Abstract
Introduction
Older patients are at increased risk for hyperkalemia (HK). This study describes the prevalence, recurrence, and clinical and economic burden of HK in Medicare patients admitted to a long-term care (LTC) setting.
Methods
Retrospective cohort study using 100% Medicare Fee-for-Service (FFS) claims identified patients aged ≥ 65 years with index admission between 2017 and 2019 to a LTC setting (skilled nursing, home health, inpatient rehabilitation, or long-term acute care). Beneficiaries were required to have 12 months continuous medical and pharmacy coverage prior to index LTC admission and ≥ 30 days after LTC discharge (follow-up). Patient characteristics, healthcare resource utilization, and costs were assessed. HK was defined as ICD-10 diagnosis code E87.5 in any claim position or Medicare Part D fill for oral potassium binder.
Results
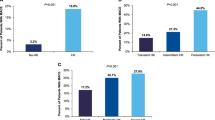
Of 4,562,231 patients with a LTC stay, the prevalence of HK was 14.7% over the full study period (pre-index, index stay, and follow-up). Excluding those with HK only during the follow-up period resulted in 4,081,103 patients. Of these, 290,567 (7.1%) had HK and 3,790,536 (92.9%) did not have HK during or within 14 days prior to index LTC stay. The HK recurrence rate during index stay and follow-up was 48.3%. Unmatched HK versus non-HK patients were more often male (43.0% vs. 35.4%), Black (13.5% vs. 8.0%), dual eligible for Medicaid (34.2% vs. 25.0%), with higher mean Charlson Comorbidity Index scores (6.2 vs. 3.9) (all p < 0.0001). After propensity matching, HK patients were 2.2 times more likely to be hospitalized, with higher mortality (30.8% vs. 21.5%) and higher total healthcare costs during both index stay (US$26,520 vs. $18,021; p < 0.0011) and follow-up ($57,948 vs. $41,744 (p < 0.0011) versus matched non-HK patients.
Conclusion
Prevalence and recurrence of HK was high among LTC patients, and HK was associated with significantly greater clinical and economic burden during and post-LTC.
Plain Language Summary
Hyperkalemia is a serious medical condition commonly occurring in nursing home residents. It is characterized by abnormally high blood levels of potassium that if untreated can be life-threatening. High levels of potassium can be the result of kidney disease and inability to remove potassium from the bloodstream; eating foods high in potassium; and/or taking medications that interfere with the kidney’s ability to remove potassium from the bloodstream. Older patients who have chronic kidney disease, heart failure, diabetes, and high blood pressure are at particularly high risk for hyperkalemia. Management is difficult as it requires reducing intake of foods high in potassium, adjusting medications that cause hyperkalemia, and potentially treating with oral potassium binders to reduce potassium blood levels. This study focused on the clinical outcomes, healthcare services use, and costs incurred by Medicare beneficiaries 65 years and older admitted to long-term care, where the occurrence of hyperkalemia is often high yet unrecognized. Patients with a diagnosis of hyperkalemia immediately before and during admission to long-term care or after discharge had an increased rate of death compared with patients without a hyperkalemia diagnosis. Hyperkalemia patients also had more hospitalizations and visits to the Emergency Department and outpatient facilities, resulting in higher total medical costs. Total costs for hyperkalemia patients were highest for those with chronic kidney disease, heart failure, and diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There is a knowledge gap regarding the prevalence, recurrence, and disease burden of hyperkalemia (HK) in elderly patients admitted to long-term care (LTC) settings, where the occurrence and recurrence of HK is high resulting in high resource utilization and costs, yet the need for prevention and treatment is underrecognized. |
Management of HK in the LTC setting is challenging, requiring a multidisciplinary approach of reducing intake of foods high in potassium, adjusting HK-inducing medications, and potentially adding oral potassium binder treatment. |
What did the study ask? |
A real-world study of 100% U.S. Medicare Fee-For-Service beneficiaries (≥65 years) between 2017 and 2019 assessed prevalence, recurrence, mortality, hospitalizations, costs, and use of oral potassium binders among propensity-matched patients with and without HK diagnosis. |
What were the study outcomes/conclusions? |
The LTC setting is associated with high HK prevalence (15%) and recurrence (48%), and HK patients had higher mortality rates, inpatient and outpatient healthcare utilization, and costs than matched controls without HK. |
What has been learned from the study? |
HK patients with chronic kidney disease, heart failure, and diabetes, conditions associated with increased risk for HK, incurred even greater costs. |
Despite high disease burden in the LTC setting, use of oral potassium binders is low. |
Introduction
Hyperkalemia (HK) is an electrolyte disorder characterized by mild (5.5–6.0 mEq/L), moderate (6.1–7.0 mEq/L), and severe (≥ 7.0 mEq/L) pathologic elevations of blood levels of potassium [1, 2]. It is associated with morbidity and mortality and increased healthcare utilization and costs, particularly in the elderly [3,4,5,6]. Older patients have increased risk of HK due to the comorbidities of chronic kidney disease (CKD), heart failure (HF), diabetes, and hypertension [4, 7]. Guideline-based treatment of these comorbidities often involves use of renin–angiotensin–aldosterone system inhibitors (RAASi), which interferes with potassium excretion [4]. Older patients with these conditions, and those treated with RAASi, have 40–50% higher occurrence of HK than the 2–3% observed in the general population [2, 4]. The higher healthcare resource utilization (HCRU) and costs of HK are the result of more emergency department (ED) visits, inpatient hospitalizations, readmissions, laboratory testing, and the use of oral binder medications [8]. In 2011, the estimated total annual hospital charges were approximately US$697 million for Medicare patients with cardiorenal disease and primary HK diagnosis [8].
By 2030, the number of people age ≥ 65 is projected to reach 71 million [4]. Of these, over two-thirds will require an average of 3 years of long-term care (LTC) [4]. Chronic diseases in the aging population (e.g., CKD and HF) will drive the need for LTC services [4]. LTC and post-acute care (PAC) settings including skilled nursing facilities (SNF), inpatient rehabilitation facilities (IRF), home health agencies (HHA), and long-term acute care hospitals (LTACH), are particularly impacted due to high prevalence of HK-related comorbidities and frequent use of RAASi therapies [4].
Treatment of HK in LTC/PAC settings presents a challenge to clinicians. Management of HK requires a multidisciplinary approach of reducing the intake of foods high in potassium, adjusting HK-inducing medications, and potentially adding oral potassium binders, to reduce potassium concentrations [8, 9]. Recent guidelines and published consensus statements recommend novel oral potassium binders, including sodium zirconium cyclosilicate (SZC) and Patiromer, for managing HK in patients with HF or CKD to enable optimization of RAASi therapy [10, 11]. As morbidity and mortality approach rates of 50% in patients on discontinued or sub-maximum doses of a RAASI, these new binder therapies could help clinicians by preventing HK, while continuing or increasing RAASi dosing to their target levels, in order to attain optimal patient outcomes [12,13,14].
Currently, there is a gap in knowledge regarding the prevalence, recurrence, and disease burden of HK in patients admitted to LTC settings, where the need for prevention and treatment alternatives for HK is underrecognized. While several publications have described the prevalence of electrolyte imbalances in SNFs, they do not specifically address the economic burden [15,16,17,18]. To date, the limited number of real-world studies assessing HK prevalence and its medical and economic burden have utilized the 5% sample Medicare Fee-For-Service (FFS) claims data [19, 20]. In particular, recurrence of HK and the impact of HK on clinical outcomes in LTC settings is rarely being studied [4, 19, 20]. Our study sought to determine HK prevalence and recurrence in the full Medicare FFS population during LTC stays and following discharge, as well as compare clinical outcomes, HCRU, and cost differences in patients with and without HK.
Methods
Study Design
We performed a retrospective observational study using the 100% Medicare FFS claims database [21] accessed through a research Data Use Agreement with the Centers for Medicare and Medicaid Services. This study did not require Institutional Review Board (IRB) approval due to the use of retrospective de-identified claims data, which is exempt from IRB review. The study quantified the prevalence and recurrence of HK and associated clinical and economic outcomes in the LTC setting and post-discharge, using inpatient and outpatient claims from Medicare Parts A/ B and Part D prescription drug claims from January 1, 2016 to December 31, 2019. Data included admission/discharge dates, diagnoses/procedure codes, source of care, durable medical equipment (DME), and date of death. Medicare enrollment files provided demographic data.
Patient Population
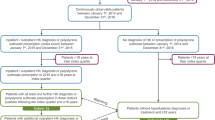
Eligible patients were ≥ 65 years of age at the time of first admission (index date) to a LTC setting (including SNFs, IRFs, HHAs, and LTACHs) between January 1, 2017 and November 30, 2019. Study patients had continuous medical and pharmacy coverage for 12 months prior to index date and ≥ 30 days after discharge. Patients were followed to the end of the study period, disenrollment (e.g., switch to Medicare Advantage, loss of Part D coverage), or death. The HK cohort included patients with International Classification of Diseases, 10th Revision (ICD-10) diagnosis code E87.5 in any claim position or Part D evidence of an oral potassium binder [sodium polystyrene sulfonate, calcium polystyrene sulfonate, Patiromer, or SZC, identified by NDC codes] within 14 days prior to or during the index LTC stay. The non-HK cohort had no claim for HK or oral potassium binder any time during the study period. Figure 1 presents the patient attrition diagram and study timeline.
Attrition diagram and study timeline. The attrition diagram presents the inclusion and exclusion criteria used to identify hyperkalemia and non-hyperkalemia Medicare patients in long-term care (LTC). The study timeline includes the period prior (Baseline) to the LTC index stay and the post LTC discharge period (Follow-up)
HK Prevalence
The overall HK prevalence was assessed in all patients with a LTC stay. Prevalence was determined by any HK event occurring 14 days prior to, during, or post-discharge a LTC stay, not including the qualifying HK event that defined the HK-related index LTC stay. Prevalence of HK and concomitant comorbidities of CKD/end stage renal disease (ESRD), HF, diabetes, and hypertension were evaluated in unmatched groups. Clinical and economic outcomes were assessed in matched cohorts of patients with ≥ 7 days index LTC stay and up to 365 days post-index LTC discharge.
Patient Variables and Covariates
Patient characteristics were assessed during the baseline period prior to the index LTC stay for the unmatched and matched cohorts. Demographics included age, gender, race/ethnicity, U.S. Census Region, original reason for Medicare entitlement (age 65 or disabled/ESRD), and dual eligibility status for Medicare and Medicaid. Clinical characteristics included the Charlson Comorbidity Index (CCI) score [22], CKD/ESRD, HF, diabetes, hypertension, overweight/obesity, and other chronic conditions.
Clinical Outcomes
Clinical outcomes included HK recurrence, HK-related hospitalizations, acute kidney injury (AKI), evidence of dialysis initiation, renal transplant, and all-cause mortality during the index LTC stay or post-discharge. An AKI event was identified with an AKI-related ICD-10 diagnosis code in any claim position that did not occur 14 days prior to the index LTC stay. Evidence of dialysis initiation and renal transplantation was identified from ICD-10 procedure codes. Unadjusted and adjusted estimates for all-cause mortality were calculated.
Economic Outcomes
All-cause HCRU included inpatient hospitalizations, ED and outpatient visits, use of DME, and medication use. Total direct expenditures included overall healthcare costs and costs stratified by expenditure category. Economic outcomes for cohorts with CKD/ESRD, HF, diabetes, and hypertension were assessed to provide context for HK-related chronic conditions.
Unadjusted and adjusted analysis using log-link function identified risk of all-cause hospitalizations and differences in costs during index LTC stay and up to 365 days post-discharge. Costs included Medicare and beneficiary out-of-pocket amounts adjusted to 2019 USD using the health component of the Consumer Price Index [23].
Statistical Analysis
Descriptive statistics assessed patient demographics and clinical characteristics during baseline. Continuous variables were expressed as mean and standard deviation (± SD), or median and interquartile range (IQR), and categorical variables were presented as counts and percentages. Bivariate analysis assessed differences in patient demographics, clinical characteristics, comorbidities, HCRU, and cost outcomes between HK and non-HK patients using Student’s t test for continuous variables and Chi-square test for categorical variables. Since outcomes were measured using the population and not only utilizers, many HCRU and cost medians are zero. Thus, population means were used for comparisons. Large SDs reflect large skew, which is not unexpected since some HK patients required long hospitalizations. Clinical outcomes were observational and did not employ statistical comparisons.
Clinical and economic outcomes were assessed in 1:1 propensity matched HK and non-HK cohorts applying an exact match on age, gender, race/ethnicity, comorbidities (CKD/ESRD, HF, CKD/ESRD and HF, none of these), geographic region, and RAASi use. Nearest-neighbor matching was applied on CCI score, original reason for Medicare entitlement, and dual eligibility status. Adjusted analysis utilized Cox regression for all-cause mortality. Logistic regression and odds ratios compared likelihood of all-cause hospitalizations. Generalized linear modeling with gamma distribution and log-link function cost ratios evaluated differences in costs.
Analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC, USA), and statistical significance was defined as p < 0.05.
Results
Patient Population and HK Prevalence
A total of 4,562,231 patients with LTC stay were identified. Excluding patients with HK only during follow-up resulted in 4,081,103 patients. Of these, 290,567 (7.1%) had evidence of HK during the index stay or 14 days prior, and 3,790,536 (92.9%) did not have evidence of HK any time during the study period (Table 1). Matching resulted in 273,343 in the HK and non-HK cohorts (Table 2).
Patient Demographics, Clinical Characteristics, and Comorbidities
Compared to non-HK patients, HK patients were of similar age (mean ± SD: age 79.1 ± 8.4 vs. 79.9 ± 8.44), more often male (43.0% vs. 35.4%), Black (13.5% vs. 8.0%), dual eligible (34.2% vs. 25.0%), with higher mean CCI scores (6.2 vs. 3.9), higher prevalence of CKD (50.0% vs. 17.2%), HF (52.7% vs. 27.6%), diabetes (60.1% vs. 38.6%), and hypertension (95.6% vs. 88.4%), with higher rates of RAASi prescription fills (61.8% vs. 49.1%) all p < 0.0001. Baseline characteristics for the matched cohorts show that they are well-matched (Table 2). Due to large sample sizes, many small differences may be statistically significant but not clinically meaningful.
Clinical Outcomes
Overall HK recurrence was 48.3%, with 26.3% having ≥ 1 and 24.1% ≥ 2 HK-related hospitalizations (Table 3). All-cause hospitalizations were higher (64.5% vs. 49.6%), with more AKI (61.9% vs. 35.2%) and all-cause mortality (30.8% vs. 21.5%) in HK patients. Adjusted analysis found all-cause mortality was 1.3 times greater in HK patients than non-HK patients (Table 4).
Economic Outcomes
During index LTC stay, population mean ± SD length of stay was longer for HK patients (65.8 ± 105.3 vs. 57.9 ± 89.5 days), with more HK patients requiring inpatient hospitalizations (29.0% vs. 16.0%), ED visits (20.0% vs. 16.0%), outpatient visits (50.0% vs. 46.0%), and DME (37.0% vs. 34.0%) (all p < 0.0001). Greater HCRU for HK resulted in higher population mean ± SD total direct costs ($26,520 ± $31,555 vs. $18,021 ± $22,739, p < 0.0001) (Table 5). While LTC and inpatient hospitalizations accounted for three-quarters of the overall costs during the index stay, costs were higher in the HK cohort (LTC $14,137 ± $17,215 vs. $10,631 ± $13,334; inpatient $6,229 ± $16,330 vs. $2,873 ± $10,398; p < 0.0001). Population mean oral potassium binder costs per HK patient ($9 ± $175) represented < 1% of mean prescription costs ($1458 ± $8429). Adjusted analyses revealed the likelihood for all-cause hospitalizations during the index LTC stay for HK patients was 2.2 times higher than non-HK and total costs 1.5 times higher (Table 4).
Within 365 days post-discharge LTC index visit, more HK than non-HK patients had ≥ 1 additional stay in a LTC setting (64.0% vs. 55.0%; p < 0.0001), with a length of stay averaging 72.5 and 73.8 days, respectively (Table 5). Significantly more HK patients required inpatient hospitalizations (53.0% vs. 42.0%; p < 0.0001), ED visits (50.0% vs. 47.0%; p < 0.0001), and DME (66.0% vs. 60.0%; p < 0.0001) resulting in higher population mean ± SD total direct costs ($57,948 ± $62,445 vs. $41,744 ± $46,383 ; p < 0.0011). LTC and inpatient hospitalizations accounted for 50.0%, and ED and outpatient visits nearly 20.0%, of total costs in the post-index discharge period. Population mean ± SD LTC costs were higher for HK than non-HK ($13,775 ± $23,273 vs. $9191 ± $17,488 ; p < 0.0001), as were inpatient costs ($16,427 ± $30,270 vs. $9679 ± $19,507 ; p < 0.0001). Population mean ± SD oral potassium binder costs per HK patient of $46 ± $486 represented < 1% of prescription costs of $7642 ± $15,774 .
We also evaluated economic outcomes for HK and non-HK matched cohorts with CKD/ESRD, HF, diabetes, and hypertension (Supplementary Material Tables 1, 2, 3, 4). Within 365 days post-index LTC discharge, population mean total costs per HK patient were highest for CKD/ESRD, followed by HF and diabetes.
Discussion
This retrospective observational study was conducted to describe the patient characteristics, prevalence, and clinical and economic burden in Medicare FFS patients with HK admitted to a LTC facility. HK-related comorbidities of CKD/ESRD, HF, diabetes, and hypertension, along with RAASi use, occurred more often in HK than non-HK patients. Among those with a LTC stay, the prevalence of HK during the pre-index, index stay, and follow-up period was almost 15%, with 7% of index stays being HK-related. While almost half of the HK patients had HK recurrence, an oral potassium binder was prescribed in only 4% of HK patients. HCRU measured by inpatient hospitalizations, readmissions, and ED visits, was greater in the HK cohort and resulted in higher total costs.
HK has long been recognized as a serious and potentially life-threatening medical condition in nursing home residents [15,16,17,18]. Elderly patients are predisposed to electrolyte abnormalities due to comorbid diseases, polypharmacy, difficulty in monitoring prescriptions, as well as functional and cognitive impairment [16, 17]. Moreover, healthcare practitioners may underappreciate the susceptibility of nursing home residents to these abnormalities, the potential for harmful outcomes, and the consequences of unnecessary hospitalizations [18]. Dharmarajan et al. illustrated this in a case study of a 70-year-old male admitted to a nursing home on a potassium-sparing diuretic (spironolactone), potassium supplementation, and low magnesium, with renal failure and diabetes [15]. He was subsequently hospitalized for severe hyperkalemia with weakness and lethargy. His hypokalemia medications were discontinued, and he was treated aggressively in intensive care. Lack of close monitoring in the nursing home had resulted in life-threatening hyperkalemia, preventable with early recognition and management [15]. Use of adverse drug event alerts and trigger tools to identify concurrent drug administration with an abnormal laboratory value could have helped with early identification of HK in the nursing home [16, 17]. Consistent with this report, our findings suggest that HK remains a significant medical challenge in LTC settings.
Our study of Medicare patients admitted to LTC facilities 2017–2019 found high HK prevalence and recurrence. Of note, these high rates are only second in frequency to the higher rates reported in the dialysis setting, which range from 20% (peritoneal dialysis) to 74% (hemodialysis) [24, 25]. A search of recent HK literature did not reveal any prior prevalence studies specific to LTC settings, suggesting that our study contributes new evidence-based findings. We did, however, identify three real-world studies published during 2017 and 2020 reporting HK prevalence using 2010–2014 data from a large commercially insured population and 5% sample of Medicare beneficiaries [19, 20, 26]. The annual HK prevalence among commercially insured adults increased yearly from 1.2% (2010) to 1.6% (2014) [26]. This increase likely reflected an aging population with HK-related comorbidities. Among these patients, almost 50% had CKD and/or HF, with a 2014 annual HK prevalence of 6.35%. For those aged ≥ 65, the 2010–2014 annual prevalence ranged 5.9%–7.6%. During the same 5-year period, Mu et al. reported that Medicare beneficiaries had overall HK prevalence of 2.6%–2.7%, and prevalence of 8.9%–9.3% among those with CKD and/or HF [19]. In a study using 2014 data, Medicare beneficiaries demonstrated an overall 2.3% HK prevalence, with a 13.3% prevalence among those with CKD [20]. Our study of overall HK prevalence in the LTC setting demonstrated higher rates than reported for the commercially insured and 5% Medicare populations. We did not determine HK prevalence for patients with CKD and HF, although we reported their frequency.
Key findings of our study highlight the clinical burden and increased HCRU and costs for Medicare FFS patients with HK during index LTC stay and up to 365 days post-discharge. We reported an increased risk for mortality and hospitalizations. Total healthcare costs were $8499 higher in the HK cohort during the index LTC stay and $16,204 higher during the 365 days post-index period. Similarly, the real-world studies cited above found significant HCRU and costs in HK cohorts [5, 19, 20]. In a commercial population, 30-day and 1-year rates of inpatient admissions, outpatient, and ED visits were higher in the HK cohort compared to those without HK [5]. Respective 30-day and 1-year total healthcare costs were $4128 and $15,983 higher for the HK cohort, and, when assessed for those with CKD and HF, costs were even higher ($5553 and $24,133, respectively). When inpatient admissions were assessed through the 1 year post-discharge, patients with HK incurred $30,379 higher costs [27]. Mu et al. reported that Medicare patients with HK had significantly higher rates of inpatient admissions, outpatient and ED visits, SNF admissions, hospice care, and use of home health over the ensuing year compared to those without HK [19]. For HK patients, this translated to increased costs of $7208 at 30 days and $19,348 at 1 year. For those with CKD and HF, respective cost differences were $7726 and $21,577. Fitch et al. reported the average allowed Medicare per-patient-per-month (PPPM) cost in patients with HK was 5 times greater than that in the total Medicare population ($5645 vs. $1035) [20]. Moreover, when adjusted for CKD severity, PPPM costs for HK patients were double those for patients without HK.
Despite the clinical and economic burden of HK observed in our study, use of oral potassium binders for treatment of HK was very low (4%). This may stem from a lack of knowledge regarding the availability of new safer options (sodium zirconium cyclosilicate and patiromer), other than sodium polystyrene sulfonate which is associated with serious gastrointestinal adverse events [28, 29]. Prescribers of oral binders are typically nephrologists who manage hyperkalemia on a more frequent basis than physicians treating patients in LTC settings. Since frequency of HK is rising in primary care, physicians and other practitioners will need to become more aggressive in its management. More studies, like our analysis, demonstrating that better control of potassium could lead to improved outcomes, should raise awareness. A 2020 PubMed review of randomized controlled studies demonstrating fewer side effects with the newer oral potassium binders, effective reduction of serum potassium, and maintenance of RAASi therapy may increase their use [29]. Our study underscores that a randomized control trial of oral potassium binder use may be an important next step.
The limitations related to the use of administrative data and those specific to our study warrant discussion. The main purpose of administrative claims is to support billing and reimbursement practices. The accuracy of identification of HK patients relies on providers to make and record diagnoses. Lack of laboratory results limits the ability to identify severity of HK and to assess other markers of kidney function. While we identified patients with CKD (as well as heart failure, diabetes, and hypertension) to assess overall HCRU and cost outcomes in LTC, we did not further address the relationship of CKD and HK. Certainly, increased mortality, higher costs, advanced CKD stages, and severe comorbidities would contribute to the clinical and economic burden. Additionally, the effect of oral binder use on healthcare and economic outcomes in LTC was not an intended study objective, but its low utility was a concerning finding. Prescription data to determine potassium binder and RAASi use may not reflect actual administration. Furthermore, our results may not be generalizable to patients without insurance or with other coverage such as commercial insurance. Ultimately, this retrospective study is hypothesis driven and cannot be used to determine cause and effect. However, despite these limitations, this study represents a large real-world patient population with representation of all U.S. states in the 100% FFS data and a comprehensive definition for HK with limited exclusions. The study was not limited to high-risk groups with HK-related comorbidities but focused on the overall LTC population. Medicare FFS claims reflect complete capture of healthcare services, and medications provided outside the inpatient setting so patients can be followed longitudinally prior to, during, and post-discharge LTC stay.
Conclusions
We found that the LTC setting is associated with high HK prevalence (15%), has the highest 1-year HK prevalence after the dialysis setting, and that annual HK recurrence is high (48%), while potassium binder use is low (4%). Compared to patients without HK, patients with HK had higher number of comorbidities (CKD, HF, diabetes, hypertension) and greater use of RAASi at baseline. HK patients were 2.2 times as likely to be hospitalized during a LTC stay and had 1.5 times higher mortality during or within 1 year of a LTC stay. Ultimately, total healthcare costs are 1.5 times higher for patients with HK during a LTC stay. Finally, as 6 in 10 patients were on RAASi prior to the LTC stay, well-tolerated long-term treatment options to manage HK and continue RAASi may be important to improve outcomes. Future studies to address the relationship of CKD and HK in LTC and effects of oral potassium binder use on clinical and economic outcomes are warranted.
References
National Kidney Foundation. Facts about high potassium in patients with kidney disease. https://www.kidney.org/atoz/content/hyperkalemia/facts. Accessed on 27 Oct 2021.
Kovesdy CP. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol. 2014;10:653–62.
Montford JR, Linas S. How dangerous is hyperkalemia? J Am Soc Nephrol. 2017;28(11):3155–65.
Kumar R, Kanev L, Woods SD, et al. Managing hyperkalemia in high-risk patients in long-term care. Am J Manag Care. 2017;23(2 Suppl):S27–36.
Betts KA, Woolley JM, Mu F, et al. The cost of hyperkalemia in the United States. Kidney Int Rep. 2017;14(3):385–93.
Desai NR, Reed P, Alvarez PJ, et al. The economic implications of hyperkalemia in a Medicaid managed care population. Am Health Drug Benefits. 2019;12:352–61.
Desai AS, Swedberg K, McMurray JJ, CHARM Program Investigators, et al. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol. 2007;50:1959–66.
Sharma A, Alvarez PJ, Woods SD, et al. Healthcare resource utilization and costs associated with hyperkalemia in a large managed care population. J Pharm Health Serv Res. 2021;12:35–41.
Harris-Kojetin L, Sengupta M, Park-Lee E, Valverde R. Long-term care services in the United States: 2013 overview. Vital Health Stat. 2013;3(37):1–107.
McDonagh TA, Metra M, Adamo M, ESC Scientific Document Group, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;42:3599–726.
Kidney Disease Improving Global Outcomes (KDIGO). KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. https://www.kidney-international.org/article/S0085-2538(20)30718-3/fulltext. Accessed on 27 Oct 2021.
Epstein M. Hyperkalemia constitutes a constraint for implementing renin-angiotensin-aldosterone inhibition: the widening gap between mandated treatment guidelines and the real-world clinical arena. Kidney Int Suppl. 2011;2016(6):20–8.
Trevisan M, de Deco P, Xu H, et al. Incidence, predictors and clinical management of hyperkalaemia in new users of mineralocorticoid receptor antagonists. Eur J Heart Fail. 2018;20:1217–26.
Ferreira JP, Butler J, Rossignol P, et al. Abnormalities of potassium in heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2836–50.
Dharmarajan TS, Nguyen T, Russell RO. Life-threatening, preventable hyperkalemia in a nursing home resident: case report and literature review. J Am Med Dir Assoc. 2005;6:400–5.
Boyce RD, Perera S, Nace DA, et al. A survey of nursing home physicians to determine laboratory monitoring adverse drug event alert preferences. Appl Clin Inform. 2014;29(5):895–906.
Marcum ZA, Arbogast KL, Behrens MC, et al. Utility of an adverse drug event trigger tool in Veterans Affairs nursing facilities. Consult Pharm. 2013;28:99–109.
Pickenhan L, Rungg C, Schiefermeier-Mach N. Electrolyte imbalances in nursing home residents: a review of prevalence, management and considerations. J Nurs Home Res. 2020;6:14–9.
Mu F, Betts K, Woolley M, et al. Prevalence and economic burden of hyperkalemia in the United States Medicare population. Curr Med Res Opin. 2020;36:1333–41.
Fitch K, Woolley JM, Engel T, et al. The clinical and economic burden of hyperkalemia on medicare and commercial payers. Am Health Drug Benefits. 2017;10:202–10.
Mues KE, Liede A, Liu J, et al. Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol. 2017;9:267–77.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–709.
Health Resources and Services Administration. Consumer Price Index (CPI) for Medical Care. Available from: https://www.hrsa.gov/sites/default/files/hrsa/get-health-care/affordable/hill-burton/cpitables.pdf. Accessed on 3 Sept 2021.
Torlén K, Kalantar-Zadeh K, Molnar MZ, et al. Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clin J Am Soc Nephrol. 2012;7:1272–84.
Rossignol P, Lamiral Z, Frimat L, et al. Hyperkalaemia prevalence, recurrence and management in chronic haemodialysis: a prospective multicentre French regional registry 2-year survey. Nephrol Dial Transplant. 2017;32:2112–8.
Betts KA, Woolley JM, Mu F, et al. The prevalence of hyperkalemia in the United States. Curr Med Res Opin. 2018;34:971–8.
Betts KA, Woolley JM, Mu F, et al. Postdischarge health care costs and readmission in patients with hyperkalemia-related hospitalizations. Kidney Int Rep. 2020;5:1280–90.
Chaitman M, Dixit D, Bridgeman MB. Potassium-binding agents for the clinical management of hyperkalemia. P T. 2016;41:43–50.
Palmer BF. Potassium binders for hyperkalemia in chronic kidney disease-diet, renin-angiotensin-aldosterone system inhibitor therapy, and hemodialysis. Mayo Clin Proc. 2020;95:339–54.
Acknowledgements
Funding
Sponsorship for this study and its publication, including the journal’s Rapid Service and Open Access Fees were funded by AstraZeneca, Gaithersburg, MD, USA.
Medical Writing/Editorial Assistance
Carol Cohen, senior medical writer employed by Inovalon Insights, provided medical writing and editorial support. This support was funded by AstraZeneca, Wilmington, DE, USA.
Authorship
All authors (1) made substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data; (2) drafted or revised the work critically for important intellectual content; (3) approved the submitted version for publication; (4) agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy of integrity of any part of the work are appropriately investigated and resolved. Their contributions meet ICJME guidelines.
Author Contributions
James Neuenschwander: Conceptualization, methodology, interpretation of data, and critically reviewing the manuscript for important intellectual content. Alison R Silverstein: Conceptualization, methodology, data acquisition, interpretation of data, drafting and critically reviewing the manuscript for important intellectual content. Christie L Teigland: Conceptualization, methodology, data acquisition, interpretation of data, drafting and critically reviewing the manuscript for important intellectual content. Shambhavi Kumar: Conceptualization, methodology, data acquisition, data analysis, interpretation of data, drafting and critically reviewing the manuscript for important intellectual content. Edric Y Zeng: Conceptualization, methodology, interpretation of data, and critically reviewing the manuscript for important intellectual content. Abiy T Agiro: Conceptualization, methodology, interpretation of data, and critically reviewing the manuscript for important intellectual content. William J Pottorf: Conceptualization, methodology, interpretation of data, and critically reviewing the manuscript for important intellectual content. W. Frank Peacock: Conceptualization, methodology, interpretation of data, and critically reviewing the manuscript for important intellectual content.
Prior Presentation
This work was presented virtually in part at the American Society of Nephrology Kidney Week 2021 Annual Meeting on the 4th of November, 2021. The corresponding poster number was 1123.
Disclosures
James F Neuenschwander: Consultant and speaker bureau: AstraZeneca, Janssen Pharmaceutical, ThermoFisher; Consultant: Ortho Diagnostics, Siemens, MeMed; Ownership: Asceptoscope; Research grant: AstraZeneca. Alison R Silverstein is an employee of Inovalon, Inc., which received research and analysis fees from AstraZeneca. Christie L Teigland is an employee of Inovalon, Inc., which received research and analysis fees from AstraZeneca. Shambhavi Kumar was an employee of Inovalon, Inc., which received research and analysis fees from AstraZeneca, at the time the work was performed. She is currently employed by Reddit, Inc. Edric Y Zeng was an employee of Avalere Health, which received research and analysis fees from AstraZeneca, at the time the work was performed. He is currently employed as an Independent Contractor. Abiy T Agiro is an employee and stockholder of AstraZeneca. William J Pottorf is an employee and stockholder of AstraZeneca. W. Frank Peacock: Research Grants: Brainbox, Instrument Labs; Consultant: Abbott, Brainbox, Instrument Labs, Janssen, Osler, Roche, Siemens, Vifor; Stock/Ownership Interests: AseptiScope Inc, Brainbox Inc, Braincheck Inc, Coagulo Inc, Comprehensive Research Associates LLC, Comprehensive Research Management Inc, Emergencies in Medicine LLC, Fast Inc, Forrest Devices, Ischemia DX LLC, Lucia Inc, Prevencio Inc, ScPharma, Trivirum Inc, Upstream Inc.
Compliance with Ethics Guidelines
Inovalon, Inc. accessed the 100% Medicare FFS claims through a research-focused data use agreement with the Centers for Medicare & Medicaid Services (CMS) and did not require Institutional Review Board approval due to use of retrospective de-identified patient/member data.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due their proprietary nature.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Neuenschwander, J.F., Silverstein, A.R., Teigland, C.L. et al. The Increased Clinical and Economic Burden of Hyperkalemia in Medicare Patients Admitted to Long-Term Care Settings. Adv Ther 40, 1204–1223 (2023). https://doi.org/10.1007/s12325-022-02420-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02420-x