Abstract
Introduction
The randomized phase III KEYNOTE-522 trial demonstrated that addition of pembrolizumab to neoadjuvant chemotherapy provided a significant improvement in event-free survival and a favorable trend in overall survival for high-risk early-stage triple-negative breast cancer (eTNBC). This analysis evaluated the cost-effectiveness of pembrolizumab in combination with chemotherapy as neoadjuvant treatment and continued as a single-agent adjuvant treatment after surgery vs. neoadjuvant chemotherapy for patients with high-risk eTNBC in the USA.
Methods
The analysis was conducted from a US third-party public healthcare payer perspective. A multistate transition model was developed using efficacy and safety data from the KEYNOTE-522 trial. The model included four mutually exclusive health states: event-free, locoregional recurrence, distant metastasis, and death to simulate patients’ lifetime disease course. Quality-adjusted life years (QALYs) were calculated on the basis of EuroQoL-5 Dimensions utility data collected in KEYNOTE-522. Costs for drug acquisition/administration, adverse events, disease management, and subsequent therapies were reported (2021 US dollars). Costs and outcomes were discounted at 3% annually. A series of sensitivity analyses were performed to test the robustness of the main results.
Results
In the base case scenario, pembrolizumab plus chemotherapy followed by pembrolizumab resulted in expected gains of 3.37 life years (LYs) and 2.90 QALYs, and an incremental cost of $79,046 versus chemotherapy. The incremental cost per QALY gained was $27,285, which is lower than all commonly cited US willingness-to-pay thresholds. Sensitivity analyses showed the results were robust over plausible values of key model inputs and assumptions.
Conclusions
Compared with neoadjuvant chemotherapy, pembrolizumab in combination with chemotherapy as neoadjuvant treatment and continued as a single-agent adjuvant treatment after surgery is considered a cost-effective option for high-risk eTNBC in the USA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Pembrolizumab in combination with chemotherapy as neoadjuvant treatment and then continued as a single-agent adjuvant treatment after surgery has been approved in the USA for high-risk early-stage triple-negative breast cancer (eTNBC) on the basis of results from the randomized phase III KEYNOTE-522 trial, demonstrating that addition of pembrolizumab to neoadjuvant chemotherapy provided significantly improved event-free survival and a favorable trend in overall survival in high-risk eTNBC. |
This study aimed to evaluate the cost-effectiveness of pembrolizumab in combination with chemotherapy as neoadjuvant treatment and continued as a single-agent adjuvant treatment after surgery in patients with high-risk eTNBC from a US third-party payer perspective. |
What was learned from this study? |
The results show that neoadjuvant pembrolizumab + chemotherapy followed by adjuvant pembrolizumab is expected to yield substantial improvements in quality-adjusted life years (QALYs) (by 2.90) and LYs (by 3.37) relative to treatment with neoadjuvant chemotherapy alone. |
The incremental cost-effectiveness ratio (ICER) was estimated at $27,285/QALY in the base case, which is lower than all commonly cited willingness-to-pay thresholds in the USA. |
Thus, pembrolizumab can be considered a cost-effective treatment option for patients with high-risk eTNBC in the USA, in addition to its higher efficacy versus chemotherapy as demonstrated in KEYNOTE-522. |
Introduction
In 2021, there were an estimated 281,550 new cases of breast cancer (BC) and 43,600 BC-related deaths among women in the USA [1]. Approximately 10–15% of cases are triple-negative BC (TNBC), a heterogenous subtype lacking expression of the estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (also known as ERBB2) [2,3,4]. These receptors are common therapeutic targets in BC, making TNBC more difficult to treat and associated with worse outcomes and higher recurrence rates than other BC subtypes [2, 5,6,7]. Additionally, molecular subtyping can identify patients with high-risk forms of TNBC [8, 9].
Definitive surgical resection is the mainstay approach for the treatment of early-stage BC. Neoadjuvant (pre-surgical) chemotherapy has been the standard of care for high-risk early stage TNBC (eTNBC) [10, 11], while adjuvant (post-surgical) chemotherapy may or may not be used in this population [12]. However, high risks of recurrence and death remain for these patients, pointing to the urgent, unmet need for novel therapies that can augment the effectiveness of chemotherapy [13].
Pembrolizumab is a humanized monoclonal programmed cell death-1 (PD-1) antibody that inhibits the interaction between PD-1 and programmed death ligand 1 (PD-L1)/PD-L2 receptors [14]. Pembrolizumab for the treatment of high-risk eTNBC in combination with chemotherapy as neoadjuvant treatment and then continued as a single-agent adjuvant treatment after surgery was approved by the US Food and Drug Administration in July 2021 and the European Medicines Agency in May 2022 [15,16,17,18]. The regulatory approval was based on the results of the phase III KEYNOTE-522 trial (Clinicaltrials.gov identifier NCT03036488) comparing the efficacy and safety of neoadjuvant pembrolizumab + chemotherapy followed by adjuvant pembrolizumab after surgery (referred to henceforth as pembrolizumab + chemotherapy/pembrolizumab) with that of neoadjuvant placebo + chemotherapy followed by adjuvant placebo after surgery (referred to as chemotherapy henceforth) in treatment-naïve patients with high-risk stage II or III TNBC [16]. The first interim analysis of KEYNOTE-522 (median follow-up 15.5 months) indicated that pathological complete response (i.e., no invasive cancer in the breast and negative nodes) was significantly higher among patients receiving neoadjuvant pembrolizumab + chemotherapy vs. chemotherapy (64.8% vs. 51.2%, p < 0.001) [14]. The fourth interim analysis of KEYNOTE-522 (median follow-up 39.1 months) indicated a significant and clinically meaningful improvement in event-free survival (EFS) among the pembrolizumab + chemotherapy/pembrolizumab group vs. the chemotherapy group (36-month EFS rate 84.3% vs. 76.2%; hazard ratio [95% confidence interval (CI)] 0.63 [0.48, 0.82]; p < 0.001) [19]. Numerical improvement on overall survival (OS) was also observed (36-month OS rate 89.7% vs. 86.9%; HR 0.72; 95% CI [0.51, 1.02]). Furthermore, the addition of pembrolizumab to neoadjuvant chemotherapy was generally well tolerated, with no new safety concerns [19].
The economic value of pembrolizumab in eTNBC has not been comprehensively assessed but is essential information for healthcare payers considering the costs and clinical benefits of a new therapy for reimbursement decisions. Thus, the objective of this study was to evaluate the cost-effectiveness of pembrolizumab + chemotherapy/pembrolizumab vs. neoadjuvant chemotherapy in patients with high-risk eTNBC from a US third-party payer perspective. The cost-effectiveness analysis (CEA) was informed by data from KEYNOTE-522.
Methods
Model Overview
A multistate transition model was developed in Microsoft® Excel 2016 to evaluate the economic value of the addition of pembrolizumab to neoadjuvant chemotherapy for high-risk eTNBC from a US third-party public healthcare payer perspective. Efficacy, safety, and utility data were derived from KEYNOTE-522 (data cutoff: March 23, 2021; median duration of follow-up: 39.1 months) [19]. The main outcome of the model was the incremental cost-effectiveness ratio (ICER) in terms of the incremental costs per quality-adjusted life year (QALY) and life year (LY) gained. A lifetime horizon (51 years) was used and based on an average starting age of 49 years from KEYNOTE-522. A weekly cycle length was used to accurately estimate clinical outcomes and treatment-related costs. Costs and effectiveness were discounted at 3% annually, as recommended by the Institute for Clinical and Economic Review [20].
Target Population and Interventions
The target population was patients with high-risk eTNBC, based on the population of KEYNOTE-522. Patients in KEYNOTE-522 were randomized to pembrolizumab + chemotherapy/pembrolizumab or placebo + chemotherapy/placebo arms. In the neoadjuvant phase, pembrolizumab (at a dose of 200 mg) or placebo was administered once every 3 weeks (q3w) up to eight cycles, and the chemotherapy consisted of four cycles of paclitaxel (80 mg/m2 once weekly [qw]) and carboplatin (area under the curve [AUC5]) q3w or AUC 1.5 qw in the first 12 weeks), followed by four cycles of doxorubicin (60 mg/m2) or epirubicin (90 mg/m2) plus cyclophosphamide (600 mg/m2 q3w in the subsequent 12 weeks). Each subject underwent definitive surgery 3–6 weeks after conclusion of the neoadjuvant treatment. In the adjuvant phase, pembrolizumab 200 mg or placebo was administered q3w for up to nine cycles. Postoperative radiation therapy was acceptable in accordance with the standard of care as applicable.
Model Structure
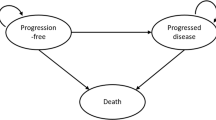
The model included four mutually exclusive health states to simulate patients’ disease course over time: event-free (EF), locoregional recurrence (LR), distant metastasis (DM), and death (Fig. 1). For each health state, costs and utility were assigned within each weekly cycle to estimate the cumulative costs and health outcomes over the modeled time horizon.
Effectiveness Inputs
Transition Probabilities (TPs) Between Health States
TPs between health states were estimated on the basis of the patient-level data from KEYNOTE-522, with extrapolation using fitted parametric functions. Parametric function fitting was carried out in line with the National Institute for Health and Care Excellence (NICE) Decision Support Unit guidelines for survival analysis [21]. Standard one- and two-piece models were explored using exponential, Weibull, log-normal, log-logistic, Gompertz, gamma, and generalized gamma distributions. Statistical tests based on the Akaike information criterion (AIC) and Bayesian information criterion (BIC) combined with visual inspection were used to select the best-fit parametric distributions for the base case. Finally, the clinical plausibility of the long-term extrapolations was evaluated for the selected distributions. Alternative parametric functions were tested in scenario analyses.
TPs from EF State
The modeled cohorts started from the EF state and were subject to the risks of three competing events: LR, DM, or death. As of the trial data cutoff for KEYNOTE-522, 15.7% of patients in the pembrolizumab + chemotherapy/pembrolizumab arm and 23.8% in the chemotherapy arm had at least one documented event. For each model cycle, the cause-specific probability of each transition (EF → LR, EF → DM, or EF → death) was calculated on the basis of the estimated probability of an EFS event, along with the probabilities of LR, DM, or death being the first event in each treatment arm. The probability of the EFS event being LR, DM, or death was estimated using the KEYNOTE-522 trial data. Competing risks among the three events were considered [22].
To estimate the EFS probability over the modeled time horizon, parametric functions were used to extrapolate the EFS data beyond the trial period. First, standard parametric models were fitted to the patient-level data in the KEYNOTE-522 trial for each treatment arm. However, they were not able to provide a good fit to the observed data. Therefore, two-phase parametric models were conducted. The Kaplan–Meier (KM) curves, hazard plots, and cumulative hazard plots of the EFS data were inspected to identify the appropriate cutoff point for the two-phase fittings. Results suggested week 50 as an optimal turning point to test in the economic model. This is because it provided plausible visual fit and a good balance of robust KM data to be used directly in the first phase and enough remaining data to be used to fit a parametric curve in the second phase. Other cutoff points were tested as scenario analyses. The area under the KM curve was used directly for the first phase of extrapolation until week 50, and parametric functions were fitted thereafter. The generalized-gamma and log-normal distribution were selected as the best-fitting distributions and were used in the model base case for the pembrolizumab + chemotherapy/pembrolizumab and the chemotherapy arm, respectively. The choice was based on lowest AIC/BIC statistics, visual inspections against the observed data, and clinical plausibility of the extrapolations. The KM curves and the base case extrapolated curves of EFS are shown in Fig. 2. Alternative parametric functions and the base case curves are presented in Fig. S1 in the supplementary material.
Modeled EFS curves. EFS event-free survival, KM Kaplan–Meier. Modeled curve for pembrolizumab + chemotherapy/pembrolizumab: KM curve followed by generalized gamma distribution from week 50 and onwards; modeled curve for chemotherapy: KM curve followed by log-normal distribution from week 50 and onwards
The estimated values of cumulative incidence of the three transitions EF → LR, EF → DM, and EF → death, as well as the cumulative incidence directly from the KEYNOTE-522 trial data, are shown in Fig. 3.
TPs from LR State
Parametric models were fitted to the data of time to DM or death following LR from the KEYNOTE-522 trial. As a result of the limited number of patients with documented LR in the trial, pooled data from both treatment arms were used. The exponential distribution was found to be the best fit. Therefore, the probability of LR → DM or death was estimated to be constant over time. For each model cycle, the transition probabilities of LR → DM and LR → death were calculated on the basis of the estimated probability of LR → DM or death, and the proportions of DM and death, respectively.
TPs from DM State
The TP from DM to death was estimated on the basis of the survival time following DM for patients who had documented DM in the KEYNOTE-522 trial. Parametric models were fitted to the patient-level data for each treatment arm and exponential distribution was found to be the best fit. Therefore, the time-constant TP of DM → death was estimated on the basis of the fitted exponential distribution.
In addition, age-specific annual probabilities of death for the general population were obtained from the US National Vital Statistics Report [23] and all individuals in the model were subject to general mortality [24].
Time on Treatment (TOT)
TOT data from KEYNOTE-522 were used to separately model the durations of neoadjuvant and adjuvant treatment, which accounted for early treatment discontinuation. No more patients remained on study interventions as of the data cutoff; therefore, the observed TOT data were used directly in the model, avoiding the need for extrapolation (Fig. S2 in the supplementary material).
Adverse Event (AE) Rates and Duration
The model considered all-cause grade 3+ AEs with an incidence of at least 5% from the combined neoadjuvant and adjuvant phases in either arm. Incidence rates, mean durations, and hospitalization rates of AEs were obtained from KEYNOTE-522 (Table 1).
Utility Inputs
Health state utility inputs were derived from the primary analyses of the EuroQoL-five-dimension-five level (EQ-5D-5L) data collected in KEYNOTE-522. The EQ-5D-5L questionnaire was administered at treatment cycle 1 of treatment 1 and cycle 1 and 4 of treatment 2 in the neoadjuvant phase, and treatment cycle 1, 5 and 9 in the adjuvant treatment phase, as well as the early discontinuation visit and long-term follow-up visits. Generic health status assessed with the EQ-5D-5L questionnaire was converted to a population-based utility value using a US scoring algorithm based on time trade-off techniques. The generic health status assessed from the EQ-5D questionnaires was converted to population-based utility values using published algorithms (US 5L value set [25] for the base case and crosswalk from 5L to three-level (3L) [26] along with US 3L value set [27] for the scenario analyses). Utilities were analyzed by health state, treatment status, and AE status. Pooled data were used as there were no significant differences between treatment arms (Table 1).
Healthcare Resource Use (HCRU) and Cost Inputs
The cost inputs used in the model, including costs of drug acquisition and administration, disease management, terminal care, and AE management were based on the US clinical practice and are listed in Table 1. It was assumed that 80% of healthcare costs would be paid by healthcare payers. All costs were updated to 2021 US dollars (USD) using the Consumer Price Index, medical care component [28].
Neoadjuvant and Adjuvant Treatment Costs
Pembrolizumab is available in single-use 100-mg vials and administered at a fixed dose of 200 mg q3w on day 1 of cycles 1–8. The list price for pembrolizumab is $5134 per 100-mg vial [29]. The average number of vials per administration for regimens which were body surface area (BSA)-based or body weight-based was estimated using a log-normal distribution of the BSA or body weight distribution, respectively, of patients in KEYNOTE-522 (mean [standard deviation], BSA 1.8 [0.2] m2; body weight 69.7 [16.3] kg). An optimal vial mix algorithm was used to minimize vial wastage in the base case. Administration costs (i.e., intravenous infusion) were derived from the Centers for Medicare and Medicaid Services Physician Fee Schedule [30].
Neoadjuvant and adjuvant treatment costs were calculated using the drug acquisition and administration costs per administration, respective TOT and relative dose intensity (RDI). The neoadjuvant and adjuvant TOT were estimated using the observed KM curves from KEYNOTE-522. The number of treatment cycles in the model was calculated on the basis of the estimated TOT and was adjusted using the RDI observed in KEYNOTE-522 to account for any delays or interruptions in administration which could be due to AEs, non-compliance, or other reasons.
Surgery and Radiation Costs
According to the KEYNOTE-522 protocol, patients who completed neoadjuvant treatment would receive definitive surgery 3 to 6 weeks later. The surgery was followed by radiation therapy, as indicated, and adjuvant treatments. Surgery and radiation costs were applied as one-time costs (Table 1). The proportion of patients receiving surgery (pembrolizumab + chemotherapy/pembrolizumab vs. chemotherapy 98% vs. 97.7%) and radiation therapy (75.9% vs. 78.5%, respectively) were obtained from KEYNOTE-522.
Disease Management and Terminal Care Costs
The average weekly costs of disease management in the EF, LR, and DM states were estimated on the basis of an Optum Health Reporting and Insights claims database study by Sieluk et al. [31]. For patients who remained EF for longer than 10 years, the disease management costs were assumed zero.
A one-time terminal care cost, assumed to include HCRU in the last 30 days of life, was applied upon patients’ death. The cost was estimated by a US Surveillance, Epidemiology, and End Results (SEER) Medicare study amongst patients with metastatic TNBC [32]. Cost elements included inpatient, emergency care, outpatient, skilled nursing facility, and hospice visits. The resultant cost updated to 2021 USD was $15,779.
Subsequent Therapy Costs
Approximately 62.5% of patients in the pembrolizumab + chemotherapy/pembrolizumab arm and 70.3% in the chemotherapy arm received systemic anticancer therapy following documented DM in KEYNOTE-522. Drug acquisition and administration costs associated with first- to fourth-line metastatic TNBC treatments were applied as a one-time cost upon entry into the DM state. The distribution and duration of subsequent treatments from the trial were used in the model. The average cost of subsequent therapy upon DM was estimated at $59,467 per patient in the pembrolizumab + chemotherapy/pembrolizumab arm and $47,804 per patient in the chemotherapy arm (Table 1).
AE Management Costs
The total average cost per patient for managing AEs for each treatment arm was calculated on the basis of AE incidence rates, rate of hospitalization, and unit costs of medical management for each AE episode in the inpatient or outpatient setting. Incidence and costs of selected grade 3–5 AEs are summarized in Table 1.
Sensitivity Analyses
Various scenario analyses were conducted to assess model uncertainty related to key model settings and assumptions. One such analysis explored the lifetime impact of chronic immune-related AEs, including hypothyroidism and adrenal insufficiency for pembrolizumab-treated patients. Another scenario analysis assumed that patients who had not experienced locoregional or metastatic recurrence 8 years after treatment were “cured”, where these patients were assumed no longer at risk for events and only subject to natural mortality. In addition, a scenario analysis was conducted under the assumption that the relative treatment effect of pembrolizumab would diminish (i.e., patients who received pembrolizumab + chemotherapy/pembrolizumab would experience the same hazard of events and death as those who received chemotherapy alone) after 8 years of treatment. The inclusion criteria of chronic AEs and the assumption of “cure” were based on input from an advisory panel review of the model. Waning of treatment effect for pembrolizumab was not supported by clinical evidence nor prompted by the advisory panel; however, this assumption was explored as it was often discussed in reimbursement reviews. Additional scenario-based sensitivity analyses adjusted the model time horizon, the discounting of costs and outcomes, the EQ-5D-5L mapping algorithm, RDI, as well as the application of alternative plausible parametric functions to EFS.
In addition, one-way deterministic sensitivity analyses (DSA) and probabilistic sensitivity analyses (PSA) were conducted to test the robustness of the model results (Table S1 in the supplementary material).
Model Validation
This model and other analogous versions were evaluated by multiple health economists and breast cancer specialists during the model development process. In addition, an expert panel consisting of eight medical oncologists and two health economists provided detailed review and feedback. Model validation covered the conceptual model as well as the model assumptions, methods, inputs, and results. The panel also provided verification to the model structure, analysis plans, clinical plausibility of clinical inputs, duration of treatment effects and modelled outcomes such as the projections of EFS and OS. Where consensus was not achieved, the panel provided guidance on the scope of sensitivity analyses.
The modeled OS was first validated with observed data from the KEYNOTE-522 trial. As shown in Fig. S3 in the supplementary material, the projected OS curve matches closely with the observed KM curve for both arms. Then the projected OS curve for chemotherapy was compared with external data presented by Walsh et al. [33] and Sikov et al. [34] (Fig. S3). The clinical plausibility of the projected long-term outcomes was also verified by independent clinical experts.
No external data are currently available to validate the modeled OS for the pembrolizumab-treated patients with eTNBC. The plausibility of the long-term OS projection for the pembrolizumab + chemotherapy/pembrolizumab arm was supported by feedback from a clinician advisory panel review of the projected treatment effects of pembrolizumab.
Compliance with Ethics Guidelines
The data used in this analysis were based on previously conducted trials and published literature; no studies with human participants or animals were performed by any of the authors. Ethics committee approval is not required.
Results
Base Case
The base case results (Table 2) projected that pembrolizumab + chemotherapy/pembrolizumab would result in a longer EFS (1.62 years) and LYs (3.37) and greater QALYs (2.90) compared to chemotherapy over the lifetime horizon. The addition of pembrolizumab was also projected to be associated with higher overall medical costs ($79,046), mainly driven by drug acquisition costs. The resultant incremental cost per QALY gained with pembrolizumab + chemotherapy/pembrolizumab vs. chemotherapy was $27,285; the incremental cost per LY gained was $23,489.
Scenario Analyses
The results of the scenario analyses are presented in Table S2 in the supplementary material. The majority of the scenarios were consistent with the base case results. However, the results were sensitive to a change in the time horizon, where a 10-year horizon resulted in an ICER of $153,574/QALY, and the consideration of “cure” after 8 years (ICER of $40,126/QALY). Assuming waning of treatment effect after 8 years of pembrolizumab treatment led to an ICER of $45,273/QALY. Alternative plausible parametric distributions for EFS for pembrolizumab + chemotherapy/pembrolizumab increased the ICER, ranging from $34,664/QALY to $57,569/QALY.
DSA and PSA
The impact of parameter variation on ICERs derived by the one-way DSA is shown in the tornado diagram in Fig. 4. The ICERs ranged from $16,384 to $75,674 per QALY gained and the results were most sensitive to variations in the parametric estimates of EFS for pembrolizumab + chemotherapy/pembrolizumab or chemotherapy.
One-way sensitivity analyses tornado diagram of ICER (Δcost/ΔQALY): Pembrolizumab + chemotherapy/pembrolizumab vs. chemotherapy. AE adverse event, DM distant metastases, EF event-free, EFS event-free survival, ICER incremental cost-effectiveness ratio, L lower bound confidence interval of the base case estimate, LR locoregional recurrence, QALY quality-adjusted life years, U upper bound confidence interval of the base case estimate
The PSA yielded an average ICER of $30,950/QALY (Fig. 5a). The cost-effectiveness acceptability curve (Fig. 5b) showed an 83% probability that ICER would be below $50,000/QALY and an 99% probability that ICER would be below $150,000/QALY.
Discussion
Over the modeled time horizon, neoadjuvant pembrolizumab + chemotherapy followed by adjuvant pembrolizumab for high-risk eTNBC is expected to yield substantial improvements in QALYs (by 2.90) and LYs (by 3.37) relative to treatment with neoadjuvant chemotherapy alone. The ICER for pembrolizumab + chemotherapy/pembrolizumab vs. chemotherapy was estimated at $27,285/QALY in the base case, which falls under all published willingness-to-pay (WTP) thresholds, including the thresholds recommended by the Institute of Clinical and Economic Review (i.e., $50,000 to $150,000 per QALY) [35, 36] and the World Health Organization (WHO) criteria of three times the gross domestic product (GDP) per capita [37]. The results were primarily driven by prevented or delayed cancer recurrence and progression with the addition of pembrolizumab in both neoadjuvant and adjuvant phases, increasing the LYs and QALYs attributed to the pembrolizumab + chemotherapy/pembrolizumab arm. The results of the scenario and sensitivity analyses supported the base case findings, attesting to their robustness. When considering the WTP threshold of $150,000/QALY, the PSA demonstrated that pembrolizumab + chemotherapy/pembrolizumab had a 99% probability of being cost-effective versus chemotherapy in the USA.
To our knowledge, this is the first study to examine the cost-effectiveness of pembrolizumab + chemotherapy/pembrolizumab vs. chemotherapy in high-risk eTNBC in the USA. A key strength of the current analysis is its use of data from KEYNOTE-522, a phase 3 randomized controlled trial comparing pembrolizumab + chemotherapy/pembrolizumab and chemotherapy in high-risk eTNBC. This permitted the use of the most accurate data for this patient population as inputs in the model, and validation of the estimated OS against the observed OS in KEYNOTE-522. EFS and OS extrapolations for the chemotherapy arm were validated against external studies [33, 34]. Additionally, an expert panel of seven medical oncologists and two health economists validated the model structure, assumptions, inputs, and outcomes.
A positive reimbursement recommendation was recently made by Canadian Agency for Drugs and Technologies in Health (CADTH) for pembrolizumab in high-risk eTNBC [38]. The recommendation highlighted that addition of pembrolizumab to neoadjuvant chemotherapy addressed unmet needs for this patient population in terms of reducing the risk of disease recurrence, controlling disease, and providing a manageable toxicity profile. The base case CEA proposed by CADTH resulted in an ICER of $81,408/QALY. As the CADTH analysis adopted different model assumptions and inputs, and reflected different clinical practices and payer systems compared to the present study, the results were not directly comparable.
The current findings are consistent with previously conducted CEAs comparing novel therapies with prior standard of care in other early-stage solid tumors in the USA. For example, a study by Bensimon et al. using data from KEYNOTE-054 found that adjuvant pembrolizumab was highly cost-effective versus observation alone following complete resection of high-risk stage III melanoma at a WTP threshold of $100,000 [39]. An ICER of $39,228/QALY (adjusted to 2021 USD) was reported by Garrison et al. for the addition of trastuzumab to chemotherapy as an adjuvant treatment in early-stage HER2-negative BC from a US payer perspective [40].
However, the results of this analysis should also be considered in the light of several limitations. First, the median follow-up of KEYNOTE-522 was 39 months at the data cutoff date for this analysis; extrapolations were necessary to model a lifetime horizon and there is inherent uncertainty in this process. To address this, multiple scenario analyses were performed to evaluate alternative extrapolation approaches for EFS, and parameter uncertainty associated with the base case extrapolation was tested in the sensitivity analyses. The results from the sensitivity analyses supported the robustness of the base case ICER. In addition, a rigorous validation process was carried out to validate the extrapolation of EFS and OS outcomes. However, longer-term follow-up of the clinical trial will be imperative to confirm the model results. Second, the model results can be sensitive to uncertainty in the duration of treatment effects for pembrolizumab. However, it is challenging to model this because of lack of evidence. Although a scenario analysis was conducted to explore the potential impact of treatment waning effect in this study, there is no evidence to indicate that the treatment effect with pembrolizumab would be lost at later follow-ups. Instead, the current KEYNOTE-522 trial shows clear separation of the survival curves after median follow-up of the trial, and the separation between the treatment arms grows more substantial over time [19]. In the absence of clinical rationale or evidence on the duration of treatment effect after stopping therapy, application of an arbitrary treatment waning effect may not be appropriate [41]. Third, the transition following the entry of the DM state was estimated from the KEYNOTE-522 data, which reflected the treatment pattern during the trial follow-up period. As TNBC care is changing rapidly, with immunotherapies and targeted therapies emerging as a new treatment paradigm, the predicted mean OS may be an underestimate compared to that in current real-world practice because more patients would be receiving more efficacious treatments in the metastatic setting. Finally, the costs of grade 3+ AEs with an incidence of less than 5% in both groups were not included in the model. Considering their low incidence, exclusion of these events was not expected to bias the results.
Conclusions
In this economic evaluation from a US third-party payer perspective, pembrolizumab in combination with chemotherapy as neoadjuvant treatment and continued as a single-agent adjuvant treatment after surgery improves patients’ life expectancy and QALYs compared with neoadjuvant chemotherapy and is projected to be a cost-effective treatment option for patients with high-risk eTNBC.
References
National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Female breast cancer 2021. https://seer.cancer.gov/statfacts/html/breast.html. Accessed 29 Sept 2021.
Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. 2019;9(2):176–98.
Keenan TE, Tolaney SM. Role of immunotherapy in triple-negative breast cancer. J Natl Compr Canc Netw. 2020;18(4):479–89.
National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Female breast cancer subtypes 2022. https://seer.cancer.gov/statfacts/html/breast-subtypes.html. Accessed 12 Sept 2022.
American Cancer Society. Triple-negative breast cancer 2021. https://www.cancer.org/cancer/breast-cancer/about/types-of-breast-cancer/triple-negative.html.
Jamdade VS, Sethi N, Mundhe NA, Kumar P, Lahkar M, Sinha N. Therapeutic targets of triple-negative breast cancer: a review. Br J Pharmacol. 2015;172(17):4228–37.
Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15):4429–34.
Hartung C, Porsch M, Stückrath K, et al. Identifying high-risk triple-negative breast cancer patients by molecular subtyping. Breast Care. 2021;16(6):637–47.
Lehmann BD, Jovanović B, Chen X, et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS ONE. 2016;11(6):e0157368.
Balmaña J, Díez O, Rubio IT, Cardoso F. ESMO Guidelines Working Group BRCA in breast cancer: ESMO clinical practice guidelines. Ann Oncol. 2011;22(suppl 6):vi31–4.
Lebert JM, Lester R, Powell E, Seal M, McCarthy J. Advances in the systemic treatment of triple-negative breast cancer. Current Oncol. 2018;25(11):142–50.
Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59.
Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–81.
Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21.
United States Food and Drug Administration. FDA approves pembrolizumab for high-risk early-stage triple-negative breast cancer 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-high-risk-early-stage-triple-negative-breast-cancer. Accessed 12 Oct 2022.
United States Food and Drug Administration. Highlights of prescribing information: KEYTRUDA (pembrolizumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf. Accessed 12 Dec 2021.
European Medicines Agency. Product Information KEYTRUDA (pembrolizumab) 2022. https://www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_en.pdf. Accessed 12 May 2022.
European Medicines Agency. CHMP post-authorisation summary of opinion for Keytruda (II-111) 2022. https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-opinion-keytruda-ii-111_en.pdf. Accessed 12 May 2022.
Schmid P, Cortes J, Dent R, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556–67.
Institute for Clinical and Economic Review. A guide to ICER's methods for health technology assessment 2018. http://icer-review.org/wp-content/uploads/2018/08/ICER-HTA-Guide_082018.pdf. Accessed 21 Sept 2021.
National Institute for Health and Care Excellence. Decision Support Unit technical support document 14: Survival analysis for economic evaluations alongside clinical trials: extrapolation with patient-level data 2013. http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf. Accessed 29 Sept 2021.
Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509.
Arias E, Xu J. United States Life Tables, 2018. https://www.cdc.gov/nchs/data/nvsr/nvsr69/nvsr69-12-508.pdf. Accessed 31 July 2020.
Arias E. United States life tables, 2017. Natl Vital Stat Rep. 2019;68(7):1–66.
Pickard AS, Law EH, Jiang R, et al. United States valuation of EQ-5D-5L health states using an international protocol. Value Health. 2019;22(8):931–41.
van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–15.
Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–20.
United States Bureau of Labor Statistics. Consumer Price Index. https://www.bls.gov/cpi/. Accessed 12 Oct 2021.
IBM Micromedex. RED BOOK®. http://www.micromedexsolutions.com/micromedex2/librarian/. Accessed 12 Oct 2021.
Centers for Medicare & Medicaid Services. CMS Physician Fee Schedule 2021. https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed 12 Oct 2021.
Sieluk J, Song Y, Freimark J, et al. The economic burden of recurrence in triple-negative breast cancer among working age patients in the United States. Adv Ther. 2021;39(2):943–58.
Aly A, Shah R, Hill K, Botteman M. Overall survival, costs and healthcare resource use by number of regimens received in elderly patients with newly diagnosed metastatic triple-negative breast cancer. Future Oncol. 2019;15(9):1007–20.
Walsh EM, Shalaby A, O’Loughlin M, et al. Outcome for triple negative breast cancer in a retrospective cohort with an emphasis on response to platinum-based neoadjuvant therapy. Breast Cancer Res Treat. 2019;174(1):1–13.
Sikov WM, Polley M-Y, Twohy E, et al. CALGB (Alliance) 40603: Long-term outcomes (LTOs) after neoadjuvant chemotherapy (NACT) +/- carboplatin (Cb) and bevacizumab (Bev) in triple-negative breast cancer (TNBC). J Clin Oncol. 2019;37(15_suppl):591.
Institute for Clinical and Economic Review. 2020–2023 value assessment framework 2020. https://icer.org/wp-content/uploads/2020/10/ICER_2020_2023_VAF_102220.pdf. Accessed 12 Oct 2022.
World Health Organization. New cost-effectiveness updates from WHO-CHOICE 2021. https://www.who.int/news-room/feature-stories/detail/new-cost-effectiveness-updates-from-who-choice. Accessed 12 Oct 2022.
Adams T, Baltussen R, Tan-Torres T, et al. Making choices in health: WHO guide to cost-effectiveness analysis 2003. http://apps.who.int/iris/bitstream/handle/10665/42699/9241546018.pdf?sequence=1. Accessed 12 Oct 2022.
Canadian Agency for Drugs and Technologies in Health. Reimbursement Review: pembrolizumab. https://www.cadth.ca/pembrolizumab-3. Accessed 12 Oct 2022.
Bensimon AG, Zhou ZY, Jenkins M, et al. Cost-effectiveness of pembrolizumab for the adjuvant treatment of resected high-risk stage III melanoma in the United States. J Med Econ. 2019;22(10):981–93.
Garrison LP, Lubeck D, Lalla D, Paton V, Dueck A, Perez EA. Cost-effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER2-positive breast cancer. ACE J. 2007;110(3):4890498.
National Institute for Health and Care Excellence. Single Technology Appraisal Atezolizumab with nab-paclitaxel for treating PD L1-positive, triple-negative, advanced breast cancer [ID1522]. 2022. https://www.nice.org.uk/guidance/ta639/evidence/final-appraisal-determination-committee-papers-pdf-8776324046. Accessed 30 May 2022.
Acknowledgements
Funding
This study, and the journal’s Rapid Service and Open Access fees, were funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Medical Writing, Editorial, and Other Assistance
Medical writing was provided by Shelley Batts, PhD, an independent contractor of Analysis Group, Inc., and funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Authorship
All authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Min Huang, Peter Fasching, Amin Haiderali, Weiguang Xue, Chelsey Yang, Wilbur Pan, Zheng-Yi Zhou, Peter Hu, Mitashri Chaudhuri, Celine Le Bailly De Tilleghem, Nicolas Cappoen and Joyce O’Shaughnessy contributed to the study conception, design, material preparation, data collection and analysis, and manuscript composition and editing.
Disclosures
Min Huang, Amin Haiderali, Peter Hu, and Wilbur Pan are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. and hold stock/options in Merck & Co., Inc., Rahway, NJ, USA. Weiguang Xue, Chelsey Yang, and Zheng-Yi Zhou are employees of Analysis Group, Ltd., which has received consulting fees from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA for this research. Celine Le Bailly De Tilleghem and Nicolas Cappoen are employees of MSD, Brussels, Belgium. Peter Fasching reports personal fees from Novartis Pfizer, Daiichi-Sankyo, AstraZeneca, Eisai, Merck Sharp & Dohme, Lilly Pierre Fabre, SeaGen, Roche, Hexal, Agendia, and grants from Biontech and Cepheid. Mitashri Chaudhuri is an employee of Complete HEOR Solutions (CHEORS), which has received consulting fees from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA for this research. Joyce O’Shaughnessy has received consulting fees and/or honorarium from AbbVie Inc., Agendia, Amgen Biotechnology, Aptitude Health, AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene Corporation, Clovis Oncology, Daiichi Sankyo, Eisai, G1 Therapeutics, Genentech, Gilead Sciences, GRAIL, Halozyme Therapeutics, Heron Therapeutics, Immunomedics, Ipsen Biopharmaceuticals, Lilly, Merck & Co., Inc, Myriad, Nektar Therapeutics, Novartis, Pfizer, Pharmacyclics, Pierre Fabre Pharmaceuticals, Puma Biotechnology, Prime Oncology, Roche, Samsung Bioepis, Sanofi, Seagen, Syndax Pharmaceuticals, Taiho Oncology, Takeda, and Synthon.
Prior Presentation
The results of this study were previously presented at the ESMO Breast Cancer Congress; Berlin, Germany; May 3–5, 2022.
Compliance with Ethics Guidelines
The data used in this analysis were based on previously conducted trials and published literature; no studies with human participants or animals were performed by any of the authors. Ethics committee approval is not required.
Data Availability
The efficacy, safety, patient-reported outcomes data used in this study were collected in the phase III KEYNOTE-522 trial. The patient-level data are not publicly available, but the results of the trials have been presented in several publications. The trial results supporting the findings of this analysis are available within the article and its supplemental material. Other data, including cost data and datasets used for model validation were obtained from published literature, for which references are provided in the article.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Huang, M., A. Fasching, P., Haiderali, A. et al. Cost-Effectiveness of Neoadjuvant Pembrolizumab Plus Chemotherapy Followed by Adjuvant Single-Agent Pembrolizumab for High-Risk Early-Stage Triple-Negative Breast Cancer in the United States. Adv Ther 40, 1153–1170 (2023). https://doi.org/10.1007/s12325-022-02365-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02365-1