Abstract
Introduction
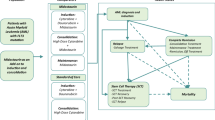
Chronic myeloid leukemia (CML) is a hematopoietic myeloproliferative disorder that accounts for 20% of all leukemias of adults. The introduction of tyrosine kinase inhibitors (TKIs) (imatinib, bosutinib, dasatinib, nilotinib, ponatinib) has yielded significant benefits for patients with CML in terms of survival and quality of life. This real-world analysis evaluated the economic burden for managing patients with CML in 2nd or ≥ 3rd TKI lines in Italian settings of clinical practice.
Methods
A retrospective observational analysis was performed exploiting the administrative databases of a sample of entities covering around 15 million inhabitants. From 2015 to 2018, the study included adult patients with at least one prescription for TKIs, (and for some TKI with at least one hospitalization discharge diagnosis for CML, or at least one prescription for BCR–ABL examination). The index date was the first TKI prescription. Healthcare resource consumption and costs for patients with CML in 2nd and ≥ 3rd line treatment with TKIs were analyzed for drug prescriptions, hospitalizations, specialist visits, and diagnostic services.
Results
In total 635 patients were included, 491 in 2nd line and 144 in 3rd line with TKIs. Dasatinib was the most frequently prescribed drug in 2nd line (28.9%) and imatinib in later lines (26.4%). With progressing lines of treatment, healthcare consumption showed a trend towards increased non-TKI prescriptions per patient (8 for 2nd line and 9.7 for ≥ 3rd line). The management of patients with CML in later lines resulted in increased overall healthcare burden, with hospitalizations accounting for about half of total expenditure, whatever the treatment line and type of TKI.
Conclusions
This analysis in Italian real-life clinical practice reported economic expenditure for patients with CML in 2nd or ≥ 3rd lines with TKIs, mostly burdened by hospitalizations. Such clinical complexity suggests that further efforts are needed to improve the therapeutic management of later lines of CML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The improvement in the survival of patients with chronic myeloid leukemia (CML) together with the life-long treatments required with tyrosine kinase inhibitors (TKI) can lead to a great impact on healthcare direct costs. |
In this setting, evidence from real life is needed to estimate the financial burden in patients treated with TKIs at later lines with long-term exposure. |
A high economic burden for patients with CML in later lines beyond the costs of specific TKI therapy was found, primarily driven by hospitalizations expenditure, suggesting a high level of comorbidity in these patients. |
Introduction
Chronic myeloid leukemia (CML) is a malignant condition of hematopoietic stem cells caused by the reciprocal translocation between chromosomes 9 and 22 (so-called Philadelphia chromosome) that led to fusion gene encoding the constitutively active BCR–ABL1 tyrosine kinase [1, 2]. According to the Global Burden of Disease Study, the CML rates of death and disability-adjusted life years (DALY, i.e., the total of years of life lost because of premature mortality plus years lost to disability/time lived not in full health) showed decreasing trends over the decades. Notably, the approval of the tyrosine kinase inhibitors (TKIs) has significantly contributed to this trend, pointing out the role of TKIs in shaping the therapeutic and disease patterns of CML [3]. Indeed, TKIs dramatically affect the lifespan of patients diagnosed with CML, increasing their probability of survival from a few years to a near-normal life expectancy [4] and becoming the standard of care for the management of such patients [5]. This enhanced survival has resulted from the use of TKIs not only in frontline treatment but also in second or later lines of treatment [1].
TKIs currently approved in Italy are imatinib (first-generation TKI), bosutinib, dasatinib, and nilotinib (second-generation TKI), and ponatinib (third-generation TKI). Except for the last of these, which is indicated for second and later lines, the other TKIs are approved as first or subsequent lines of therapy. The selection of TKI therapy relies on clinical decision and on a patient-centered approach [5,6,7]. Failure of TKI therapies represents a major challenge in the management of CML [8]. Patients may require sequential treatments to overcome inadequate response, resistance, or side effects and to limit the risk of disease progression and death [9]. It has been estimated that over 25% of patients with CML could switch TKIs at least once because of intolerance or resistance [10]. Furthermore, the rate of treatment failure increases while moving through lines of therapy [11].
The burden of TKI treatment failure increases with line of treatment [11]. Indeed, progression to later lines often leads to an impaired health-related quality of life and significantly affects the economic burden of patients with CML. Negi et al. have shown that the switch to subsequent therapy is associated with an increase of healthcare costs as well as a higher resource consumption [12] while an American study based on a claims database showed that the economic burden in the 1 year after TKI treatment failure increases with line of therapy [8]. By exploiting Italian administrative databases, we depict a detailed picture of the economic burden of CML management in later lines in Italy with the evaluation of the healthcare resource consumption and related direct costs of patients with CML in 2nd or ≥ 3rd TKI lines of therapy in Italian settings of clinical practice.
Methods
Data Sources
A retrospective observational analysis was performed on the basis of data from the administrative databases of a sample of Italian Local Health Units (LHUs) covering around 15 million inhabitants (25% of the Italian population) across Italy. The databases used were a demographics database to collect age and sex data; pharmaceuticals database for retrieving data on the drugs prescribed as the anatomical therapeutic code (ATC), prescription date, number of packages, costs per package; hospitalization database, which contains date of hospitalization, diagnoses identified by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), Diagnosis Related Group (DRG), and DRG-related costs provided by the Italian National Health System (INHS); outpatients specialist service database which provides data on the type of laboratory test or specialist visit and prescription date.
To guarantee patient privacy, an anonymous univocal numeric code (Patient ID) allowed electronic linkage between databases. The anonymous code of the patient ensures the anonymity of the extracted data in full compliance with UE Data Privacy Regulation 2016/679 (GDPR) and Italian D.lgs. n. 196/2003, as amended by D.lgs. n. 101/2018. All the results of the analyses were produced as aggregated summaries, which could not be connected, either directly or indirectly, to individual patients. Informed consent was not required since obtaining it is impossible for organizational reasons (pronouncement of the Data Privacy Guarantor Authority, General Authorisation for personal data treatment for scientific research purposes – n. 9/2014). The analysis was approved by the Ethics Committee of the LHUs involved in the project, as reported in the ethics approval section. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Study Population
The study population selection was already described [13]. Briefly, adult patients (≥ 18 years old) were screened for eligibility based on prescription for TKIs used to treat CML (identified by the ATC codes in force at the time of study period: bosutinib, ATC code L01XE14; dasatinib, ATC code L01XE06; imatinib, ATC code L01XE01; nilotinib, ATC code L01XE08; ponatinib, ATC code L01XE24) between January 1, 2010 and December 31, 2018 (inclusion period). Because some TKIs can be prescribed also for the treatment of patients without CML, the following additional criteria were applied in patients treated with such TKIs in order to reduce the possible bias and to properly identify only patients with CML: (i) at least a hospitalization discharge diagnosis for CML (ICD-9-CM code 205.1) or (ii) at least one prescription for BCR–ABL examination (codes 91.36.5, 91.29.4, 91.29.3) without hospitalization discharge diagnosis of lymphoid acute leukemia (ICD-9-CM code 204.0). Fewer than three patients (we cannot disclose the number because of data privacy) that were included in the analysis underwent allogeneic stem cell transplantation.
The line of treatment was determined by counting how many different TKI prescriptions every single patient had over the entire data availability period of the databases at the time of extraction.
Only patients in 2nd line and ≥ 3rd lines of TKI treatment during January 2015-December 2018 were included in the analysis, and the dates of first prescription for 2nd or ≥ 3rd lines during the inclusion period were defined as index date. Patients were followed up from index date until death, exiting the database or end of study period (whatever occurred first).
Healthcare Resource Consumption and Costs
The analyses of healthcare resource consumption and costs were performed on patients in 2nd line (from now on 2nd L cohort) and patient in ≥ 3rd line (from now on ≥ 3rd L cohort). Healthcare resource consumption was reported as annual mean (SD) number per patient of drug treatments prescribed, hospitalizations, specialist visits, and diagnostic services. Direct medical costs in euros (€), derived from all aforementioned healthcare resource consumption variables, were evaluated during the first year of follow-up and reported as annual mean (SD) cost per patient in terms of drugs other than TKI (excluded), all visits, all tests, and all-cause hospitalizations. The costs considering TKI expenditure were also reported. Healthcare direct costs were evaluated from the perspective of the INHS. Drug costs were evaluated on the basis of the INHS purchase price, i.e., costs after deduction of discount available for each LHUs. Hospitalization costs were determined using DRG tariffs, which represent the reimbursement levels by the INHS to healthcare providers. The costs of outpatient services (visits/tests) were defined according to tariffs applied by each region. The annualized healthcare cost of TKI treatment was estimated during the treatment-exposure period (calculated as costs for TKI prescription dispensed during a period from first to last prescription) and reproportioned for a 12-month period.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD), and categorical variables as numbers and percentages. Generalized linear models (GLM) with a gamma distribution were developed to evaluate the impact of TKI therapy, treatment lines, and comorbidities on total annual healthcare costs. A separate analysis was carried out for healthcare expenditure with and without TKI costs.
A P value less than 0.05 was considered statistically significant. All analyses were performed using Stata SE version 12.0 (StataCorp, College Station, TX, USA).
Results
The 2nd L cohort comprised 491 patients: 201 (40.9%) were treated with dasatinib, 142 (28.9%) with nilotinib, 60 (12.2%) with bosutinib, 50 (10.2%) with ponatinib, and 38 (7.8%) with imatinib (Fig. 1a). A total of 144 patients were included in the ≥ 3rd L cohort (Fig. 1b): 38 (26.4%) received imatinib, 32 (22.2%) ponatinib, 27 (18.8%) nilotinib, 24 (16.7%) bosutinib, and 23 (16.0%) dasatinib. Characteristics of patients were described elsewhere [13].
In the 2nd L cohort, an overall annual mean of 8 drug prescriptions (TKI excluded) per patient was reported, from 6.8 for nilotinib, 6.9 for dasatinib, 8.6 for imatinib, 9.7 for ponatinib, and up to 12.4 for bosutinib patients. On average, 2 BCR tests per year per patient were prescribed in each group (except for imatinib patients that had an annual mean of 1.2 BRC tests) (Table 1). In the ≥ 3rd L cohort the mean number of non-TKI drug prescription was 9.7 for overall patients: specifically, nilotinib patients had a mean number of 7.9 non-TKI prescriptions, dasatinib patients 9.0, ponatinib ones 9.8, imatinib 10.5, and bosutinib 10.7. The mean annual number of BCR tests was 1.3 and 1.5 for dasatinib and bosutinib patients, respectively, 1.7 for the imatinib cohort, and 2.1 for nilotinib and ponatinib cohorts (Table 2).
Overall mean annual costs per patient during the first year of follow-up in the 2nd L cohort (TKI excluded) were €12,068 for ponatinib, €6196 for bosutinib, €5098 for dasatinib, €4899 for nilotinib, and €3837 for imatinib (Fig. 2a). Costs were mainly driven by hospitalizations (€8843 for ponatinib, €3020 for bosutinib, €2619 for dasatinib, €2438 for nilotinib, and €1704 for imatinib) and tests (€2264 for ponatinib, €1726 for bosutinib, €1719 for dasatinib, €1646 for nilotinib, and €1247 for imatinib). The same trend was observed for the mean annual costs/patient in the ≥ 3rd L cohort (TKI excluded): €7198 for ponatinib, €5883 for bosutinib, €4974 for bosutinib, €4147 for nilotinib, €3972 for imatinib patients. Similarly to what was reported for the 2nd L cohort, hospitalization and tests accounted for the more expensive items, being respectively €3687 and €1780 for ponatinib, €2742 and €1330 for bosutinib, €1945 and €1887 for dasatinib, €1566 and €1312 for nilotinib, and €1897 and €1084 for imatinib patients (Fig. 2b).
An estimation of the mean annualized cost for TKI was also provided, based on the treatment length within each group. In the 2nd L cohort, the mean annualized cost of TKI therapy tended to be higher among ponatinib patients (€51,559, mean treatment length of 1.50 years) followed by dasatinib (€39,278, mean treatment length of 3.21 years), nilotinib (€35,404, mean treatment length of 3.24 years), bosutinib (€34,801, mean treatment length of 1.42 years), and imatinib (€10,622, mean treatment length of 1.84 years) (Table 3). In the ≥ 3rd L cohort, the annualized mean cost of TKI therapy was €49,909 for ponatinib patients (mean treatment length of 1.50 years), €38,943 for bosutinib patients (mean treatment length 1.14 years), €36,710 for nilotinib patients (mean treatment length of 3.16 years), €31,837 for dasatinib patients (mean treatment length of 2.78 years), and €11,887 for imatinib patients (mean treatment length of 2.42 years) (Table 4).
GLM analyses for total annualized healthcare direct costs (excluding and including drug expense for TKIs) and stratifying by treatment line costs after adjusting for confounding variables (age and gender) are reported in Table 5. Imatinib therapy was chosen as reference. In the estimation of overall cost per year excluding TKIs, treatment with ponatinib was the only significant predictor of cost increase (P = 0.043). The separate analysis of treatment lines revealed that metabolism disorders (P = 0.046) and anemia (P = 0.018) led to significantly increased overall cost per year (excluding TKIs) for 2nd line and ≥ 3rd line, respectively. When including TKIs in the cost evaluation, a rise in total annualized healthcare direct costs was significantly associated to all TKIs treatments analyzed here (P < 0.001 for all TKIs, namely dasatinib, nilotinib, bosutinib, ponatinib), gastrointestinal disease (P = 0.042), and anemia (P = 0.001). Higher overall costs per year for 2nd line TKI treatment were also significantly correlated with all TKI treatments (P < 0.001 for all TKIs) and cardiovascular disease (P = 0.035). For 3rd line treatment, overall annualized costs increased significantly with all TKIs treatments (P < 0.001 for all TKIs) and decreased with anemia (P = 0.017).
Discussion
With the development of TKI drugs, the expected survival of patients diagnosed with CML is approaching that of the general population [14]. Such improvement resulted in a trend of increasing prevalence that, together with the life-long treatments required with TKI, can lead to a great impact on healthcare direct costs. TKIs are associated with high healthcare resource utilization (HRU) and costs, and evidence from real life is needed to estimate the financial burden in patients with later lines with long-term exposure and outside the strict criteria of clinical trials. In this context, the present real-world study investigated the resource consumption and related costs for patients with CML in therapy with later lines of TKI by using administrative data to provide a realistic scenario of the economic burden in settings of daily clinical practice in Italy.
To the best of our knowledge, to date there are no such analyses performed for Italy, as studies are mainly focused on cost-effectiveness analysis estimated by comparing specific TKIs in second or third line based, however, on models and on hypothetical cohorts whose characteristics are assumed by data from clinical trials [15, 16].
Notably the most used TKI in the 3rd L cohort was imatinib. The sequence pattern of this subset of patients [13] shows that patients treated with first line imatinib, after receiving a different TKI in 2nd line, switched back to imatinib. Consistently, other real-world studies on patients with CML, have reported this trend, showing that a sizeable portion of patients in their later lines, at some point, switch back to imatinib [17]. Even though administrative databases do not allow one to retrieve the clinical reasons behind this trend, it is reasonable to speculate that the wide usage of imatinib in later lines might be mainly driven by intolerance to the previous TKIs [18].
Our findings showed a high economic burden for patients with CML in later lines beyond the costs of specific TKI therapy, which could underline a high degree of complexity for the therapeutic management of the disease. This is particularly evident in the cost item distribution, since hospitalizations account for around half of total expenditure in both cohorts, suggesting a high level of comorbidity. This trend was also reported by McGarry et al. [8] with 51.3% and up to 81.1% of mean costs for second and third line related to costs for medical (non-pharmacologic) services. The extensive use of outpatient services (as tests or specialist visits) may be an indicator of the close monitoring of patients with CML during the chronic phase of the disease, in order to prevent/delay progression of the disease, which could further exacerbate healthcare costs. The economic burden of CML progression to the blast phase was evaluated in literature to be indeed mainly driven by extensive use of hospitalization, while outpatient medical service represented only around 6% of the total healthcare costs and reached up to 62.2% for patients with CML without progression [19]. The expenditure for TKIs was excluded from the healthcare cost analysis; however, their economic burden was reported as mean annualized cost based on treatment length. Since only a few patients were found to be treated with generic imatinib, we are not able to provide data regarding the economic impact of generic TKIs versus the branded; however, we foresee that generics will play crucial role in reshaping the economic burden associated with CML [20].
Regarding the mean number of drugs (TKI excluded) reported in the analysis, it should be noted that a TKI could be chosen over others on the basis of the patient’s underlying comorbidities; therefore patients with multiple comorbidities might receive a certain TKI rather than others. Indeed, as reported in our previous work, we found that certain TKIs tended to be prescribed to older patients that may have a multi-comorbid profile [13].
We acknowledge some limitations of the study, mainly due to the data source used, i.e., administrative databases. The first limitation is the lack of clinical information, such as data related to the severity or to the CML phase, evidence of remissions, reason for therapy discontinuation (e.g., intolerance, resistance, treatment-free remission). The second limitation concerns the overall estimation of costs, because administrative data do not capture out of pocket or unreimbursed medical expenses, nor indirect costs or quality of life that may greatly impact the burden of disease. The GLM for cost predictions was based on baseline characteristics retrieved from administrative databases; thus the impact of other variables was not evaluated. Ultimately, we have limited the follow-up to 1 year to understand the short-term impact in terms of direct medical costs. The results of the study refer to the study population and may not be generalizable to the entire national population.
Conclusion
The present real-world study provided the economic scenario of resource consumption in the Italian clinical practice setting, with a focus on expenditure outside the cost of therapy. Our results showed a heavy economic burden for patients in 2nd or ≥ 3rd lines, especially in terms of hospitalizations that underlined a complex disease and comorbid profile, suggesting the need for novel therapeutic options for management of later lines of CML.
References
Chopade P, Akard LP. Improving outcomes in chronic myeloid leukemia over time in the era of tyrosine kinase inhibitors. Clin Lymphoma Myeloma Leuk. 2018;18:710–23.
De Santis S, Monaldi C, Mancini M, Bruno S, Cavo M, Soverini S. Overcoming resistance to kinase inhibitors: the paradigm of chronic myeloid leukemia. Onco Targets Ther. 2022;15:103–16.
Lin Q, Mao L, Shao L, et al. Global, regional, and national burden of chronic myeloid leukemia, 1990–2017: a systematic analysis for the global burden of disease study 2017. Front Oncol. 2020;10:580759.
Sasaki K, Strom SS, O’Brien S, et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015;2:e186–193.
Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84.
Hochhaus A, Saussele S, Rosti G, et al. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv41–51.
Baccarani M, Abruzzese E, Accurso V, et al. Managing chronic myeloid leukemia for treatment-free remission: a proposal from the GIMEMA CML WP. Blood Adv. 2019;3:4280–90.
McGarry LJ, Chen YJ, Divino V, et al. Increasing economic burden of tyrosine kinase inhibitor treatment failure by line of therapy in chronic myeloid leukemia. Curr Med Res Opin. 2016;32:289–99.
Hochhaus A, Breccia M, Saglio G, et al. Expert opinion—management of chronic myeloid leukemia after resistance to second-generation tyrosine kinase inhibitors. Leukemia. 2020;34:1495–502.
Patel AB, O’Hare T, Deininger MW. Mechanisms of resistance to ABL kinase inhibition in CML and the development of next generation ABL kinase inhibitors. Hematol Oncol Clin North Am. 2017;31:589–612.
Zhang S, Maegawa R, Nandal S, Patwardhan P. Targeted literature review of patient reported outcomes (PROs) in chronic myeloid leukemia (CML) patients receiving second and later lines of treatment. Blood. 2020;136:26–7.
Negi H, Agrawal R, Vieira J, Ryan J, Thakur D, Viana R. PCN231 humanistic and economic burden in patients with chronic myeloid leukemia â—a review of the literature. Value Health. 2021;24:S63.
Breccia M, Chiodi F, Nardozza AP, et al. Real-world analysis of the therapeutic management and disease burden in chronic myeloid leukemia patients with later lines in Italy. JCM. 2022;11:3597.
Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM-L. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34:2851–7.
Lucioni C, Iannazzo S, Mazzi S, Saporiti G, Chiroli S. Cost-effectiveness of ponatinib in chronic myeloid leukemia in Italy: Valutazione di costo-efficacia di ponatinib nella terapia della leucemia mieloide cronica in Italia. Global Reg Health Technol Assess. 2015;2:1–16.
Bonifacio M, Maheshwari V, Tran D, Agostoni G, Filioussi K, Viana R. Economic model to evaluate the cost-effectiveness of second-line nilotinib versus dasatinib for the treatment of Philadelphia chromosome-positive chronic myeloid leukemia (CML-CP) in Italy. Pharmacoecon Open. 2021;6:95–104.
Guérin A, Guo A, Williams D, et al. Treatment patterns of chronic myelogenous leukemia patients with suboptimal responses to imatinib. J Clin Oncol. 2009;27:7090–7090.
Campiotti L, Suter MB, Guasti L, et al. Imatinib discontinuation in chronic myeloid leukaemia patients with undetectable BCR–ABL transcript level: a systematic review and a meta-analysis. Eur J Cancer. 2017;77:48–56.
Jabbour EJ, Lin J, Siegartel LR, Lingohr-Smith M, Menges B, Makenbaeva D. Evaluation of healthcare resource utilization and incremental economic burden of patients with chronic myeloid leukemia after disease progression to blast phase. J Med Econ. 2017;20:1007–12.
Erçalışkan A, Seyhan Erdoğan D, Eşkazan AE. Current evidence on the efficacy and safety of generic imatinib in CML and the impact of generics on health care costs. Blood Adv. 2021;5:3344–53.
Acknowledgements
Funding
Novartis Farma S.p.A. purchased the study report that is the basis for this manuscript and funded the journal’s Rapid Service and Open Access Fees. This manuscript was developed with Novartis Farma S.p.A and CliCon S.r.l. Società Benefit. The agreement signed by Clicon S.r.l. Società Benefit and Novartis Farma S.p.A does not create any entityship, joint venture or any similar relationship between parties. Clicon S.r.l. Società Benefit is an independent company. Neither CliCon S.r.l. Società Benefit nor any of their representatives are employees of Novartis Farma S.p.A for any purpose.
Medical Writing and Editorial Assistance
The authors would like to thank Paola Amore, BSc, of Novartis Farma S.p.A. for support in the review process.
Author Contributions
Conceptualization, Massimo Breccia, Francesca Chiodi, Diletta Valsecchi, Valentina Perrone and Luca Degli Esposti; Data curation, Valentina Perrone, Diego Sangiorgi and Luca Degli Esposti; Methodology, Valentina Perrone, Diego Sangiorgi and Luca Degli Esposti; Supervision, Luca Degli Esposti; Validation, Massimo Breccia, Francesca Chiodi, Aurelio Pio Nardozza, Diletta Valsecchi, Valentina Perrone, Maria Chiara Rendace, Paola Coco, Eleonora Premoli. and Luca Degli Esposti; Visualization, Valentina Perrone and Luca Degli Esposti; Writing—original draft, Valentina Perrone and Elisa Giacomini; Writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Disclosures
Massimo Breccia is a medical consultant for Novartis, Pfizer, BMS, Incyte, Abbvie. Francesca Chiodi, Diletta Valsecchi, Maria Chiara Rendace, Paola Coco, Aurelio Pio Nardozza and Eleonora Premoli are employees of Novartis Farma S.p.A., Italy. Valentina Perrone, Diego Sangiorgi, Elisa Giacomini and Luca Degli Esposti are employees of CliCon S.r.l., Italy.
Compliance with Ethics Guidelines
The analysis has been approved by the following ethics committees: Comitato Etico Campania del Sud (protocol number 129951, approval date 02/09/2020 and protocol number 152534, approval date 03/11/2020), Comitato Etico Lazio (protocol number 1166, approval date 12/10/2020), Comitato Etico Lazio 2 (protocol number 0087354, approval date 15/05/2019), Comitato Etico Regionale dell’Umbria (protocol number 19414/20/ON, approval date 16/09/2020), Comitato Etico di Bergamo (protocol code ONCO/EM, approval date 12/10/2018), Comitato Etico per la Sperimentazione Clinica di Verona e Rovigo (protocol number 52048, approval date 25/07/2018), Comitato Etico Lecce (protocol number 25, approval date 29/10/2018), Comitato Etico Indipendente Azienda Ospedaliero Universitaria Consorziale Policlinico (protocol number 0007020, approval date 15/01/2020), Comitato Etico Sezione Area Centro Regione Calabria (protocol number 186, approval date 19/07/2018), Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana (protocol number 20190211, approval date 12/09/2019).
Data Availability
All data used for the current study are available upon reasonable request to CliCon S.r.l., which is the body responsible for data treatment and analysis by Local Health Units.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Breccia, M., Chiodi, F., Nardozza, A.P. et al. The Economic Burden of Chronic Myeloid Leukemia in Patients with Later Lines: Findings from a Real-World Analysis in Italy. Adv Ther 40, 961–974 (2023). https://doi.org/10.1007/s12325-022-02398-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02398-6