotvall J, et al. Dose effect of once-daily fluticasone furoate in persistent asthma: a randomized trial. Respir Med. 2012;106:642–50.
Busse WW, O'Byrne PM, Bleecker ER, Lotvall J, Woodcock A, Andersen L, et al. Safety and tolerability of the novel inhaled corticosteroid fluticasone furoate in combination with the beta2 agonist vilanterol administered once daily for 52 weeks in patients >=12 years old with asthma: a randomised trial. Thorax. 2013;68:513–20.
O'Byrne PM, Woodcock A, Bleecker ER, Bateman ED, Lotvall J, Forth R, et al. Efficacy and safety of once-daily fluticasone furoate 50 mcg in adults with persistent asthma: a 12-week randomized trial. Respir Res. 2014;15:88.
Tukiainen H, Taivainen A, Majander R, et al. Comparison of high and low dose of the inhaled steroid, budesonide, as an initial treatment in newly detected asthma. Respir Med. 2000;94:678–83.
Masoli M, Holt S, Weatherall M, Beasley R. Dose-response relationship of inhaled budesonide in adult asthma: a meta-analysis. Eur Respir J. 2004;23:552–8.
Sekibag Y, Borekci S, Gemicioglu B. Adherence, quality of life, and satisfaction with conventional fix combined therapy versus maintenance and reliever therapy in patients with asthma after inhaler training. J Asthma. 2022;59:1819–30.
Chapman KR, An L, Bosnic-Anticevich S, et al. Asthma patients’ and physicians’ perspectives on the burden and management of asthma. Respir Med. 2021;186:106524.
Chapman KR, Canonica GW, Lavoie KL, et al. Patients’ and physicians’ perspectives on the burden and management of asthma: results from the APPaRENT 2 study. Respir Med. 2022;201:106948.
O’Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med. 2018;378:1865–76.
Bateman ED, Reddel HK, O’Byrne PM, et al. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med. 2018;378:1877–87.
Beasley R, Holliday M, Reddel HK, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med. 2019;380:2020–30.
Hardy J, Baggott C, Fingleton J, et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394:919–28.
Plaza V, Alobid I, Alvarez C, et al. [Translated article] Spanish Asthma Management Guidelines (GEMA) v.5.1. highlights and controversies. Arch Bronconeumol. 2022;58:T150–8.
O’Byrne PM, Jacques L, Goldfrad C, et al. Integrated safety and efficacy analysis of once-daily fluticasone furoate for the treatment of asthma. Respir Res. 2016;17:157.
Averell CM, Laliberte F, Germain G, et al. Impact of adherence to treatment with inhaled corticosteroids/long-acting beta-agonists on asthma outcomes in the United States. Ther Adv Respir Dis. 2022;16:17534666221116996.
Williams LK, Peterson EL, Wells K, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol. 2011;128(1185–91):e2.
Wilson AM, Lipworth BJ. Dose-response evaluation of the therapeutic index for inhaled budesonide in patients with mild-to-moderate asthma. Am J Med. 2000;108:269–75.
Toogood JH, Baskerville J, Jennings B, Lefcoe NM, Johansson SA. Bioequivalent doses of budesonide and prednisone in moderate and severe asthma. J Allergy Clin Immunol. 1989;84:688–700.
Allen A, Schenkenberger I, Trivedi R, et al. Inhaled fluticasone furoate/vilanterol does not affect hypothalamic-pituitary-adrenal axis function in adolescent and adult asthma: randomised, double-blind, placebo-controlled study. Clin Respir J. 2013;7:397–406.
Derendorf H, Nave R, Drollmann A, Cerasoli F, Wurst W. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. Eur Respir J. 2006;28:1042–50.
Acknowledgements
Funding
This study was funded by GSK, the study sponsor is also funding the journal’s Rapid Service Fee and the Open Access Fee.
Medical Writing/Editorial Assistance
Medical writing and editorial assistance (in the form of conducting the literature search, performing data extraction/analysis for the literature review/modelling analyses, and manuscript development [drafting of manuscript content, collating author comments, grammatical editing and support with figures/tables]) was provided by Joanna Wilson, PhD, and Malgorzata Urbacz, of Ashfield MedComms, an Inizio company, and was funded by GSK. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Peter Daley-Yates devised the concept of this literature review, assisted with the literature search and data analysis, and performed the airway efficacy and systemic activity modelling; all authors contributed to drafting and/or critically revising the work. Dave Singh is supported by the National Institute for Health Research Manchester Biomedical Research Centre.
Disclosures
Peter Daley-Yates was an employee of and shareholder in GSK at the time of study; Dave Singh declares receipt of personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Genentech, GSK, Glenmark, Gossamerbio, Menarini, Novartis, Pfizer, Pulmatrix, Teva, Theravance and Verona; Juan M Igea declares receipt of personal fees for Allergopharma, Allergy Therapeutics, AstraZeneca, Chiesi, FAES Farma, GSK, Imnmunotek, Menarini, Merck, Mundi Pharma, Novartis, Pfizer and TEVA; Luigi Macchia reports personal conference fees from AstraZeneca, GSK and Sanofi, and research grants from Chiesi, GSK and Sanofi; Manish Verma and Maximillian Plank are employees of GSK and hold shares; Norbert Berend holds shares in GSK, and reports speaker fees from GSK, AstraZeneca, Boehringer Ingelheim and Pfizer.
Compliance with Ethics Guidelines
This article is based on in silico modelling and simulation studies as well as previously conducted studies. It does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Peter Daley-Yates affiliation as GSK, Uxbridge, UK at time of study.
This article was revised due to update in text.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Daley-Yates, P., Singh, D., Igea, J.M. et al. Assessing the Effects of Changing Patterns of Inhaled Corticosteroid Dosing and Adherence with Fluticasone Furoate and Budesonide on Asthma Management. Adv Ther 40, 4042–4059 (2023). https://doi.org/10.1007/s12325-023-02585-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02585-z