Abstract
Introduction
For high responders with polycystic ovary syndrome (PCOS), there is no clear recommendation for the initial follicle-stimulating hormone (FSH) dosage to ensure an optimal number of retrieved oocytes and avoid ovarian hyperstimulation syndrome (OHSS). The aim of this study was to determine the ideal initial FSH dosage of in patients with PCOS undergoing in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) using the gonadotropin-releasing hormone antagonist (GnRH-ant) protocol to obtain the optimal number of retrieved oocytes and minimize the risk of OHSS.
Methods
The data of 1898 patients with PCOS aged 20–40 years from January 2017 to December 2020 were retrospectively analyzed to explore the factors related to the number of retrieved oocytes. Statistically significant variables were used to construct a dose nomogram and it was then validated using an independent cohort of patients with PCOS from January 2021 to December 2021.
Results
Multivariate analyses demonstrated that body mass index (BMI) was the most significant factor to predict the number of retrieved oocytes compared to body weight (BW) and body surface area (BSA). Among patients with PCOS aged 20–40 years undergoing their first IVF cycles with the GnRH-ant protocol, age was not a significant predictor of the initial FSH dosage. We developed a nomogram based on BMI, basal FSH, basal luteinizing hormone (bLH), anti-Müllerian hormone (AMH), and antral follicle count (AFC) to calculate the ideal initial FSH dosage for patients with PCOS undergoing IVF/ICSI using the GnRH-ant protocol. In addition, low BMI and high bLH and AMH levels and AFC appear to be risk factors for OHSS.
Conclusions
We clearly demonstrated that the initial FSH dosage for patients with PCOS undergoing IVF/ICSI with the GnRH-ant protocol may be calculated on the basis of the woman’s BMI and ovarian reserve markers. The nomogram will help guide clinicians in the selection of the most appropriate initial FSH dose in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
The aim of this study was to determine the ideal initial FSH dosage of in patients with PCOS undergoing IVF/ICSI with GnRH-ant protocol to obtain the optimal number of retrieved oocytes and minimize the risk of OHSS |
What was learned from the study? |
BMI was the most significant factor to predict the number of retrieved oocytes compared to BW and BSA |
Age was not a significant predictor of the initial FSH dosage among patients with PCOS aged 20–40 years undergoing their first IVF cycles with the GnRH-ant protocol |
A nomogram based on BMI, basal FSH, basal LH, AMH, and AFC was developed to calculate the ideal initial FSH dosage for patients with PCOS undergoing IVF/ICSI using the GnRH-ant protocol |
A low BMI, high basal LH and AMH levels, and a high AFC appear to be risk factors for ovarian hyperstimulation syndrome |
Introduction
Polycystic ovary syndrome (PCOS) is the most common reproductive endocrine and metabolic disorder among women and affects 5–18% thereof [1,2,3,4]. The clinical presentation of PCOS is highly heterogeneous [5]. Approximately, 15–20% of infertile women suffer from PCOS [6], and it is reportedly the leading cause of anovulatory infertility in 80% of patients [7].
In vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI)-embryo transfer is an effective treatment for patients with PCOS having fertility desires who have not become pregnant after receiving ovulation stimulation with clomiphene citrate/letrozole or who have combined tubal and/or male factor infertility [8]. The gonadotropin-releasing hormone antagonist (GnRH-ant) protocol is preferred for women with PCOS because of its short ovarian stimulation time, reduced treatment dosage, and low incidence of ovarian hyperstimulation syndrome (OHSS) [9, 10]. A lower starting dosage of follicle-stimulating hormone (FSH) results in poor follicle growth, significantly reduced number of retrieved oocytes, and significantly higher cycle cancellation rates. However, a higher initial FSH dosage greatly increases the risk of an excessive response and OHSS, and high levels of estradiol may also affect endometrial receptivity. Therefore, an appropriate starting dosage of FSH is thought to improve IVF/ICSI clinical outcomes in patients with PCOS. On the basis of existing domestic and foreign evidence, 15 retrieved oocytes maximizes the live birth rate during fresh embryo transfer [11,12,13,14,15]. As stated in a recent meta-analysis, 15 oocytes was the most common suggestion to be the optimal number of oocytes at which fresh live birth seems to be maximized [16]. Steward et al. found that more than 15 retrieved oocytes are the best predictor of OHSS risk with an analysis of 256,381 cycles [12]. Thus, we considered 15 retrieved oocytes to be the optimal oocyte yield to both maximize the live birth rate of fresh embryo transfer and minimize the risk of OHSS.
Clinicians commonly determine the initial FSH dosage according to age, individual body weight (BW), body mass index (BMI), ovarian response to previous IVF cycles, and markers of ovarian reserve including FSH, anti-Müllerian hormone (AMH), and antral follicle count (AFC) [17,18,19,20,21,22,23]. Few researchers proposed models to calculate the most appropriate starting FSH dosage in IVF cycles with a long GnRH agonist protocol based on patient age, basal FSH level, and AFC or AMH level to help patients achieve an optimal response [24,25,26]. The aim of the present retrospective analysis was to identify the factors that affect the number of retrieved oocytes and to develop a dose nomogram to suggest the appropriate initial FSH dosage for women with PCOS undergoing their first IVF/ICSI cycles with the GnRH-ant protocol.
Methods
Study Population
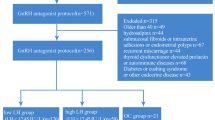
Women with PCOS who underwent their first, fresh IVF/ICSI-ET cycles from January 2017 to December 2021 at the Reproductive Center of Peking University Third Hospital were evaluated. Patient data and information on cycles were extracted from the electronic record system and medical records. In the primary cohort, 1898 patients that were treated from 2007 to 2020 were assigned to the training group and used to develop the nomogram model. An independent cohort of 593 patients treated from 2020 to 2021 were assigned to the testing group and used to validate the model. This study was approved by the Ethics Committee of Reproductive Medicine at Peking University Third Hospital (No. 2021SZ-011). The study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from the patients for the IVF procedure and to record and use their clinically relevant information for research purposes.
Inclusion Criteria
The inclusion criteria were as follows: (1) reproductive-age women between 20 and 40 years old; (2) women with a PCOS diagnosis according to the revised 2003 Rotterdam diagnostic criteria, meeting at least two of the three criteria—oligo- and/or anovulation, clinical and/or biochemical signs of hyperandrogenism (androstenedione > 11.5 nmol/L, testosterone > 2.53 nmol/L used in our center), and polycystic ovaries (≥ 12 follicles per ovary)—with the exclusion of other etiologies (congenital adrenal hyperplasia, androgen-secreting tumors, Cushing’s syndrome); (3) women undergoing their first IVF/ICSI cycle; (4) women undergoing controlled ovarian stimulation treatment with the GnRH-ant protocol; and (5) women with complete patient information on clinical and IVF cycle characteristics.
Exclusion Criteria
The exclusion criteria were as follows: (1) chromosomal abnormality in one member of the couple; (2) diseases or surgeries of the ovaries, such as endometriotic cysts of the ovary, ovarian tumor, ovarian drilling, or history of ovarian cyst or tumor removal surgery; and (3) in vitro maturation cycles.
GnRH-Ant Protocol
Gonadotropin (Gn, FSH in this study) was injected daily beginning on the second or third day of the menstrual cycle. Transvaginal ultrasound will be done from the fifth day of ovarian stimulation to monitor the development of the ovarian follicles to adjust the dose of medication according to the ovarian response. There were two options for the timing of GnRH-ant administration: (1) a fixed dosing regimen, in which an antagonist was added on the fifth to seventh days after Gn administration; and (2) a flexible dosing regimen, in which the antagonist was added when the dominant follicle reached 12 mm in diameter until the trigger day. Patients with PCOS with a high risk of OHSS from GnRH-ant cycles were commonly administered GnRH-a (0.2 mg) as a trigger or in combination with low-dose hCG when at least three follicles were larger than 17 mm in diameter. Recombinant urinary human chorionic gonadotropin (hCG) was injected only in those patients with a lower risk of OHSS. Oocyte retrieval was performed after 36–38 h.
Statistical Analysis
All statistical analyses were performed using the IBM SPSS software (version 25.0, IBM SPSS Inc., Chicago, IL). Continuous variables are expressed as mean and standard deviation (SD). Categorical variables are presented as frequencies and percentages. Body surface area (BSA) was calculated using Stevenson’s formula for Chinese women as follows:
Univariate and multivariate analyses were performed to assess risk factors for the number of retrieved oocytes using general and multiple linear regression models. Regression coefficients or partial regression coefficients (β) and 95% confidence intervals (CIs) were used to estimate the effects on the number of retrieved oocytes. We established a multiple regression model using retrieved oocyte numbers, initial FSH dosage, and other factors influencing the number of retrieved oocytes. Stepwise backward selection using p values was applied to the multiple regression model for variable selection. To estimate the ideal initial FSH dosage, we set the optimal number of retrieved oocytes as 15. Furthermore, we analyzed the difference between the actual and ideal initial FSH dosages among different oocyte retrieval groups using a t test. Statistical significance was considered at p < 0.05.
Results
Population Characteristics
We included 2491 patients with PCOS who had undergone IVF/ICSI cycles with the GnRH-ant protocol from January 2017 to December 2021 (Fig. 1). Patient and IVF cycle characteristics are summarized in Table 1 including 1898 patients in training set and 593 patients in testing set. The majority of patients with PCOS in this study were under 35 years of age (89.4% and 88.0%, respectively). Almost half of the population were overweight (BMI 24–28 kg/m2) and obese (BMI ≥ 28 kg/m2). The mean numbers of retrieved oocytes were 17.31 ± 9.88 (range 1–76) and 17.67 ± 9.88 (range 1–77) in the training set and testing set, respectively. More than half of patients underwent frozen embryo transfer (57.2% and 65.1%, respectively).
Factors Associated with Number of Retrieved Oocytes
A linear regression model was constructed to identify the factors that affected the number of retrieved oocytes (Table 2). This study first compared three indicators, namely BW, BMI, and BSA, and determined which was the best indicator for calculating the initial FSH dosage for patients with PCOS undergoing IVF/ICSI with the GnRH-ant protocol. Model 1 showed that the independent variables BW, BMI, and BSA were all significantly related to the number of retrieved oocytes. After adjustments for age, type of infertility, infertility duration, parity, basal FSH (bFSH) level, basal LH (bLH) level, AMH level, AFC, initial FSH dosage, duration of stimulation, total Gn dosage, serum estrogen, LH, and progesterone on the trigger day, only BMI was significantly related to the number of retrieved oocytes (p = 0.007) (Table 2). Therefore, BMI was regarded as the most significant predictor of the number of retrieved oocytes compared to BW and BSA and was subsequently chosen to develop the initial FSH dosage nomogram.
As listed in Table 3, independent variables, including age, parity, BMI, bFSH level, bLH level, AMH level, and AFC, significantly predicted the number of retrieved oocytes. Age, parity, BMI, and bFSH were negatively correlated with oocyte number, whereas bLH, AMH levels and AFC were positively correlated. However, in multivariate analysis, age and parity were no longer significant. Furthermore, we divided the age group into < 35 and ≥ 35 years, and age group was still not an significant independent factor in the multivariate analysis (p = 0.510) (Supplementary Table 1). Finally, BMI, bFSH level, bLH level, AMH level, and AFC were included in the equation to calculate the initial FSH dosage.
Nomogram for Initial FSH Dosage
The model presented in Table 4 was used to develop the initial FSH dosage nomogram to achieve the desired number of retrieved oocytes. According to this model, the initial FSH dosage for PCOS depends mainly on BMI and ovarian reserve markers, including bFSH, bLH, and AMH levels and AFC (Fig. 2).
To test the utility of this nomogram, we applied this model to the same population and another independent cohort stratified by the number of retrieved oocytes (Fig. 3a, b). The lowest initial FSH dosage was set at 112.5 IU/day for women with PCOS to prevent the absence of follicle growth and cycle cancellation in clinical practice. Among patients with 15 retrieved oocytes, our predicted FSH dosage was almost the same as the actual dosage. When the number of retrieved oocytes was less than 15, the actual dosage was significantly lower than the predicted dosage. Similarly, when the number of retrieved oocytes exceeded 15, the actual dosage was significantly higher than the predicted dosage. These results further verify the reliability of the proposed model.
Predicted initial FSH dosage calculated by the dose nomogram from the present study. a Differences between the actual initial FSH dosage and the predicted initial FSH dosage among the same population. b Differences between the actual initial FSH dosage and the predicted initial FSH dosage among another independent cohort. c Predicted initial FSH dosage stratified by BMI groups with normal-weight (< 24 kg/m2), overweight (24–28 kg/m2), and obese (≥ 28 kg/m2) patients
Considering the notable effect of BMI on the calculation of the initial FSH dosage, we divided the population into normal-weight (< 24 kg/m2), overweight (24–28 kg/m2), and obese (≥ 28 kg/m2) groups according to the guidelines for the prevention and control of overweight and obesity in Chinese adults [27, 28]. As shown in Fig. 3c, the predicted initial dose of FSH was positively correlated with BMI. A total of 34.7% of obese patients with PCOS had a predicted dosage greater than 225 IU/day, while the percentages of normal-weight and overweight patients with this predicted dosage were 1.8% and 8.7%, respectively. In addition, 89.5% of patients with normal weight had a predicted dosage of less than 150 IU/day.
Risk Factors Associated with Moderate–Severe OHSS
Patients with PCOS are at a high risk of developing OHSS. OHSS, a potentially lethal iatrogenic complication of ovarian stimulation, could be classified into mild, moderate, and severe according to its severity [29]. Most cases of OHSS are mild and self-limited with mild abdominal distension/discomfort, mild nausea/vomiting, mild dyspnea, and diarrhea due to enlarged ovaries [30]. Moderate-to-severe OHSS, which is characterized by a further increase in vascular permeability resulting in a loss of protein-rich fluid, ascites, hydrothorax, and hemoconcentration, affects 2–5% of patients with IVF cycles [29, 31, 32]. On the basis of the independent variables involved in the nomogram, we analyzed and compared the characteristics of patients hospitalized for moderate-to-severe OHSS (n = 43) and those with the number of retrieved oocytes exceeding 20 (n = 866) in this cohort. As shown in Table 5, the average levels of bLH and AMH and AFC were higher than the average levels in the total PCOS population in this study, while the average BMI values were lower than those in the total PCOS population. The proportion of all patients with PCOS who were lean or had normal weight was about 50%, while the proportion of patients hospitalized for moderate-to-severe OHSS and those with the number of retrieved oocytes exceeding 20 were 60.5% and 58.9%, respectively. A low BMI, high levels of bLH and AMH, and a high AFC appeared to be risk factors for OHSS.
Discussion
Multivariate analyses demonstrated that BMI was the most significant reference to predict the number of retrieved oocytes compared to BW and BSA. In addition, among patients with PCOS aged 20–40 years undergoing their first IVF cycles with the GnRH-ant protocol, age was not a significant predictor of the initial FSH dosage. We developed a nomogram based on BMI, bFSH, bLH, and AMH levels, and AFC to calculate the ideal initial FSH dosage for patients with PCOS undergoing IVF/ICSI using the GnRH-ant protocol. In addition, a low BMI, high bLH and AMH levels, and a high AFC appear to be risk factors for OHSS.
In assisted reproductive technology cycles, exogenous FSH induces multiple follicular growths among women for whom the FSH sensitivity threshold is exceeded [33]. However, the sensitivity to exogenous FSH varies among different populations. Clinicians should not only account for the number of retrieved oocytes to improve clinical pregnancy rates but also take measures to avoid serious complications from ovarian stimulation, such as OHSS. In other words, optimally retrieved oocytes are preferred over maximally retrieved oocytes. Therefore, ovarian stimulation programs for individuals with different ovarian responses must be considered. Women with PCOS have a large pool of antral follicles, indicating that they are highly sensitive to FSH therapy. In recent years, the GnRH-ant protocol has been proven to reduce the risk of excess stimulation, and choosing the optimal initial dosage of FSH further decreases the incidence of OHSS, especially in patients with PCOS [34, 35].
Although ovarian stimulation with FSH during IVF cycles has been widely used for several decades, it is unclear how the most appropriate initial FSH dosage should be selected in daily clinical practice. Clinicians commonly determine the initial FSH dosage according to the ovarian response to stimulation in previous IVF cycles, and if there are no previous cycles for use as a reference, criteria such as patient age, BW, BMI, and markers of ovarian reserve are considered [17,18,19,20,21,22]. This study is applicable to women with PCOS who have undergone their first IVF cycle using the GnRH-ant protocol.
Some researchers have proposed that patients with a high ovarian response should receive an initial FSH dose of 100–150 IU during the GnRH-ant protocol. An initial FSH dose of 150 IU has more significant benefits in oocyte retrieval than an initial dose of 100 IU, but at the same time, the risk of OHSS is relatively increased [15]. Lensen et al. found that a decreased Gn dose in predicted high responders seemed to reduce the risk of moderate-to-severe OHSS [36]. However, a low initial FSH dosage may result in the absence of follicle growth and cycle cancellation; thus, in clinical practice, full-dose initiation is recommended, and the lowest initial FSH dosage is set to 112.5 IU/day for women with PCOS in the present institution. Therefore, it is important to choose an appropriate initial FSH dosage in IVF cycles to maximize clinical outcomes and minimize the risk of OHSS. As stated above, the desired number of optimal oocytes was set to 15.
The initial dosage of FSH should be comprehensively determined according to patient age, BW, BMI, AFC, bFSH level, and AMH level, among other characteristics. Women with PCOS have abnormal glucose and lipid metabolism, mainly manifesting as impaired glucose tolerance, hyperinsulinemia, insulin resistance, and metabolic syndrome. The reported rate of obesity among women with PCOS ranges from 50% to 80% [37]. Our population was consistent with the proportion of overweight and obese individuals. Drug doses are usually calculated on the basis of BW, BMI, or BSA; however, it is unclear which one makes more sense for patients with PCOS. In the present study, we found that each of the three indicators was significantly correlated with the number of retrieved oocytes. However, when adjustments were made for all other variables, only BMI remained statistically significant compared to BW and BSA. Therefore, we considered BMI to be the most significant predictor of involvement in the subsequent calculations.
Our results showed that the independent variables, namely age, parity, BMI, bFSH, bLH, and AMH levels, and AFC, were significantly correlated with the number of retrieved oocytes. Multiple analyses showed that the initial dosage of FSH depended mainly on BMI and markers of ovarian reserve, including bFSH, bLH, and AMH levels and AFC (p < 0.05). Almost all previous predictive models of initial FSH dosage were based on patients’ age [38]. Olivennes et al. created a model to calculate the starting dosage of recombinant human FSH in IVF cycles using a GnRH agonist long protocol, in which bFSH level, BMI, age, and AFC were included in the dosing algorithm [39]. Notably, age, which had been reported as an important and significant predictor in other studies, was no longer a significant independent variable for tailoring the initial dosage of FSH for patients with PCOS aged between 20 and 40 years in the present study. In women older than 35 years, age-related decline in fertility mainly manifests as a decreased number of follicles and a declining ovarian response to exogenous Gn [40], with significantly decreased pregnancy and live birth rates [41]. Although we divided the age group into over 35 and under 35 years, age group did not contribute to the final construction of the FSH dosage nomogram. A plausible explanation is that patients with PCOS have a high ovarian reserve and that age does not add to the value derived from other markers in the multivariate regression model. However, the number of patients with PCOS and advanced age in this study was indeed low (201/1898 cases), so a certain sample size needs to be accumulated for subsequent stratified analysis.
Both AFC and AMH have a strong linear relationship with the number of retrieved oocytes [42, 43]. La Marca et al. used AMH level and AFC combined with age and FSH level to develop a nomogram to predict the FSH starting dosage in their previous studies [24, 26]. However, there was no absolute consistency between AMH and AFC. AMH is produced by granulosa cells of early developing follicles and is positively related to the number of recruited follicles [20]. AFC refers to the number of antral follicles measuring approximately 2–9 mm in diameter in the ovary, as determined by transvaginal ultrasound, in the early follicular phase and is another marker of ovarian reserve. AMH has many distinct advantages in the assessment of ovarian reserve and response, and it is an accurate biomarker of ovarian aging that is superior to FSH and AFC [18, 44, 45]. Combining AMH and AFC can predict the maximum responsiveness of the ovary [46].
Many women diagnosed with PCOS exhibit an elevated LH pulse frequency and an increased LH-to-FSH ratio, which inhibits follicle recruitment and maturation. Elevated LH levels in PCOS promote theca cell hyperplasia and insulin-like growth factor 1 (IGF-1) activity and, thus, the secretion of excessive androgen [47]. In the present study, LH concentration was found to be another significant independent predictor of individual initial FSH dosage in the GnRH-ant protocol for patients with PCOS. Specifically, a 1 IU/L increase in LH concentration was associated with a 0.291-fold increase in the number of retrieved oocytes (95% CIs 0.163–0.418; p < 0.001).
On the basis of the present dose nomogram, we attempted to identify factors that might lead to OHSS. Compared with those of all patients with PCOS, those hospitalized for moderate-to-severe OHSS and more than 20 retrieved oocytes had high average levels of bLH and AMH and AFC and low BMI. In addition, most patients were lean or had normal weight (BMI < 24 kg/m2). These results were consistent with those of previous studies which showed that a low BMI, high levels of bLH and AMH, and a high AFC are particularly associated with an increased risk of OHSS [30, 48, 49]. The above results further illustrate the reliability of this nomogram and validate its clinical application value in avoiding OHSS and reducing the cancellation rate of a fresh embryo transplantation cycle.
The present study is the first to construct a dose nomogram based on BMI and ovarian reserve markers for the individualization of the initial FSH dosage for patients with PCOS undergoing IVF/ICSI with the GnRH-ant protocol. In addition, this study had a large sample size of 2491 patients with PCOS from 2017 to 2021, which makes the results highly stable and reliable for application in clinical practice. The current study also has several limitations, including a single-center design, which might have caused selection bias; thus, evidence from other medical centers is required for validation. In addition, patients over 40 years of age were excluded from our study (n = 52); thus, our nomogram is not applicable to patients with PCOS over 40 years of age. Future studies should investigate whether age > 40 years might increase the initial FSH dosage appropriately.
Conclusions
The concept of personalizing initial FSH dosage helps improve the clinical outcomes of patients with PCOS undergoing the GnRH-ant protocol during IVF. This study demonstrates that the initial FSH dosage for patients with PCOS aged between 20 and 40 years who are undergoing IVF/ICSI with the GnRH-ant protocol depends on BMI and ovarian reserve markers, whereas age is not a significant factor. The optimal FSH dosage reached the optimal stimulation level to obtain an appropriate number of retrieved oocytes and prevent OHSS. This nomogram allows clinicians to modulate the initial FSH dosage for patients with PCOS to easily obtain an optimal number of 15 retrieved oocytes in clinical practice. The present nomogram has been well validated in patients with PCOS at Peking University Third Hospital; however, it is necessary to verify these findings at other reproductive centers and to test them in future prospective studies.
References
Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. 2021;106(3):e1071–83.
Joham AE, Norman RJ, Stener-Victorin E, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10(9):668–80.
Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–84.
Mimouni NEH, Paiva I, Barbotin AL, et al. Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab. 2021;33(3):513–30e8.
Fernandez RC, Moore VM, Rumbold AR, et al. Diagnosis delayed: health profile differences between women with undiagnosed polycystic ovary syndrome and those with a clinical diagnosis by age 35 years. Hum Reprod. 2021;36(8):2275–84.
Deswal R, Nanda S, Dang AS. Single nucleotide polymorphisms in treatment of polycystic ovary syndrome: a systematic review. Drug Metab Rev. 2019;51(4):612–22.
Quaresima P, Saccone G, Morelli M, et al. Stillbirth, potentially preventable cases: an Italian retrospective study. Ital J Gynaecol Obstetr. 2022;34(2):89–102.
Weiss NS, Kostova E, Nahuis M, et al. Gonadotrophins for ovulation induction in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2019;1:CD010290.
Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22(6):687–708.
Al-Inany HG, Youssef MA, Ayeleke RO, et al. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4:CD001750.
Sunkara SK, Rittenberg V, Raine-Fenning N, et al. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26(7):1768–74.
Steward RG, Lan L, Shah AA, et al. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril. 2014;101(4):967–73.
Ji J, Liu Y, Tong XH, et al. The optimum number of oocytes in IVF treatment: an analysis of 2455 cycles in China. Hum Reprod. 2013;28(10):2728–34.
Professional Committee on Reproductive Medicine. Cumulative delivery/live birth rate of complete ovarian stimulation cycle consensus. Chin J Reprod Contracep. 2018;38(12):963–8.
Expert Consensus Compilation Group of Reproductive Medicine Committee of China Medical Women's Association. Expert consensus on standardized application of antagonist protocol in assisted reproductive technology. Chin J Reprod Contracep. 2022(02):109–16.
Law YJ, Zhang N, Kolibianakis EM, et al. Is there an optimal number of oocytes retrieved at which live birth rates or cumulative live birth rates per aspiration are maximized after ART? A systematic review. Reprod Biomed Online. 2021;42(1):83–104.
Fleming R, Seifer DB, Frattarelli JL, Ruman J. Assessing ovarian response: antral follicle count versus anti-Mullerian hormone. Reprod Biomed Online. 2015;31(4):486–96.
La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update. 2014;20(1):124–40.
Iliodromiti S, Nelson SM. Ovarian response biomarkers: physiology and performance. Curr Opin Obstet Gynecol. 2015;27(3):182–6.
Li R, Gong F, Zhu Y, et al. Anti-Mullerian hormone for prediction of ovarian response in Chinese infertile women undergoing IVF/ICSI cycles: a prospective, multi-centre, observational study. Reprod Biomed Online. 2016;33(4):506–12.
Alviggi C, Humaidan P, Ezcurra D. Hormonal, functional and genetic biomarkers in controlled ovarian stimulation: tools for matching patients and protocols. Reprod Biol Endocrinol. 2012;10:9.
Abbara A, Patel A, Hunjan T, et al. FSH requirements for follicle growth during controlled ovarian stimulation. Front Endocrinol (Lausanne). 2019;10:579.
Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129–40.
La Marca A, Papaleo E, Grisendi V, et al. Development of a nomogram based on markers of ovarian reserve for the individualisation of the follicle-stimulating hormone starting dose in in vitro fertilisation cycles. BJOG. 2012;119(10):1171–9.
Allegra A, Marino A, Volpes A, et al. A randomized controlled trial investigating the use of a predictive nomogram for the selection of the FSH starting dose in IVF/ICSI cycles. Reprod Biomed Online. 2017;34(4):429–38.
La Marca A, Grisendi V, Giulini S, et al. Individualization of the FSH starting dose in IVF/ICSI cycles using the antral follicle count. J Ovar Res. 2013;6(1):11.
Chen C, Lu FC, Department of Disease Control Ministry of Health PRC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1–36.
Wang H, Zhai F. Programme and policy options for preventing obesity in China. Obes Rev. 2013;14(Suppl 2):134–40.
Nelson SM. Prevention and management of ovarian hyperstimulation syndrome. Thromb Res. 2017;151(Suppl 1):S61–4.
Practice Committee of the American Society for Reproductive Medicine. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. 2016;106(7):1634–47.
Humaidan P, Nelson SM, Devroey P, et al. Ovarian hyperstimulation syndrome: review and new classification criteria for reporting in clinical trials. Hum Reprod. 2016;31(9):1997–2004.
Budev MM, Arroliga AC, Falcone T. Ovarian hyperstimulation syndrome. Crit Care Med. 2005;33(10 Suppl):S301–6.
Abbara A, Clarke SA, Dhillo WS. Novel concepts for inducing final oocyte maturation in in vitro fertilization treatment. Endocr Rev. 2018;39(5):593–628.
Dewailly D, Robin G, Peigne M, et al. Interactions between androgens, FSH, anti-Mullerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update. 2016;22(6):709–24.
Lambalk CB, Banga FR, Huirne JA, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. 2017;23(5):560–79.
Lensen SF, Wilkinson J, Leijdekkers JA, et al. Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI). Cochrane Database Syst Rev. 2018;2:CD012693.
McCartney CR, Marshall JC. Clinical practice. Polycystic ovary syndrome. N Engl J Med. 2016;375(1):54–64.
Popovic-Todorovic B, Loft A, Lindhard A, et al. A prospective study of predictive factors of ovarian response in “standard” IVF/ICSI patients treated with recombinant FSH. A suggestion for a recombinant FSH dosage normogram. Hum Reprod. 2003;18(4):781–7.
Olivennes F, Howies CM, Borini A, et al. Individualizing FSH dose for assisted reproduction using a novel algorithm: the CONSORT study. Reprod Biomed Online. 2011;22(Suppl 1):S73-82.
Ferraretti AP, La Marca A, Fauser BC, et al. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–24.
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633–4.
Sermondade N, Sonigo C, Sifer C, et al. Serum antimullerian hormone is associated with the number of oocytes matured in vitro and with primordial follicle density in candidates for fertility preservation. Fertil Steril. 2019;111(2):357–62.
Kotanidis L, Nikolettos K, Petousis S, et al. The use of serum anti-Mullerian hormone (AMH) levels and antral follicle count (AFC) to predict the number of oocytes collected and availability of embryos for cryopreservation in IVF. J Endocrinol Invest. 2016;39(12):1459–64.
Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21(6):698–710.
Moolhuijsen LME, Visser JA. Anti-Mullerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab. 2020;105(11):3361.
Broer SL, Dolleman M, van Disseldorp J, et al. Prediction of an excessive response in in vitro fertilization from patient characteristics and ovarian reserve tests and comparison in subgroups: an individual patient data meta-analysis. Fertil Steril. 2013;100(2):420–97.
Coyle C, Campbell RE. Pathological pulses in PCOS. Mol Cell Endocrinol. 2019;498: 110561.
Sun B, Ma Y, Li L, et al. Factors associated with ovarian hyperstimulation syndrome (OHSS) severity in women with polycystic ovary syndrome undergoing IVF/ICSI. Front Endocrinol (Lausanne). 2021;11: 615957.
Ocal P, Sahmay S, Cetin M, et al. Serum anti-Mullerian hormone and antral follicle count as predictive markers of OHSS in ART cycles. J Assist Reprod Genet. 2011;28(12):1197–203.
Acknowledgements
We thank Lixue Chen for help with the electronic database, and all the staff in the reproductive center of Peking University Third Hospital for their work for this study.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81701407) and the National Key Research and Development Program of China (No. 2022YFC2702901). The Rapid Service Fee and Open Access Fee were supported by these funding sources.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their final approval for the version to be published.
Author Contributions
Xiaoyu Long and Jie Qiao conceived and designed the study. Manfei Si and Xinyu Qi collected and extracted data. Manfei Si, Chen Yang, and Tian Tian were responsible for statistical analysis. Manfei Si, Xinyu Qi, Xiumei Zhen, Xiaoyu Long, and Jie Qiao interpreted and discussed results. Manfei Si wrote the manuscript. All authors contributed to critical revision of the manuscript and approved the final version of the manuscript.
Disclosures
Manfei Si, Xinyu Qi, Xiumei Zhen, Chen Yang, Tian Tian, Xiaoyu Long and Jie Qiao declare that they have no competing interests. Jie Qiao is an expert on reproductive medicine at the Chinese Academy of Engineering and the head of the Center for Reproductive Medicine, Department of Obstetrics and Gynecology, Peking University Third Hospital, No. 49 North Huayuan Road, Haidian District, Beijing 100191, China.
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee of Reproductive Medicine at Peking University Third Hospital (No. 2021SZ-011). The study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from the patients for the IVF procedure and to record and use their clinically relevant information for research purposes.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Si, M., Qi, X., Zhen, X. et al. Dose Nomogram of Individualization of the Initial Follicle-Stimulating Hormone Dosage for Patients with Polycystic Ovary Syndrome Undergoing IVF/ICSI with the GnRH-Ant Protocol: A Retrospective Cohort Study. Adv Ther 40, 3971–3985 (2023). https://doi.org/10.1007/s12325-023-02582-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02582-2