Abstract
Introduction
Transdermal cannabinoids may provide better safety and bioavailability profiles compared with other routes of administration. This single-arm, open-label study investigated a novel topical transdermal delivery system on the pharmacokinetics of cannabidiol (CBD) and tetrahydrocannabinol (THC).
Methods
Participants were 39.5 ± 7.37 years old and healthy, based on a review by the Medical Director. Blood was collected pre-dose and 10, 20, 30, and 45 min, and 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, and 12 h after topical application of 100 mg CBD:100 mg THC. Psychoactive effects were assessed prior to each timepoint. Area-under-the-curve (AUC0–12 h), maximum concentration (Cmax), time to maximum concentration (Tmax), area-under-the-curve to infinity (AUCI), terminal elimination rate constant (λ), terminal half-life (t½), and absorption rate constant (ka) were measured individually for CBD and THC. Safety was assessed by clinical chemistry, hematology, and adverse events.
Results
AUC0–12 h for CBD and THC was 3329.8 ± 3252.1 and 2093.4 ± 2090.6 pg/mL/h, with Cmax of 576.52 ± 1016.18 and 346.57 ± 776.85 pg/mL, respectively. Tmax for CBD and THC was 8 h, ranging from 2.5 h to 12 h and 10 min to 12 h, respectively. AUCI for CBD and THC was 6609.2 ± 7056.4 and 3721.0 ± 3251.7 pg/mL/h, with t1/2 of 5.68 ± 1.5 and 5.38 ± 1.25 h, respectively. CBD was absorbed at a faster rate compared with THC (123.36 ± 530.97 versus 71.5 ± 1142.19 h−1) but with similar λ (0.12 ± 0.029 versus 0.13 ± 0.03 h−1). No psychoactive effects were reported. Transdermal cannabinoid delivery was safe and well tolerated in the population studied.
Conclusion
To our knowledge, this is the first pharmacokinetic study in humans that demonstrated CBD and THC entering systemic circulation via transdermal administration . This study represents an important contribution to understanding the pharmacokinetics of transdermal cannabinoids.
Clinical Trial Registration
ClinicalTrials.gov Identifier—NCT05121506 (November 16, 2021).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Transdermal administration of cannabinoids may provide safety and bioavailability advantages over other routes of administration. |
There is no previous literature on transdermal cannabinoid pharmacokinetics. |
What was learned from this study? |
The investigated technology delivering CBD and THC was found to be safe and well tolerated, with no associated psychoactive effects. |
There was large variability in the pharmacokinetics of CBD and THC between individuals when delivered transdermally. |
Future double-blind, randomized, placebo-controlled pharmacokinetic studies are needed to confirm the preliminary findings of this open-label, exploratory study. |
Introduction
Transdermal delivery of cannabinoids may provide advantages over other more traditional routes of administration. Rapid entry of cannabinoids into systemic circulation via inhalation involves risks related to toxicity and exposure to carcinogenic byproducts [1], and oral consumption can lead to a greater likelihood of overconsumption [2]. It has been suggested that transdermal cannabinoids may provide diminished psychotropic effects and more constant cannabinoid plasma levels [3, 4], benefiting those with certain medical conditions and individual lifestyles. However, transdermal cannabinoid delivery is challenging. Cannabinoids are hydrophobic molecules, which prevents them from easily diffusing through the aqueous layer of the skin [5]. In general, cannabinoids are molecules that have low transdermal uptake, build up in a reservoir in the stratum corneum (outermost layer of the skin), and thought not to reach the blood [6]. To our knowledge, there are no published studies in humans that have demonstrated that cannabinoids can enter systemic circulation via transdermal administration.
The objective of this study was to investigate the systemic bioavailability of cannabidiol (CBD) and tetrahydrocannabinol (THC) following acute topical administration using Gefion GT4 technology, a novel transdermal delivery system, in a single-arm, open-label study of 18 healthy adults.
Methods
Ethical Approval and Trial Registration
This study was conducted at KGK Science Inc. (London, Canada) from November 2021 to January 2022, and approved by the Therapeutic Products Directorate (TPD) (Health Canada, Ottawa, Canada), and the Institutional Review Board Services (Aurora, Canada) (Pro00058904). The study was conducted in accordance with the 1964 Declaration of Helsinki guidelines and its subsequent amendments, and in compliance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Guideline for Good Clinical Practice. The trial was registered at Clinicaltrials.gov (NCT05121506). Participants provided written informed consent before the initiation of study procedures.
Study Procedures
Participants attended a screening visit and a 12-h in-clinic visit. A follow-up phone call assessed adverse events (AEs) for up to 1 week after product administration. At screening, current health status, concomitant medications, medical history, and inclusion/exclusion criteria were reviewed. Height, weight, heart rate (HR), and blood pressure (BP) were measured, and urine tests determined pregnancy status (females only) and the presence of drugs of abuse. The health of participants was assessed by blood collected for clinical chemistry, hematology, hemoglobin A1c (HbA1c), human immunodeficiency virus (HIV), and hepatitis B/C. Eighteen eligible participants (nine female, nine male) returned to the clinic. Urine tests confirmed pregnancy status and presence of drugs of abuse, vital signs were measured, and body mass index (BMI) calculated. Participants washed their left hand, wrist, and forearm with soap and water within 30 min of topical administration, and immediately before application the area was wiped with alcohol disinfectant. Study personnel rubbed a topical dose of 100 mg CBD and 100 mg THC into the left hand, wrist, and forearm until absorbed (approximately 90 s) and the timing of scheduled blood draws was dependent on the time of complete absorption. Blood was collected pre-dose (t = 0) and at 12-h post-dose for safety analysis of clinical chemistry and hematology. Blood was collected pre-dose (t = 0) and at 10, 20, 30, 45 min, and 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, and 12 h after cannabinoid administration for analysis of CBD and THC concentrations in plasma. Psychoactive assessments were conducted immediately before each post-dose blood draw timepoint. Participants were asked “Are you experiencing a ‘high’ feeling?” and their answer determined whether they were subsequently asked “On a scale of 1 (least) to 10 (most), how intense is that feeling?” and “Is this ‘high’ feeling (other than intensity) different than past high experiences? If yes, please describe.”
Participants
Participants were included if they were between the ages of 25–65 years, met study criteria for contraception; had a BMI between 18.5 and 29.9 kg/m2; consumed cannabis at least once in the past 6 months, but not more than three times per week, without experiencing severe AEs; agreed to washout of cannabis and tobacco/nicotine products 48 and 96 h prior to dosing, respectively; and were determined by the Medical Director (MD) as healthy, via medical history and laboratory results.
Individuals were excluded if they were pregnant, breast feeding, or planning to become pregnant; were a habitual user of cannabis (≥ 4 times/week); had an acute or chronic skin disease or dermatological condition on the proposed area of application; had shaving, waxing, or laser hair removal on the proposed area of application within 14 days of dosing; were positive for drugs of abuse at screening or baseline; self-reported serious psychological disorder(s) diagnosis or had an immediate family member with a history of psychosis; used prescription or over-the-counter medications or supplements that may have affected study results or participant safety; or clinically significant abnormal laboratory results or recent or active unstable medical condition that may have adversely affected their ability to fully participate in the study or posed significant risk, as assessed by the QI.
Investigational Product
The investigational product (IP) was a novel Gefion GT4 technology that used emulsion technology containing penetrating agents, basement membrane disruptors, and vasodilators to overcome hydrophilic and lipophilic structures to open channels and transport cannabinoids deep into the dermis layer of the skin [7]. Once in the dermis, vasodilators dilate the capillary bed to increase fluid dynamic flow into and out of the application site, delivering cannabinoids into the blood stream [7]. The Gefion GT4 transdermal delivery system has been used previously to deliver gabapentin and naproxen sodium for the treatment of pain, with meaningful efficacy and no reported AEs (H. Crowley, personal communication, 16 June 2019).
Laboratory Analyses
Blood collected for CBD and THC analysis was centrifuged immediately for 10 min at 1500g at 4 °C. An aliquot of 1 mL of plasma was separated into three cryogenic vials and stored at −80 °C until being shipped for analysis. An aliquot of 300 µL of human plasma was used for the analysis of CBD and THC by Altasciences Company Inc. (Laval, Canada). The compounds were identified and quantified using reverse-phase high performance liquid chromatography (HPLC) with triple quadrupole mass spectrometry (LC–MS/MS API 6500) and an injection volume of 10 µL. The detection limits were 25.0–7500.0 pg/mL for CBD and 50.0–30,000.0 pg/mL for THC. Screening blood work and safety endpoints were analyzed by LifeLabs (London, Canada) using standard procedures. Urine pregnancy tests for females of childbearing potential and drugs of abuse analysis was conducted at KGK Science (London, Canada).
Statistical Analysis
The pharmacokinetic (PK) outcomes for CBD and THC were evaluated for intention-to-treat (ITT) and per protocol (PP) populations. The ITT population consisted of all participants who received the IP, and on whom any PK information was available. The PP population consisted of all participants who received IP, did not have any major protocol violations, and completed all study visits and procedures connected with measurement of primary outcomes.
PK outcomes included: area under the curve over a 12-h period (AUC0–12 h); area under the curve to infinity (AUCi); time to maximum concentration (tmax); peak concentration (Cmax); terminal elimination rate constant (λ); terminal half-life (t1/2); and absorption rate constant (ka). AUC0–12 h was calculated using the trapezoid approximation. Cmax and Tmax were determined directly from the concentration–time curve. AUCI was calculated by AUC0–12 h + CT/λ, where CT was the last quantifiable concentration. λ was calculated as the slope of points on the terminal log-linear end of the concentration versus time curve. t1/2 was calculated as ln(2)/λ = 0.693/λ and ka was calculated using the feathering method. Psychoactive assessment data was summarized using descriptive statistics (means, standard deviations, medians, minimums, maximums). All data is presented as mean ± standard deviation.
Results
Participants
A total of 30 potential participants were screened and 18 participants were enrolled in this study. Enrolled participants completed all study procedures (Fig. 1).
Participants were an average of 39.5 ± 7.37 years old, with 89% of the population Eastern/Western European White. The average BMI of participants was slightly overweight (25.95 ± 2.35 kg/m2), with 39% classified as normal weight (< 25 kg/m2) and 61% classified as overweight (≥ 25 kg/m2) (Table 1).
Four participants were removed from the PP analysis due to detectable cannabinoids pre-dose and one participant was removed due to concentration values exceeding the upper limit of quantification at 10 min post-dose. This single participant was excluded from the PP population after their samples were diluted and analyses repeated for accuracy, as verified by Altasciences Company Inc.
Pharmacokinetic Outcomes
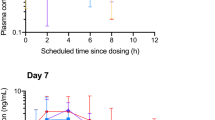
In the PP population, AUC0–12 h for CBD and THC was 3329.8 ± 3252.1 and 2093.4 ± 2090.6 pg/mL/h, respectively, with Cmax of 576.52 ± 1016.18 and 346.57 ± 776.85 pg/mL. The Tmax for CBD and THC was 8 h, with Tmax ranging from 2.5 to 12 h and 10 min to 12 h, respectively. At 12-h post-dose, concentrations of CBD and THC had not returned to pre-dose levels, demonstrating that the blood sampling period was not long enough to capture the entire PK curve. AUCI for CBD and THC was 6609.2 ± 7056.4 and 3721.0 ± 3251.7 pg/mL/h, respectively, with comparable t1/2 of 5.68 ± 1.5 and 5.38 ± 1.25 h. CBD was absorbed at a faster rate compared with THC (123.36 ± 530.97 versus 71.5 ± 1142.19 h−1) but with similar λ (0.12 ± 0.029 versus 0.13 ± 0.03 h−1) (Fig. 2). PK outcomes for the ITT population can be found in Tables S1 and S2. Individual time-concentration curves are provided in Figs. S1–S18.
Psychoactive Assessment
None of the participants in ITT population reported feeling psychoactive effects at any post-dose blood draw timepoint. One hundred percent of participants responded to “Are you experiencing a ‘high’ feeling?” with “No” and therefore were not asked the remaining psychoactive assessment questions.
Safety
There were two post-emergent AEs reported by two participants, each reporting a headache following dosing. The post-emergent AEs were classified as mild in intensity and unrelated to the IP. One participant recovered on the same day as IP administration, and the other had recovered by the following day. No AEs were reported in the 7 days following cannabinoid delivery. There were no statistically significant or clinically relevant changes in clinical chemistry or hematology parameters, as confirmed by the MD.
Discussion
Transdermal delivery of cannabinoids may provide advantages over other more traditional routes of administration. Cannabis smoking rapidly yields very high concentrations of cannabinoids in the bloodstream, but comes with risks associated with toxicity, exposure to carcinogenic byproducts, and respiratory conditions, as well as loss of activity through combustion (inactive and degraded cannabinoids due to excess heat) [1, 8,9,10,11]. When consumed orally, cannabinoids undergo first-pass metabolism, resulting in reductions in bioavailability and other unwanted effects associated with liver metabolizing enzymes, most notably, the cytochrome P450 system [12]. THC metabolism via cytochrome P450 (CYP450) produces a greater accumulation of 11-hydroxytetrahydrocannabinol, a potent, longer-lasting psychoactive metabolite that that may go beyond the desired effects of a novice or medical consumer [12]. Furthermore, CBD metabolism by CYP450 is associated with potentially dangerous drug–drug interactions that may be minimized when CBD is delivered through the skin, owing to delayed cannabinoid accumulation [13]. This can be particularly important in the treatment of chronic conditions over long periods of time or where a treatment regimen involves significant quantities of CBD as it relates to the patient’s body weight, for example in pediatrics. It is suggested that by avoiding first-pass metabolism and applying cannabinoids to the skin, psychotropic impacts may be reduced, and more constant cannabinoid plasma levels can be achieved [3, 4]. A challenge with transdermal delivery is that cannabinoids are hydrophobic molecules, preventing them from easily diffusing through the aqueous layer of the skin [5]. In general, cannabinoids are molecules that have low transdermal uptake, build up in a reservoir in the stratum corneum, and thought not to reach the blood [6]. Using emulsion technology containing penetrating agents, basement membrane disruptors, and vasodilators, Gefion GT4 technology targets hydrophilic and lipophilic structures to open channels and transport cannabinoids deep into the dermis layer of the skin, while vasodilators dilate the capillary bed to increase fluid dynamic flow to deliver cannabinoids into the blood stream [7].
To our knowledge, this is the first PK study that has demonstrated the ability of CBD and THC to successfully enter systemic circulation via transdermal administration in humans. Importantly, the successful delivery of cannabinoids via Gefion GT4 technology was safe and well tolerated by all participants. There were no clinically significant changes in clinical chemistry or hematology, or any related AEs in the 7-day monitoring period following dosing. This study represents an important step in understanding cannabinoid PKs, opening the door for novel cannabinoid delivery systems, and therapeutic interventions. Previous in vitro studies investigating cannabinoid permeability in human skin found that CBD had an approximately 10 × higher permeability than THC [14, 15], aligning with research that determined that CBD is relatively less lipophilic than its psychoactive counterpart [15]. These findings are consistent with the greater AUC and Cmax found with CBD in the current study, although to a lesser degree than what has been previously reported [15]. This preliminary data suggests that to deliver equal doses of CBD and THC via the skin, a greater dose of THC may be required. In the current study, Tmax for CBD and THC occurred later in the blood sampling period compared to what is expected from other routes of administration. Previous literature has demonstrated that Tmax for CBD and THC in inhaled and orally consumed forms occurred within only a few minutes to approximately 4 h post-dose [16,17,18]. Inhalation provides a faster Tmax because of avoidance of the gastrointestinal tract and first-pass metabolism [5, 19]. Given the challenges of transdermal delivery of cannabinoids (hydrophobic molecules required to pass through aqueous layers of the skin) and preclinical research demonstrating a Tmax of up to 38 h post-dose [20], it is not surprising that delayed absorption and a longer Tmax for CBD and THC was found in this study. Furthermore, transdermal delivery of CBD and THC appeared to result in a smaller Cmax than has been found for inhaled and orally consumed cannabis. When occasional cannabis consumers were given a maximal 50.6 mg dose of THC via smoked, vaporized, and ingested routes of administration, Cmax was found to be 52, 48, and 10 ng/mL, respectively [19]. In another study of occasional users, administration of only 1.35 mg of CBD in a liquid extract capsule resulted in a Cmax of 0.93 ng/mL [21]. Comparatively, in the current study topical application of 100 mg of CBD and THC resulted in a Cmax of 576.52 pg/mL (0.576 ng/mL) and 346.57 pg/mL (0.346 ng/mL), respectively. The Cmax of cannabinoids delivered transdermally appear to be lower than that of inhaled or oral routes of administration, despite a higher delivered dose. However, the results of this study need to be considered in the context of large interindividual variation and a blood sampling period that was not sufficient to capture the full descending arm of the PK curve.
For both CBD and THC, there was large interindividual variation across all PK parameters except for t1/2 and λ, suggesting that once successfully in the blood stream, the cannabinoids were removed from systemic circulation at similar rates between participants. However, the magnitude and rate at which cannabinoids were able to diffuse through the skin appeared to be highly dependent on the individual participant.
Several reasons may explain the variability found between individual participants in this study. Transdermal drug distribution tends to present in more erratic patterns with less symmetrical bell-shaped curves [22] due to biological differences that affect the structure and integrity of the skin, leading to variation in the quantity absorbed [23,24,25]. The quality of an individual’s skin may be influenced by gender, ethnicity, metabolism, and perhaps most notably, age [23]. As humans get older, the epidermis and dermis layers of the skin begin to thin, and there are critical changes in keratinocytes, melanocytes, type-3 collagen fibers, and Langerhans cells [26]. Changes to aging skin has contributed to significant reductions in skin permeability and drug absorption [23]. However, those that are more lipophilic, such as testosterone and estradiol, were less impacted by aging skin [27]. Considering that participants in the current study ranged in age from 27 to 57 years, the integrity and quality of skin of individual participants may have contributed to large interindividual variation in cannabinoid absorption. Further adding to the large variation may have been differences between males and females, which made up an equal 50% of the enrolled study population. Men typically have larger keratinocytes [28] and sweat and sebaceous glands [29], and a lower skin pH than females [30], which could result in varying levels of transdermal permeation [23]. Although most studies have not demonstrated any significant differences between males and females in transdermal drug delivery, this previous research should not be generalized to cannabinoids without further investigation. Cannabinoids are highly lipophilic molecules that are readily absorbed into fatty tissues [5, 31] and contain unique molecular structures. Although BMI is a poor predictor of body fat [32], there was a range between 20.6 and 29.1 kg/m2 in study participants, suggesting there may have been large variations in body fat percentage. Depending on the amount of body fat, particularly subcutaneous deposits at the application site, body fat may have contributed to the variation in PK parameters since this adipose layer can sequester CBD and THC molecules. This also could account for the PK penetration differential between CBD and THC as THC is substantially more lipophilic.
The findings of this study must be considered in the context of certain limitations. The study design was chosen to provide adequate information on the transdermal delivery of cannabinoids in humans. As this is the first study to demonstrate the PKs of CBD and THC following transdermal application, the length required for blood sampling was unclear. The preliminary results clearly demonstrate that the 12-h sampling period was not sufficient to capture a full PK curve, owing to the delayed rate at which cannabinoids entered the blood stream. Health Canada guidance on the conduct of bioequivalence studies recommends that to accurately estimate PK parameters, a minimum of three timepoints should be collected on the descending arm of the PK curve [33]. In total, 61% (8/13) of participants in the PP population had a Tmax of 12 h, indicating that their true Tmax may have been 12 h, or any number of hours after that final timepoint. Furthermore, only one participant had their concentration of THC drop to pre-dose levels by the end of blood sampling. In the same vein, the true Cmax and AUC for both CBD and THC may have been larger than reported in the current study. Although there was less variability in t1/2 and λ among participants, a complete representation of elimination is not possible without a longer sampling period. Furthermore, this study enrolled a relatively small sample size. The findings of this study will need to be confirmed with a larger double-blind, randomized, placebo-controlled PK trial that utilizes a longer blood sampling period, of at least 48–72 h. Future studies should evaluate differences in transdermal cannabinoid PK profile based on sex, age, and BMI, as well as cannabis use history. Emerging evidence has demonstrated that cannabinoid PKs differ based on years of recreational use, with those reporting more years of use having a greater AUC and Cmax, and longer t1/2 for major cannabinoids and analytes [34].
The successful delivery of CBD and THC to the blood stream highlights the advantages of transdermal cannabinoid delivery. Although more research is needed, more constant and lower plasma cannabinoid concentrations without concerns of psychoactive effects may be beneficial for certain therapeutic indications and individual lifestyles [3]. The results from the psychoactive assessment suggest that the concentration of THC that reached the brain was not sufficient to produce a feeling of being “high.” In the case of using cannabinoid therapy in the treatment of vulnerable populations, the lower concentration of delivered THC could be viewed as a safety mechanism to prevent psychoactive effects. However, the psychoactive effects of transdermally delivered THC should be explored in isolation from CBD. CBD acts as a negative allosteric modulator to THC and may have diminished the psychoactive effects reported in this trial [35]. Based on the extended delivery of CBD and/or THC demonstrated in this study, the GT4 transdermal delivery system may be more applicable for the treatment of chronic compared with acute conditions that require a more rapid Cmax for symptom relief.
Conclusions
The results of this open-label PK study demonstrated that major cannabinoids CBD and THC can successfully permeate through human skin and enter systemic circulation. Cannabinoid delivery occurred over an extended period of time, with peak concentration levels occurring later in the blood sampling period. However, these results need to be considered in the context of a 12-h blood sampling period that did not capture the full PK curve and large interindividual variation in the absorption of delivered cannabinoids. Gefion GT4 technology was safe and well tolerated by all participants, with no AEs related to the IP and no clinically relevant changes to clinical chemistry or hematology parameters. Gefion GT4 technology may provide lower, more steady-state cannabinoid concentrations that minimize psychoactive effects in participants. This could represent a significant benefit in the treatment of chronic conditions over longer periods of time, or in the treatment of diseases where high concentrations of cannabinoids are needed in relation to the body weight of vulnerable populations, such as pediatrics. This study represents an important step forward in cannabinoid pharmacokinetics.
References
Grotenhermen F. Harm reduction associated with inhalation and oral administration of cannabis and THC. J Cannabis Ther. 2001;1(3–4):133–52.
Light K, Karboune S. Emulsion, hydrogel and emulgel systems and novel applications in cannabinoid delivery: a review. Crit Rev Food Sci Nutr. 2021;62:1–31.
Mahmoudinoodezh H, Telukutla SR, Bhangu SK, Bachari A, Cavalieri F, Mantri N. The transdermal delivery of therapeutic cannabinoids. Pharmaceutics. 2022;14(2):438.
Bruni N, Della Pepa C, Oliaro-Bosso S, Pessione E, Gastaldi D, Dosio F. Cannabinoid delivery systems for pain and inflammation treatment. Molecules. 2018;23(10):2478.
Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770–804.
Hess C, Krämer M, Madea B. Topical application of THC containing products is not able to cause positive cannabinoid finding in blood or urine. Forensic Sci Int. 2017;272:68–71.
Gefion Canada. Technology Platform. 2020. https://www.gefioncanada.com/technology-platform/. Accessed April 2021.
Solowij N, Broyd SJ, van Hell HH, Hazekamp A. A protocol for the delivery of cannabidiol (CBD) and combined CBD and 9-tetrahydrocannabinol (THC) by vaporisation. BMC Pharmacol Toxicol. 2014;16(15):58.
Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802.
Heuberger JA, Guan Z, Oyetayo O-O, Klumpers L, Morrison PD, Beumer TL, et al. Population pharmacokinetic model of THC integrates oral, intravenous, and pulmonary dosing and characterizes short-and long-term pharmacokinetics. Clin Pharmacokinet. 2015;54(2):209–19.
Ohlsson A, Lindgren JE, Andersson S, Agurell S, Gillespie H, Hollister LE. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed Environ Mass Spectrom. 1986;13(2):77–83.
Barrus DG, Capogrossi KL, Cates SC, Gourdet CK, Peiper NC, Novak SP, et al. Tasty THC: promises and challenges of cannabis edibles. Methods Rep RTI Press. 2016.
Patel PM, Lio PA. Safety and sourcing of topical cannabinoids: many questions, few answers. J Clin Aesthet Dermatol. 2021;14(8):49.
Challapalli PV, Stinchcomb AL. In vitro experiment optimization for measuring tetrahydrocannabinol skin permeation. Int J Pharm. 2002;241(2):329–39.
Stinchcomb AL, Valiveti S, Hammell DC, Ramsey DR. Human skin permeation of Δ8-tetrahydrocannabinol, cannabidiol and cannabinol. J Pharm Pharmacol. 2004;56(3):291–7.
Millar SA, Stone NL, Yates AS, O’Sullivan SE. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol. 2018;9:1365.
Stott CG, White L, Wright S, Wilbraham D, Guy GW. A phase I study to assess the single and multiple dose pharmacokinetics of THC/CBD oromucosal spray. Eur J Clin Pharmacol. 2013;69(5):1135–47.
Joerger M, Wilkins J, Fagagnini S, Baldinger R, Brenneisen R, Schneider U, et al. Single-dose pharmacokinetics and tolerability of oral delta-9-tetrahydrocannabinol in patients with amyotrophic lateral sclerosis. Drug Metab Lett. 2012;6(2):102–8.
Newmeyer MN, Swortwood MJ, Barnes AJ, Abulseoud OA, Scheidweiler KB, Huestis MA. Free and glucuronide whole blood cannabinoids’ pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration in frequent and occasional cannabis users: identification of recent cannabis intake. Clin Chem. 2016;62(12):1579–92.
Paudel KS, Hammell DC, Agu RU, Valiveti S, Stinchcomb AL. Cannabidiol bioavailability after nasal and transdermal application: effect of permeation enhancers. Drug Dev Ind Pharm. 2010;36(9):1088–97.
Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk E-M, et al. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Δ9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit. 2005;27(6):799–810.
Schwartz JB. The influence of sex on pharmacokinetics. Clin Pharmacokinet. 2003;42(2):107–21.
Singh I, Morris AP. Performance of transdermal therapeutic systems: effects of biological factors. Int J Pharm Investig. 2011;1(1):4.
Meidan VM, Roper CS. Inter-and intra-individual variability in human skin barrier function: a large scale retrospective study. Toxicol In Vitro. 2008;22(4):1062–9.
Farahmand S, Maibach HI. Transdermal drug pharmacokinetics in man: interindividual variability and partial prediction. Int J Pharm. 2009;367(1–2):1–15.
Fore J. A review of skin and the effects of aging on skin structure and function. Ostomy Wound Manag. 2006;52(9):24–35 (quiz 6).
Roskos KV, Maibach HI, Guy RH. The effect of aging on percutaneous absorption in man. J Pharmacokinet Biopharm. 1989;17(6):617–30.
Murdan S. Transdermal and topical drug delivery. From theory to clinical practice. Pharm Educ. 2004;4(1):49–50.
Giacomoni PU, Mammone T, Teri M. Gender-linked differences in human skin. J Dermatol Sci. 2009;55(3):144–9.
Jacobi U, Gautier J, Sterry W, Lademann J. Gender-related differences in the physiology of the stratum corneum. Dermatology. 2005;211(4):312–7.
Izgelov D, Shmoeli E, Domb AJ, Hoffman A. The effect of medium chain and long chain triglycerides incorporated in self-nano emulsifying drug delivery systems on oral absorption of cannabinoids in rats. Int J Pharm. 2020;580: 119201.
Arroyo M, Rocandio AM, Ansotegui L, Herrera H, Salces I, Rebato E. Comparison of predicted body fat percentage from anthropometric methods and from impedance in university students. Br J Nutr. 2004;92(5):827–32.
Health Canada. Guidance document—Conduct and analysis of comparative bioavailability studies. In: Health Canada, editor. Ottawa; 2018.
Berl V, Hurd YL, Lipshutz BH, Roggen M, Mathur EJ, Evans M. A randomized, triple-blind, comparator-controlled parallel study investigating the pharmacokinetics of cannabidiol and tetrahydrocannabinol in a novel delivery system, solutech, in association with cannabis use history. Cannabis Cannabinoid Res. 2022. Ahead of print.
Laprairie R, Bagher A, Kelly M, Denovan-Wright E. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172(20):4790–805.
Acknowledgements
The authors wish to thank our volunteers who participated in this study, and for their compliance to the conduct of the study.
Funding
This study was funded by Gefion Canada, who also funded the journal’s Rapid Service Fee and the Open Access Fee for this publication.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
G. Varadi: formal analysis plan, data interpretation; Z. Zhu: conceptualization; H.D. Crowley: study design, data interpretation; M. Moulin: data interpretation, visualization, writing—original draft preparation, review, and editing; R. Dey: formal analysis; E.D. Lewis: writing—review and editing; M. Evans: study design, data interpretation, writing—original draft preparation, review and editing, supervision.
Disclosures
G. Varadi and Z. Zhu are employed as consultants to Gefion Canada. H.D. Crowley serves as the Senior Vice President and Chief Medical Officer of Gefion Canada. M. Moulin, R. Dey, E.D. Lewis, and M. Evans are employees of KGK Science, Inc., the Contract Research Organization contracted to conduct the presented study. No further personal or financial relationships exist with Gefion Canada or the studied investigational product.
Compliance with ethics guidelines
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board Services (Aurora, Canada; Pro00058904). Participants provided written informed consent before the initiation of study procedures.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Varadi, G., Zhu, Z., Crowley, H.D. et al. Examining the Systemic Bioavailability of Cannabidiol and Tetrahydrocannabinol from a Novel Transdermal Delivery System in Healthy Adults: A Single-Arm, Open-Label, Exploratory Study. Adv Ther 40, 282–293 (2023). https://doi.org/10.1007/s12325-022-02345-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02345-5