Abstract
Introduction
This study investigated factors associated with the antihypertensive effects of esaxerenone and the incidence of serum potassium elevation in patients with hypertension.
Methods
Using pooled data from seven phase III studies, the study analyzed factors associated with changes in office systolic (SBP) and diastolic (DBP) blood pressure from baseline to 12 weeks, and factors associated with incidence of serum potassium levels ≥ 5.5 mEq/L in esaxerenone-treated patients.
Results
Overall, 1466 and 1472 patients were included in the full analysis and safety analysis sets, respectively. Male sex (4.02/2.40 mmHg), weight ≥ 78.4 kg (4.62/2.09 mmHg), hypertension duration ≥ 10 years (2.66/1.71 mmHg), prior antihypertensive treatment (2.38/1.40 mmHg), plasma aldosterone concentration ≥ 120 pg/mL (1.66/1.17 mmHg), urinary albumin-to-creatinine ratio (UACR) ≥ 300 mg/gCr (8.94/4.85 mmHg) or 30–299 mg/gCr (5.17/4.15 mmHg), and smoking (2.62/1.27 mmHg) were associated with mean changes in SBP and DBP. Fasting blood glucose ≥ 126 mg/dL (− 2.73 mmHg) was associated with the mean change in SBP only, and older age (65–74 years, − 2.12 mmHg; and ≥ 75 years, − 3.06 mmHg) with mean change in DBP only. Factors significantly associated with incidence of serum potassium levels ≥ 5.5 mEq/L were higher baseline serum potassium (≥ 4.5 mEq/L, odds ratio [OR] 6.702); lower estimated glomerular filtration rate (≥ 90 mL/min/1.73 m2, OR 0.148; 60–89 mL/min/1.73 m2, OR 0.331 vs 30–59 mL/min/1.73 m2, respectively); higher UACR (30–299 mg/gCr, OR 7.317); higher DBP (≥ 100 mmHg, OR 3.248); and grade I hypertension (OR 2.168).
Conclusion
Esaxerenone is effective in patients with a broad range of backgrounds, though some factors may predict increased benefit. Regarding elevated serum potassium, careful therapeutic management is recommended for patients with higher baseline serum potassium and reduced renal function.
Clinical trial registration: UMIN000047026.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Esaxerenone, an oral, nonsteroidal mineralocorticoid receptor blocker, is well tolerated and effective at managing blood pressure (BP); however, the specific patient groups who would most benefit from esaxerenone treatment are unknown. |
This study aimed to identify factors associated with the antihypertensive effect of esaxerenone, and to identify factors associated with an increased incidence of serum potassium elevation. |
What was learned from the study? |
Several factors including female sex, lower body weight, lower plasma aldosterone concentration, shorter duration of hypertension, no antihypertensive treatment, lower urinary albumin-to-creatinine ratio (UACR), and non-smoking were associated with a stronger reduction in BP under esaxerenone treatment; in addition, factors associated with serum potassium elevation were mainly higher baseline serum potassium, lower estimated glomerular filtration rate (eGFR), and higher UACR. |
Esaxerenone is generally effective in all patient groups; however, a stronger BP-lowering effect may be observed in some patients according to their background characteristics. |
Higher baseline serum potassium level and reduced renal function including lower eGFR and higher UACR were associated with a higher risk of serum potassium elevation, indicating the need for careful therapeutic management in affected patients. |
Introduction
Hypertension is one of the major risk factors for developing cerebrovascular and cardiovascular diseases [1,2,3]. Additionally, hypertension often occurs with comorbidities such as diabetes mellitus, dyslipidemia, and chronic kidney disease (CKD), which can further exacerbate the risk of affected patients developing cerebrovascular and cardiovascular diseases [4,5,6]. Therefore, blood pressure (BP) management in patients with hypertension is important for preventing the development of these diseases.
Mineralocorticoid receptor blockers (MRBs) are one of the options for treating hypertension, and are effective in controlling BP in patients who are unresponsive to conventional antihypertensive therapies when used in combination with first-line antihypertensive agents [7]. However, hyperkalemia may develop in patients who are receiving MRB treatment, particularly in patients with risk factors such as CKD or diabetes mellitus, or if therapies that interfere with potassium homeostasis are used [8]. Nonsteroidal MRBs are third-generation MRBs with both high potency and selectivity against mineralocorticoid receptors [9]. Esaxerenone is an oral, nonsteroidal MRB that has been shown through multiple phase III studies to be generally well tolerated and effective at controlling BP [10,11,12,13,14,15,16], with a recent review summarizing the patient background characteristics in which serum potassium levels may be elevated [17].
The efficacy of antihypertensive therapies can differ depending on the background of the patient. For example, renin–angiotensin system (RAS) inhibitors have been shown to be less effective in treating low-renin hypertension, which is a common form of hypertension in elderly patients [18, 19]. A subgroup analysis of the CS3150-A-J301 (ESAX-HTN) study suggested that women and individuals with lower body mass indices had an improved response to esaxerenone treatment [10]; however, the patients in the ESAX-HTN study had similar backgrounds, and therefore additional characteristics associated with different responses to esaxerenone treatment were not identified. In addition, the results of an exposure–response analysis using population pharmacokinetics analysis suggested that the presence of diabetes mellitus with albuminuria or proteinuria, severe renal dysfunction (estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2), and moderate renal dysfunction (eGFR 30 to < 60 mL/min/1.73 m2) was associated with serum potassium elevation following esaxerenone treatment [20]. However, it is still unknown which specific patient populations will most benefit from the addition of esaxerenone, and whether other patient characteristics affect the antihypertensive effect and incidence of serum potassium elevation with esaxerenone treatment.
We hypothesized that, following treatment with esaxerenone, there will be a group of patients with significantly improved BP control and a group of patients with increased serum potassium. Using the pooled data from the phase III studies of esaxerenone, this study aimed to identify the factors associated with a stronger reduction in BP in hypertensive patients with diverse backgrounds who were receiving esaxerenone treatment. In addition, we examined whether any unknown factors were associated with an increased incidence of serum potassium elevation.
Methods
Study Design and Patients
This was a post hoc analysis of pooled data from seven previously published Japanese phase III studies of esaxerenone: ESAX-HTN, CS3150-A-J302 (J302), CS3150-A-J305 (J305), CS3150-A-J306 (J306), CS3150-A-J307 (J307), CS3150-B-J308 (ESAX-DN), and CS3150-B-J309 (J309) [10,11,12,13,14,15,16]. CS3150-A-J304 was not included in this analysis as the study period was only 8 weeks and not appropriate to include in the pooled analysis [21]. The study designs of each included study and the respective registration identification numbers are shown in Table 1.
Consent was obtained in writing from the participants in each of the previous phase III studies. As this pooled analysis was a secondary use of data obtained from the seven studies, and in accordance with the Ethical Guidelines for Life Science and Medical Research Involving Human Subjects [22], no new consent was required or obtained from the research participants. The study was conducted in accordance with the principles of the Declaration of Helsinki, the Ethical Guidelines for Human Life Science and Medical Research, and the Act on the Protection of Personal Information. Approval was provided by the ethical review committee at the Kitamachi Clinic (Tokyo, Japan) and the study was registered with the University hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000047026).
Efficacy Endpoint
The efficacy endpoint in this study was the factors associated with a change in office systolic and diastolic BP in a sitting position from baseline to 12 weeks in patients receiving esaxerenone. This time period was chosen to ensure a fixed dosing period of at least 4 weeks of esaxerenone administration, as the majority of the phase III studies were designed to titrate esaxerenone over the first 8 weeks of treatment, and was considered to be a sufficient duration to evaluate the BP-lowering effect. This endpoint was analyzed in the overall population and in a subgroup with both office systolic BP (SBP) ≥ 140 mmHg and diastolic BP (DBP) ≥ 90 mmHg at baseline.
Safety Endpoint
The safety endpoint was the factors associated with incidences of serum potassium elevation in patients, pre-defined as serum potassium levels ≥ 5.5 mEq/L during the treatment period.
Statistical Analyses
For the efficacy endpoints, change in office SBP and DBP from baseline to 12 weeks after esaxerenone treatment was used as the objective variable. Subgroup analyses were performed on data from patients with baseline SBP/DBP ≥ 140/90 mmHg. The effect of each factor on the change in BP, its 95% confidence interval (CI), and p value were calculated using univariate and multivariate linear regression models.
In addition, for the occurrence of serum potassium levels ≥ 5.5 mEq/L during the esaxerenone treatment, the effect of each background factor and its 95% CI and p value were calculated using univariate and multivariate logistic regression models. All models, including univariate models, had the baseline value and method of administration (starting dose and whether the study design included titration or a set dose of esaxerenone) as covariates.
When missing BP measurements were present after the start of esaxerenone administration, the last observation carried forward method was applied to impute missing values. Missing background factors were categorized as “unknown”. Both the ESAX-HTN and J302 studies excluded patients with a urinary albumin-to-creatinine ratio (UACR) of ≥ 30 mg/gCr by study design. Thus, all the patients from these studies were considered to have a UACR < 30 mg/gCr. As esaxerenone is contraindicated in patients with either eGFR < 30 mL/min/1.73 m2 or serum potassium levels ≥ 5.0 mEq/L, these patients were excluded from this analysis.
The significance level for hypothesis testing was 5% two-sided, with a two-sided 95% CI. SAS System Release 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Patients
Patients treated with esaxerenone in the full analysis set (FAS) of each study were included in the efficacy analysis (N = 1466). For each study, the number of patients was as follows: ESAX-HTN (n = 667), J302 (n = 368), J305 (n = 58), J306 (n = 51), J307 (n = 44), ESAX-DN (n = 222), and J309 (n = 56). The safety analysis set of each study was used for analysis of the safety endpoint (N = 1472). For each study, the number of patients treated with esaxerenone in the safety analysis set was as follows: ESAX-HTN (n = 669), J302 (n = 368), J305 (n = 58), J306 (n = 51), J307 (n = 44), ESAX-DN (n = 226), and J309 (n = 56). The subgroup analysis included patients who had both office SBP ≥ 140 mmHg and DBP ≥ 90 mmHg at baseline from the FAS of each study (1192/1466; 81.3%).
Patient characteristics in the overall FAS population and subgroups are shown in Table 2. In the overall FAS population, the mean age ± standard deviation was 58.2 ± 10.5 years; 74.0% were male; body weight was 70.4 ± 13.2 kg; SBP/DBP was 152.8/94.6 ± 11.4/8.6 mmHg; eGFR was 75.5 ± 15.4 mL/min/1.73 m2; serum potassium was 4.2 ± 0.3 mEq/L; complication of diabetes mellitus was 34.7%; prior use of antihypertensives was 68.8%, of which 51.2% used RAS inhibitors, and 47.1% used calcium channel blockers (CCB). There was no prior use of thiazide or loop diuretics.
Efficacy Endpoint
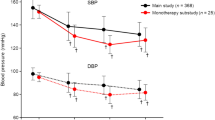
In the overall population, a significant BP reduction was observed in patients treated with esaxerenone. The mean change from baseline at week 12 in SBP and DBP was − 14.4 (95% CI − 15.0, − 13.7) mmHg and − 6.9 (95% CI − 7.3, − 6.5) mmHg, respectively; the change from baseline at week 12 and at the end of study in SBP and DBP for each study is shown in Table 1. The multivariate analysis of factors associated with a change in office SBP/DBP in a sitting position is shown in Table 3, and the univariate analysis of factors is shown in Table S1 in the supplementary material. The estimates (95% CI) indicate the absolute difference in BP change compared to the reference category; negative estimates indicate that BP reduction is larger relative to the reference (i.e., a relatively stronger antihypertensive effect), and positive estimates indicate that the BP reduction is smaller relative to the reference (i.e., a relatively weaker antihypertensive effect). Factors that were significantly associated with a positive estimated change in office SBP in a sitting position from baseline to 12 weeks after esaxerenone treatment relative to the reference (i.e., these factors may be associated with a weaker BP-lowering effect) included male sex (estimate 4.02 mmHg; p < 0.001); higher weight ≥ 78.4 kg (quartile 4) (estimate 4.62 mmHg; p = 0.002); duration of hypertension ≥ 10 years (estimate 2.66 mmHg; p < 0.001); prior treatment with antihypertensive drugs (estimate 2.38 mmHg; p = 0.002); plasma aldosterone concentration (PAC) ≥ 120 pg/mL (estimate 1.66 mmHg; p = 0.016); UACR ≥ 300 mg/gCr (estimate 8.94 mmHg; p < 0.001) or 30–299 mg/gCr (estimate 5.17 mmHg; p = 0.020); and current smoking (estimate 2.62 mmHg; p = 0.002). Conversely, fasting blood glucose ≥ 126 mg/dL was associated with a significantly negative estimated change in office SBP and a stronger BP-lowering effect of esaxerenone relative to the reference (estimate − 2.73 mmHg; p = 0.029).
Similar factors were associated with a change in office DBP, except for age and fasting blood glucose (Table 3). Age ranges of 65–74 years (estimate − 2.12 mmHg; p < 0.001) and ≥ 75 years (estimate − 3.06 mmHg; p = 0.001) were associated with a negative estimated change in office DBP, indicating a stronger BP-lowering effect of esaxerenone relative to the reference. A fasting blood glucose level of ≥ 126 mg/dL was not associated with changes in office DBP. Additionally, office SBP ≥ 160 mmHg was associated with a positive estimated change in office DBP (estimate 1.61 mmHg; p = 0.009), while office DBP ≥ 100 mmHg was not associated with changes in office SBP.
Subgroup Analysis
The results of the subgroup analysis of patients with office SBP ≥ 140 and DBP ≥ 90 mmHg (n = 1192) are shown in Table 4 and Table S2 in the supplementary material. Overall, the factors associated with significant differences observed in office SBP in the subgroup were similar to those seen in the overall population, with the exception of patient age, plasma renin activity (PRA), fasting blood glucose, and UACR: an age of 65–74 years was associated with a negative estimated change in office SBP and indicated a stronger BP-lowering effect of esaxerenone (estimate − 2.21 mmHg; p = 0.022); and PRA ≥ 1.0 ng/mL/h was associated with a positive estimated change in office SBP and indicated a weaker BP-lowering effect (estimate 2.40 mmHg; p = 0.001) in the subgroup analysis. Additionally, higher UACR has a non-significant (p = 0.074) but associates with a positive change in office SBP (i.e., a weaker BP-lowering effect of esaxerenone) and fasting blood glucose ≥ 126 mg/dL has a non-significant (p = 0.073) but negative estimated change in the subgroup analysis; these factors were significantly associated with changes in office SBP in the overall population.
The following factors were associated with a negative estimated change in office DBP (indicatiing a stronger BP-lowering effect) in the subgroup but not in the overall group: the presence of grade II hypertension compared with grade I hypertension (estimate − 1.95 mmHg; p = 0.008), and eGFR of 60–89 and ≥ 90 mL/min/1.73 m2 compared with 30–59 mL/min/1.73 m2 (estimate − 3.86 mmHg, p = 0.013; and estimate − 3.87 mmHg, p = 0.018, respectively). Additionally, as well as in SBP, PRA was identified as a factor associated with a weaker reduction in office DBP for the subgroup, but not in the overall population.
Safety Endpoint
The number of patients in the overall population who experienced serum potassium levels ≥ 5.5 mEq/L during the treatment was 116 out of 1472 patients (7.9%). The incidence of serum potassium levels ≥ 5.5 mEq/L for each study are shown in Table 1, and the factors associated with incidence of serum potassium ≥ 5.5 mEq/L are shown in Table 5 and Table S3 in the supplementary material. For each factor, an odds ratio (OR) greater than 1 (95% CI) indicates higher risk factors and an OR below 1 indicates lower risk factors. The factors associated with an incidence of serum potassium ≥ 5.5 mEq/L were higher baseline serum potassium; lower eGFR; and higher UACR; higher DBP; type of hypertension (grade I vs normotensive) based on the multivariate analysis. Higher baseline serum potassium (≥ 4.5 vs < 4.5 mEq/L, OR 6.702, p < 0.001), higher UACR (30–299 vs < 30 mg/gCr, OR 7.317, p = 0.007; ≥ 300 vs < 30 mg/gCr, OR 4.360, p = 0.067), and higher DBP (≥ 100 vs < 100 mmHg, OR 3.248, p = 0.020) were observed to be associated with high-risk for the incidence of serum potassium ≥ 5.5 mEq/L. Additionally, eGFR of 30–59 mL/min/1.73 m2 was also observed to be associated with high-risk for incidence of serum potassium ≥ 5.5 mEq/L (≥ 90 vs 30–59 mL/min/1.73 m2, OR 0.148, p = 0.001; 60–89 vs 30–59 mL/min/1.73 m2, OR 0.331, p = 0.002).
Discussion
This study aimed to identify the factors associated with a weaker or stronger reduction in BP in patients receiving esaxerenone treatment, as well as the factors associated with the high incidence of serum potassium ≥ 5.5 mEq/L, using data derived from seven phase III clinical trials [10,11,12,13,14,15,16]. We identified the following factors that may be associated with a stronger antihypertensive effect of esaxerenone both in the overall population and subgroup with SBP/DBP ≥ 140/90 mmHg: female sex, lower weight, shorter duration of hypertension, treatment-naïve patients on antihypertensives, lower PAC, and non-smokers. Moreover, lower UACR may also be associated with a stronger BP-lowering effect of esaxerenone in the overall population. Patients who are older may also experience a stronger antihypertensive effect of esaxerenone, as evidenced by a greater reduction in DBP. Furthermore, higher eGFR and lower PRA may be associated with a stronger antihypertensive effect of esaxerenone, particularly in the subgroup of patients with high baseline SBP and DBP.
In the 2019 Japanese Society of Hypertension (JSH) guideline, MRB is positioned as the fourth-line antihypertensive drug and currently recommended for treatment-resistant hypertension [23]; however, our findings suggest that treatment-naïve patients who receive esaxerenone as first-line therapy may achieve better antihypertensive efficacy. To date, esaxerenone monotherapy has been shown to have a comparable antihypertensive effect in combination with a RAS inhibitor and CCB [11]. Future studies are needed to investigate the antihypertensive effect of esaxerenone through comparative studies in the monotherapy, RAS combination, and CCB combination groups.
The finding that the stronger BP reduction in patients with lower PRA levels compared to those with higher levels provides insight into the patients who are best suited to receive esaxerenone treatment. Hypertensive patients who are elderly or have excessive salt intake have lower PRA levels [24, 25]. The present study suggests that a stronger antihypertensive effect is expected in patients aged over 65 years compared with those under 65 years, suggesting that patients with PRA < 1 ng/mL/h may be an optimal patient population for esaxerenone administration.
In the current analysis, the estimated effect on BP was calculated as absolute values to show the change in specific BP values. We believe that presenting the data in this way will be easier for clinicians to understand the clinical effect of each factor on BP, compared to relative measures such as odds ratio. Furthermore, we considered changes in SBP and DBP of 5 mmHg or more to be clinically significant. While not reaching statistical significance in all analyses, patients with UACR < 30 mg/gCr tended to experience a stronger antihypertensive effect that reached our definition of clinical significance, compared with patients with higher UACR. Esaxerenone has previously been reported to improve UACR [12, 13, 15, 16, 26]; this may indicate that the use of esaxerenone during the early stages of diabetes (i.e., in the absence of albuminuria) can provide a stronger antihypertensive effect while simultaneously delaying the transition to microalbuminuria [27]. In addition, this study revealed that the antihypertensive effect of esaxerenone was constant irrespective of the presence or absence of diabetes or the hemoglobin A1c levels, and was partially enhanced at higher fasting glucose levels in office SBP in the overall population. In conjunction with the JSH guidelines recommending RAS inhibitors as treatment for hypertensive patients with diabetes [23], esaxerenone may have a stronger antihypertensive effect in hypertensive patients with diabetes who have an inadequate response to RAS inhibitors.
Previous analyses of the phase III esaxerenone studies have shown that the presence of diabetes mellitus with albuminuria or proteinuria, moderate renal dysfunction (eGFR ≥ 30 to < 60 mL/min/1.73 m2), elderly patients, patients with higher baseline serum potassium levels, and patients treated with RAS inhibitors were at risk of serum potassium elevation during esaxerenone treatment, resulting in these factors being listed as potential risks on the package insert [17, 20]. These risk factors are generally consistent with those reported in previous studies of other MRBs, such as eplerenone [28, 29], finerenone [30], and spironolactone [29, 31]. In the present analysis, some of these known risk factors for esaxerenone and other MRBs, such as elderly patients and diabetes, were not associated with serum potassium elevation. However, patients with higher baseline serum potassium (≥ 4.5 mEq/L), lower eGFR (30–59 mL/min/1.73 m2), as well as UACR (30–299 mg/gCr) had significantly higher ORs, and patients with higher UACR (≥ 300 mg/gCr) also had higher OR, although not reaching statistical significance (OR 4.360, p = 0.067). Thus, many of the known risk factors listed on the package insert of esaxerenone were also detected in the present analysis. Patients with lower eGFR and higher UACR have impaired renal function [32, 33], suggesting that these patients may still require careful monitoring and management while receiving esaxerenone treatment.
We acknowledge the limitations of this study. We conducted this study using the data from seven phase III studies with different study designs (e.g., inclusion/exclusion criteria, concomitant medication use, etc.) to include a broad patient population and to retain a larger sample size, although an integrated analysis using only studies with similar designs might more precisely estimate the effect of each factor. Therefore, there are not only differences in patient backgrounds between studies but also potential differences that are not necessarily fully accounted for, which may bias the estimated the effect of the background factors considered in this study. For example, the phase III protocol for esaxerenone specifies that hypertensive patients with diabetes mellitus should be treated with a RAS inhibitor and that those with essential hypertension should be treated with CCB. Thus, patients categorized with the background factor of “prior antihypertensive drug treatment” would include their unknown medical history as a potential hidden factor. Similarly, the background factor of “diabetes mellitus” may hide the influence of concomitant antihypertensive medications. Conversely, combining studies can increase the statistical power to detect the smaller effect because of the larger data set, even if there are differences in study designs. Regarding the safety endpoint, the total number of incidences of serum potassium levels ≥ 5.5 mEq/L was 116 (7.9%), which might be a somewhat small number of occurrences to detect risk factors. Integration of a larger data set would provide further information and greater precision regarding risk factors associated with serum potassium elevation following esaxerenone treatment.
Conclusions
This post hoc analysis from seven previously published Japanese phase III studies of esaxerenone investigated the factors that may be associated with the antihypertensive effect of esaxerenone and incidence of serum potassium elevation following esaxerenone treatment. Esaxerenone treatment results in a robust antihypertensive effect in patients with a broad range of backgrounds; however, we found a stronger BP reduction in female patients, patients with lower body weights, with PAC < 120 pg/mL, with a shorter duration of hypertension, who had not received hypertensive treatment before, and with UACR < 30 mg/gCr. Additionally, in the subgroup analysis of patients with baseline SBP/DBP > 140/90 mmHg, patients with PRA < 1 experienced a stronger BP reduction under the esaxerenone treatment. Patients with baseline serum potassium ≥ 4.5 mEq/L, eGFR levels 30–59 mL/min/1.73 m2, or UACR of 30–299 mg/gCr may be at higher risk for the incidence of serum potassium elevation via reduced renal function. Careful therapeutic management of patients with reduced renal function is recommended when administering esaxerenone.
References
Fujiyoshi A, Ohkubo T, Miura K, et al. Blood pressure categories and long-term risk of cardiovascular disease according to age group in Japanese men and women. Hypertens Res. 2012;35:947–53.
Takashima N, Ohkubo T, Miura K, et al. Long-term risk of BP values above normal for cardiovascular mortality: a 24-year observation of Japanese aged 30 to 92 years. J Hypertens. 2012;30:2299–306.
Asayama K, Satoh M, Murakami Y, et al. Cardiovascular risk with and without antihypertensive drug treatment in the Japanese general population: participant-level meta-analysis. Hypertension. 2014;63:1189–97.
Hirayama A, Konta T, Kamei K, et al. Blood pressure, proteinuria, and renal function decline: associations in a large community-based population. Am J Hypertens. 2015;28:1150–6.
Kokubo Y, Okamura T, Watanabe M, et al. The combined impact of blood pressure category and glucose abnormality on the incidence of cardiovascular diseases in a Japanese urban cohort: the Suita Study. Hypertens Res. 2010;33:1238–43.
Kokubo Y, Nakamura S, Okamura T, et al. Relationship between blood pressure category and incidence of stroke and myocardial infarction in an urban Japanese population with and without chronic kidney disease: the Suita Study. Stroke. 2009;40:2674–9.
Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53–90.
Lakkis JI, Weir MR. Hyperkalemia in the hypertensive patient. Curr Cardiol Rep. 2018;20:12.
Wan N, Rahman A, Nishiyama A. Esaxerenone, a novel nonsteroidal mineralocorticoid receptor blocker (MRB) in hypertension and chronic kidney disease. J Hum Hypertens. 2021;35:148–56.
Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, Yamakawa S. Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN Study). Hypertension. 2020;75:51–8.
Rakugi H, Ito S, Itoh H, Okuda Y, Yamakawa S. Long-term phase 3 study of esaxerenone as mono or combination therapy with other antihypertensive drugs in patients with essential hypertension. Hypertens Res. 2019;42:1932–41.
Ito S, Itoh H, Rakugi H, Okuda Y, Iijima S. Antihypertensive effects and safety of esaxerenone in patients with moderate kidney dysfunction. Hypertens Res. 2021;44:489–97.
Itoh H, Ito S, Rakugi H, Okuda Y, Nishioka S. Efficacy and safety of dosage-escalation of low-dosage esaxerenone added to a RAS inhibitor in hypertensive patients with type 2 diabetes and albuminuria: a single-arm, open-label study. Hypertens Res. 2019;42:1572–81.
Satoh F, Ito S, Itoh H, et al. Efficacy and safety of esaxerenone (CS-3150), a newly available nonsteroidal mineralocorticoid receptor blocker, in hypertensive patients with primary aldosteronism. Hypertens Res. 2021;44:464–72.
Ito S, Kashihara N, Shikata K, et al. Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN): phase 3 randomized controlled clinical trial. Clin J Am Soc Nephrol. 2020;15:1715–27.
Ito S, Kashihara N, Shikata K, et al. Efficacy and safety of esaxerenone (CS-3150) in Japanese patients with type 2 diabetes and macroalbuminuria: a multicenter, single-arm, open-label phase III study. Clin Exp Nephrol. 2021;25:1070–8.
Rakugi H, Yamakawa S, Sugimoto K. Management of hyperkalemia during treatment with mineralocorticoid receptor blockers: findings from esaxerenone. Hypertens Res. 2021;44:371–85.
Weinberger MH, White WB, Ruilope LM, et al. Effects of eplerenone versus losartan in patients with low-renin hypertension. Am Heart J. 2005;150:426–33.
Belmin J, Lévy BI, Michel JB. Changes in the renin-angiotensin-aldosterone axis in later life. Drugs Aging. 1994;5:391–400.
Japanese package insert of esaxerenone. 2022; ver.5. https://pins.japic.or.jp/pdf/newPINS/00070243.pdf [In Japanese]. Accessed 2 Nov 2022.
Rakugi H, Ito S, Ito H, Okuda Y, Iijima S. The efficacy and safety of esaxerenone for patients with grade III hypertension. Prog Med. 2020;40:755–60 (In Japanese).
Japanese Ministry of Education, Culture, Sports, Science and Technology, Ministry of Health, Labour Standards, Ministry of Economy, Trade and Industry. Ethical Guidelines for Life Science and Medical Research Involving Human Subjects, Ministry of Education, Culture, Sports, Science and Technology, Ministry of Health, Labor and Welfare, and Ministry of Economy, Trade and Industry Notification No. 1. 2022. https://www.meti.go.jp/press/2021/03/20220310006/20220310006-1.pdf [In Japanese]. Accessed 2 Nov 2022.
Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Nanba K, Vaidya A, Rainey WE. Aging and adrenal aldosterone production. Hypertension. 2018;71:218–23.
Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2020;12:CD004022.
Uchida HA, Nakajima H, Hashimoto M, et al. Efficacy and safety of esaxerenone in hypertensive patients with diabetic kidney disease: A multicenter open-label prospective study. Adv Ther. 2022;39:5158–75.
Vodošek Hojs N, Bevc S, Ekart R, Piko N, Petreski T, Hojs R. Mineralocorticoid receptor antagonists in diabetic kidney disease. Pharmaceuticals (Basel). 2021;14:561.
Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail. 2014;7:51–8.
Trevisan M, de Deco P, Xu H, et al. Incidence, predictors and clinical management of hyperkalaemia in new users of mineralocorticoid receptor antagonists. Eur J Heart Fail. 2018;20:1217–26 (Erratum in: Eur J Heart Fail. 2019; 21:540).
Agarwal R, Joseph A, Anker SD, et al. Hyperkalemia risk with finerenone: results from the FIDELIO-DKD trial. J Am Soc Nephrol. 2022;33:225–37.
Gwoo S, Kim YN, Shin HS, Jung YS, Rim H. Predictors of hyperkalemia risk after hypertension control with aldosterone blockade according to the presence or absence of chronic kidney disease. Nephron Clin Pract. 2014;128:381–6.
Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–85.
Fox CS, Matsushita K, Woodward M, et al. Chronic Kidney Disease Prognosis Consortium. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–73.
Acknowledgements
Funding
This study was funded by Daiichi Sankyo Co., Ltd. Daiichi Sankyo Co., Ltd. funded the journal’s Rapid Service and Open Access fees.
Medical Writing
Medical writing support was provided by Hannah Read, PhD, of Edanz (www.edanz.com), and funded by Daiichi Sankyo Co, Ltd., in accordance with Good Publication Practice 2022 guidelines (https://www.ismpp.org/gpp-2022).
Author Contributions
All authors contributed to the study design and planning of data analysis, conduct of the study, data interpretation, writing/reviewing the manuscript, and gave their final approval of the manuscript for submission.
Disclosures
Sadayoshi Ito has received personal fees from Daiichi Sankyo Co., Ltd. Yasuyuki Okuda, and Kotaro Sugimoto are employees of Daiichi Sankyo Co., Ltd.
Compliance with Ethics Guidelines
Consent was obtained in writing from the participants in each of the previous phase III studies. As this pooled analysis was a secondary use of data obtained from the seven studies, and in accordance with the Ethical Guidelines for Life Science and Medical Research Involving Human Subjects [32], no new consent was required or obtained from the research participants. The study was conducted in accordance with the principles of the Declaration of Helsinki, the Ethical Guidelines for Human Life Science and Medical Research, and the Act on the Protection of Personal Information. Approval was provided by the ethical review committee at the Kitamachi Clinic (Tokyo, Japan) and the study was registered with the University hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000047026).
Data Availability
The anonymized data underlying the results presented in this manuscript may be made available to researchers upon submission of a reasonable request to the corresponding author. The decision to disclose the data will be made by the corresponding author and the funder, Daiichi Sankyo Co., Ltd. Data disclosure can be requested for 36 months from article publication.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ito, S., Okuda, Y. & Sugimoto, K. Factors Associated with the Antihypertensive Effect of Esaxerenone and Serum Potassium Elevation: A Pooled Analysis of Seven Phase III Studies. Adv Ther 40, 1242–1266 (2023). https://doi.org/10.1007/s12325-022-02393-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02393-x