Abstract
Antipsychotics are the cornerstone of schizophrenia treatment. Lack of treatment adherence encouraged the development of injectable long-acting antipsychotics. However, second-generation or atypical antipsychotics require a loading dose at the start of treatment and eventually oral supplementation to achieve therapeutic plasma levels. This review discusses the evidence emerging from studies evaluating the pharmacokinetics, efficacy and safety of the intramuscular formulation of risperidone based on in situ microparticles (ISM). ISM® technology applied to risperidone allows therapeutic levels of the active moiety to be achieved within 2 h of intramuscular administration without the need for loading doses or oral supplementation, leading to a constant release over the whole dosing period. Risperidone ISM showed significant antipsychotic efficacy versus placebo in the Positive and Negative Syndrome Scale (PANSS) total score (p < 0.0001) and on the subscales of positive symptoms after 8 days, negative symptoms in 8 weeks, and general psychopathology during the 12 weeks of treatment. The improvement was also statistically significant (p < 0.0001) against placebo in the Clinical Global Impressions-Severity of Illness scale (CGI-S) score at the end of the treatment. Risperidone ISM was generally well tolerated and the most frequently reported adverse events were similar to those observed with other risperidone formulations. There is clinical evidence that these results are maintained in the long term. In conclusion, four-weekly risperidone ISM (75 mg and 100 mg) is an adequate antipsychotic for treating schizophrenia, both in the short term when an exacerbation has recently occurred and for long-term maintenance, since it provides rapid onset of action and sustained efficacy, as well as being safe and well tolerated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Lack of adherence in schizophrenia led to the development of injectable long-acting formulations of antipsychotic drugs. |
Second-generation or atypical antipsychotics require loading doses at the start of treatment and in some cases oral supplementation to achieve therapeutic plasma levels. |
Risperidone based on in situ microparticles (ISM) is a four-weekly injectable antipsychotic. Treatment with this regimen in adults with acutely exacerbated schizophrenia showed a rapid and sustained decrease in symptomatology and a disease severity improvement without the need for loading doses or oral antipsychotic supplementation. |
The safety profile of risperidone ISM is adequate and similar to that described with other risperidone formulations. |
Introduction
Schizophrenia is a severe, chronic, disabling illness with a prevalence of 0.7–1.5%. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), schizophrenia is characterised by the presence of at least two of the following symptoms: delusional ideas, hallucinations, disorganised language, disorganised or catatonic behaviour, and negative symptoms, accompanied by socio-occupational or self-care impairment for a period of 6 months, with at least 1 month of active symptoms [1]. This pathology is associated with unemployment, homelessness and lack of social integration [2]. Furthermore, substance dependence and abuse are high in these patients [3], and their life expectancy is reduced by 10–25 years [4, 5].
Since the 1950s, classical antipsychotics known as neuroleptics or first-generation antipsychotics, such as chlorpromazine and haloperidol, have been available. From 1990 onwards, the pharmacodynamic and clinical peculiarities of clozapine led to the second-generation or “atypical” antipsychotics, the first representative of which was risperidone. These drugs are of great relevance in the control of acute psychotic symptoms, maintenance treatment, functional and quality of life improvement, and reducing hospitalisation rates [6,7,8]. In a context where adherence seriously matters, the development of long-acting injectable (LAIs) formulations has increased the efficacy of these drugs over oral counterparts. LAIs are advantageous in terms of claim-based comparisons [9, 10].
A limitation of some second-generation LAI formulations of antipsychotic drugs is that they require a concomitant dose of oral medication or a loading dose to achieve therapeutic plasma levels after the first administration. The development of a novel formulation of risperidone meets the need for a safe and well-tolerated antipsychotic with rapid onset of action and sustained efficacy over time.
This review discusses the evidence emerging from studies evaluating the pharmacokinetics, efficacy and safety of risperidone in situ microparticles (ISM), an intramuscular (IM) formulation.
Methods
For this narrative review, we searched the MEDLINE, Scopus, ClinicalTrials.gov, and Google Scholar databases for studies of risperidone ISM and articles related to second-generation LAI formulations of antipsychotic drugs. The key terms used in this search were “paliperidone palmitate”, or “aripiprazole” or “risperidone” or “risperidone ISM” and “LAI” or “long-acting injectable”. To ensure the literature was relevant, the search was restricted to articles published between the dates 1 January 2002 and 1 December 2021.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by the author.
Adherence to Antipsychotic Treatment
Although antipsychotics are considered the cornerstone of schizophrenia treatment, the Achilles’ heel of their efficacy is lack of or poor adherence to treatment [8]. Their partial and total non-adherence rates are greater than 40–60% [11] and 60% [12], respectively. In contrast to clinical trials, non-adherence in clinical practice is even greater, leading to poor symptomatology control, frequent relapses, patient deterioration, poorer quality of life, increased risk of suicide and hospitalisation with consequent increased healthcare costs.
This lack of adherence necessitated the introduction, from 1960 onwards, of the first LAI, the so-called depot formulations (fluphenazine, haloperidol, pimozide and zuclopenthixol). However, as a result of their oily formulation, they had variable plasma levels that were associated with excessive peaks (resulting in adverse events [AEs]) and troughs (responsible for possible relapses) [13].
With the development of second-generation or atypical antipsychotics, fewer extrapyramidal disabling AEs were observed, although some are associated with more endocrine metabolic AEs [14, 15]. LAIs have been developed with various active ingredients, such as risperidone, olanzapine, paliperidone and aripiprazole, which maintain therapeutic blood levels between administrations, usually every 2–4 weeks, except for two formulations of paliperidone palmitate which can be administered every 13 weeks/quarterly [16] or a recently approved injectable formulation that can be administered every 6 months [17].
From a pharmacokinetic point of view, LAIs have a more stable bioavailability than oral forms because they do not have to be absorbed at the gastrointestinal level and do not undergo initial hepatic metabolism [18]. In addition, because LAIs require administration by a healthcare professional, they facilitate regular contact between patients and mental health services, improve the therapeutic alliance and adherence, and facilitate monitoring and action in the event of relapse due to non-compliance [16, 19]. Numerous studies in clinical practice have shown that second-generation LAIs decrease psychopathology [9, 20, 21], relapses resulting in hospitalisations [22], and mortality [23].
Barriers to Use of Long-Acting Antipsychotics
Despite the benefits of LAIs, some barriers exist regarding their use. One of the major barriers to the use of LAIs reported by psychiatrists is patient aversion to needles [24]. In addition, some patients and clinicians perceive the use of LAIs as coercive and stigmatising. Other barriers are the need for resources for their administration, reduced flexibility of administration that slows titration [24, 25], and the difficulty in monitoring the occurrence and management of AEs. Also, the higher cost of second-generation LAIs can be a barrier for healthcare administration and private medicine [6, 24, 26]. Another barrier is the under-representation of LAIs in clinical practice guidelines, despite their efficacy and safety being assessed in several controlled clinical trials with adequate designs. However, other important areas are not well understood, such as indications in patient profiles, somatic and psychiatric comorbidities, use in special populations (children, adolescents, the elderly, or pregnant women), treatment initiation guidelines and switching from one LAI to another.
Despite this, several European guidelines recommended LAIs. The French Association of Biological Psychiatry and Neuropsychopharmacology clinical guideline recommends the use of second-generation LAIs as first-line treatment in the maintenance of patients after a first psychotic episode and in recent-onset schizophrenia [27]. The clinical consensus of Spanish experts entitled “Adherence to treatment of schizophrenia” recommends second-generation LAIs as a first-line strategy to improve adherence in outpatients with a recent diagnosis of psychosis with a course of less than 2 years [28]. However, the British National Institute for Health and Care Excellence (NICE) guideline recommends using LAIs in patients with first psychotic episodes only in those who request it [29]. Alternatively, the American Psychiatric Association’s (APA) practice guideline for treating patients with schizophrenia does not recommend LAIs in recent-onset psychosis, limiting their use to patients with poor adherence or recurrent exacerbations [30].
In the absence of consistent recommendations between different clinical guidelines, the choice of the LAI should be based on shared decisions between the clinician and the patient, according to their preferences and clinical characteristics [31]. There is no doubt that such different recommendations influence prescribing rates. In Spain and other European countries, only 15% of all antipsychotics prescribed are LAIs and the remaining 85% are oral formulations. In Europe, second-generation LAIs represent 34% of all LAIs, with the UK having the lowest corresponding proportion (14%) and Spain having the highest prescription rate (74%) [6].
Pharmacokinetics of Long-Acting Antipsychotics
In addition to their heterogeneous pharmacodynamics, the pharmacokinetic characteristics of LAIs contribute significantly to their efficacy and clinical utility, and thus to appropriate prescribing [26]. LAIs should reach therapeutic plasma concentrations rapidly and maintain them without excessive fluctuations throughout the time interval between administrations [32]. However, some second-generation LAIs do not reach therapeutic plasma levels rapidly after the first administration, making it necessary to provide a loading dose of the same active substance, either with the oral formulation or with a second supplementary injectable administration [33]. Thus, risperidone, aripiprazole monohydrate and paliperidone palmitate require supplementary oral or loading doses during the initial phase of treatment [33]. In particular, the most commonly used long-acting risperidone is the injectable microsphere formulation which requires additional oral administration during the first 3 weeks to achieve sufficient antipsychotic coverage [32, 34].
Against this background, a second-generation LAI—one without safety or tolerability issues that achieves therapeutic levels for 1 month without the need for an injectable loading dose or oral supplementation at the start of treatment—could address the shortcomings shown by other similar antipsychotics and improve adherence. The new LAI formulation of risperidone using ISM® technology (Laboratorios Farmacéuticos Rovi, S.A.) eliminates both the supplementation barrier and the potential drawback of the post-injection syndrome described with olanzapine pamoate [35, 36].
Risperidone ISM Pharmaceutical Technology
The ISM® technology platform, patented by Laboratorios Farmacéuticos Rovi, is a new technology developed to release injected drugs, which allows sustained release throughout the dosing period. It is based on forming a solid and stable polymeric matrix system that in situ entraps microparticles of the active ingredient. The formulation contains drug microparticles suspended in a polymeric solution. An injectable suspension is formed with the active substance and two excipients, a biocompatible copolymer of poly lactic-co-glycolic acid (PLGA) and dimethyl sulfoxide (DMSO) acting as a solvent. After administration by IM injection, it precipitates in situ to form a small matrix, by solvent diffusion to body fluids. During this process, the solvent, which carries part of the active ingredient in solution, is displaced from the matrix and first released rapidly into the bloodstream. Conversely, by hydrolysis, the matrix releases the immobilised fraction of the active substance in a sustained manner over time [36,37,38].
The first galenic development of this type was achieved with risperidone [37,38,39], although Rovi is also developing a long-acting injectable letrozole (an aromatase inhibitor for the treatment of hormone-dependent breast cancer) based on the ISM® technology [40].
Pharmacokinetics of Risperidone ISM
The pharmacokinetics of risperidone ISM and its safety and tolerability have been evaluated by three phase 1 studies [35, 38, 41] and one phase 2 study [37] (Table 1).
In the first phase 1 study, risperidone ISM in a single dose of 25 mg and 37.5 mg was administered to 17 healthy volunteers to evaluate its pharmacokinetics, safety and tolerability. The mean plasma concentration of the active moiety, consisting of risperidone and its active metabolite (9-hydroxyrisperidone), was detected from 2 h to 30 days after injection. No tolerability issues were noticed [41].
Subsequently, a second phase 1 study (PRISMA-1) was conducted in 36 patients with schizophrenia or schizoaffective disorder to characterize the pharmacokinetics and to evaluate the safety of risperidone ISM [35]. This was a multicentre, open-label, three parallel-arm and randomised trial. Patients received a single gluteal injection of three different doses of risperidone ISM: 50 mg, 75 mg or 100 mg. The results showed that the mean (standard deviation, SD) plasma concentration of the active moiety at 24 h after injection was 21.45 (8.34) ng/ml, 24.60 (11.65) ng/ml and 29.68 (11.77) ng/ml in the 50, 75 and 100 mg group, respectively, indicating that risperidone ISM rapidly achieves therapeutic plasma levels and does not require any oral supplementation at the start of treatment [35].
The BORIS study was a phase 1, open-label, one-sequence trial that evaluated the steady-state comparative bioavailability of risperidone ISM and orally administered risperidone in 58 patients with schizophrenia on stable treatment with orally administered risperidone (4 mg) [38]. Patients continued on the oral regimen for 1 week to reach steady-state risperidone concentrations. This was followed by four IM injections of 100 mg risperidone ISM every 4 weeks. After patients switched from the oral formulation to risperidone ISM, the bioavailability of the injectable form every 4 weeks was comparable to that obtained with the oral formulation (Fig. 1) [38].
Mean (± SD) plasma concentrations versus time profiles for risperidone active moiety during oral risperidone 4 mg treatment (7th dose) and after switching to risperidone ISM 100 mg (PK population). Once daily risperidone 4 mg was administered orally for 7 days, an intense oral PK analysis was conducted on day 7 (last day of the treatment), including samples at pre-dose (within 0.5 h relative to the dose time), 1, 2, 3, 4, 6, 8 and 12 h, post-dose (black line). Twenty-four hours after the last oral dose of risperidone (day 8), a single IM dose of risperidone ISM 100 mg was administered and PK samples were obtained at pre-dose and 12 h post-dose, as well as at days 10, 15, 22, 29 and 36 (blue line). IM, intramuscular; PK, pharmacokinetic; SD, standard deviation (Drug Design, Development and Therapy 2021;15:4371–4382, Originally published by, adapted and used with permission from Dove Medical Press Ltd.) [38]
The PRISMA-2 study was a phase 2, multicentre, open-label, parallel trial conducted in 36 patients with schizophrenia and a Positive and Negative Syndrome Scale (PANSS) total score of 70 or less that characterized the pharmacokinetics, safety and tolerability of risperidone ISM [37]. These patients received four gluteal or deltoid administrations of risperidone ISM 75 mg at 4-week intervals. After administration at each injection site, the mean concentration of the active moiety was higher than 10 ng/ml from 2 h onwards and peak concentration was reached between 24 and 48 h (39.6–53.2 ng/ml and 54.1–61 ng/ml, when given in gluteal or deltoid muscle, respectively). No accumulation of the active moiety was detected throughout treatment [37].
Therefore, given the positive pharmacokinetic results obtained in the PRISMA-1 and PRISMA-2 studies, it was recommended that the 75 mg and 100 mg doses of risperidone ISM be used in further studies to evaluate its efficacy and safety in patients with schizophrenia [35, 37]. In addition, these doses were considered on the basis of the results of the population pharmacokinetic analysis and the population pharmacokinetic/pharmacodynamic models [35, 42].
Clinical Development of Risperidone ISM: Efficacy and Safety
The PRISMA-3 study was a phase 3, double-blind, multicentre, randomised, placebo-controlled, double-blind trial designed to evaluate the efficacy and safety of risperidone ISM [36] (Table 1). The study population consisted of 438 patients with acute exacerbation of schizophrenia, with a PANSS total score between 80 and 120 at the baseline visit. The study duration was 12 weeks and patients were randomised to receive three IM injections of 75 mg or 100 mg risperidone ISM or placebo (1:1:1) every 4 weeks. The primary endpoint was the change in PANSS total score (Fig. 2). Secondary endpoints assessed were PANSS subscales total score, Clinical Global Impression-Severity (CGI-S) score mean change from baseline to week 12, patient well-being (Subjective Well-being under Neuroleptic Treatment [SWN-20]) and social functioning (Personal and Social Performance [PSP]) scale from baseline to the end of the study. Safety and tolerability were also assessed [36].
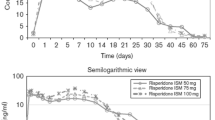
Least-squares (LS) mean change from baseline at each time point (mITT population) in a PANSS total score. Mean PANSS score at baseline for placebo = 96.40 (SD 7.21), for risperidone ISM 75 mg = 96.30 (SD 8.47) and for risperidone ISM 100 mg = 96.10 (SD 8.42). The error bars represent SE and P values are for risperidone ISM 75 mg and risperidone ISM 100 mg dose group versus placebo (*p < 0.01, **p < 0.001, ***p < 0.0001). mITT, modified intent-to-treat; PANSS, Positive and Negative Syndrome Scale; SD, standard deviation; SE, standard error. Figure reproduced under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0) (Correll CU et al. NPJ Schizophr. 2020;6:37) [36]
Compared with placebo, risperidone ISM (75 mg and 100 mg) was associated with a significant decrease (p < 0.0001) in the primary (PANSS total) and key secondary efficacy variables (CGI-S), at the end of treatment. Mean difference of PANSS total score from baseline to day 85 with risperidone ISM (75 and 100 mg) and placebo was − 13.0 and − 13.3 (p < 0.0001), respectively. Mean changes for CGI-S score from baseline to day 85 for both doses of risperidone ISM compared with placebo was − 0.7 (p < 0.0001), for both doses. Furthermore, in patients with higher severity (PANSS total score ≥ 95), risperidone ISM, particularly at the 100 mg dose, led to a significant reduction in the PANSS total score at the end of treatment (15.6-point decrease versus placebo). In addition, risperidone ISM 100 mg showed significant superiority over placebo from day 8 of administration on the PANSS subscales of general psychopathology (7.3 and 6.8 points versus placebo, for each dose respectively) and positive symptoms (3.9 and 4.6 points versus placebo, for each dose respectively), and 15 days onwards on the PANSS subscale of negative symptomatology (2.1 and 2.0 points versus placebo, for each dose respectively). The overall response rate improved over placebo from day 8 with risperidone ISM 100 mg (p < 0.005) and from day 15 for the 75 mg dose (p < 0.0001). From week 2 onwards, significant differences remained with both drug doses versus placebo. At the end of treatment, the difference versus placebo in the overall response rate was 39.2% with risperidone ISM 75 mg and 33.8% with the 100 mg dose (p < 0.0001) (Fig. 2) [36].
To complement the significance obtained in the PRISMA-3 study, a post hoc analysis assessed the effect size of both doses of risperidone ISM versus placebo. It concluded that both doses were associated with clinically relevant improvements in symptoms, with medium to large effect sizes (0.5–0.8) [43]. Similarly, risperidone ISM resulted in a rapid and progressive improvement in PSP scale scores both at the end of the double-blind (12-week) phase of the study and the end of the extension (12-month) study [44]. Furthermore, a substudy concluded that both doses of the drug are effective in treating of relapse symptoms, irrespective of whether the patient had previously been treated with risperidone or another antipsychotic drug [45].
A multicentre open-label extension (OLE) of the PRISMA-3 study has examined the long-term efficacy and safety of risperidone ISM in the treatment of schizophrenia [46] (Table 1). Those patients in the PRISMA-3 study who were receiving placebo (unstable) or risperidone ISM (stabilised), together with de novo patients, received monthly (once every 4 weeks) IM injections of risperidone ISM 75 mg or 100 mg for 12 months. Long-term efficacy assessment included the PANSS, the CGI-S and the Clinical Global Impression-Improvement (CGI-I) scales. Safety assessments included AEs, injection site reactions, laboratory tests and various safety assessments. A total of 215 patients (55 unstable, 119 stabilised and 41 stable) were included. Most of them (74.9%) completed the study and discontinuation rates were very similar between the three groups. PANSS total and subscale scores decreased from baseline to endpoint in all groups, with a greater decrease in unstable patients (Fig. 3). Similarly, an improvement from baseline to 12 months was observed in CGI-S and CGI-I scores in unstable and stabilised patients. Both scores remained almost unchanged in the stable group, while the relapse rate after 1 year was 10.7% [46].
Mean (SD) PANSS total score at each time point in unstable, stabilized and stable patients treated with monthly risperidone ISM® (pooled 75 and 100 mg). PANSS, Positive and Negative Syndrome Scale; SD, standard deviation. Figure reproduced under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0) (Filts Y et al. Schizophr Res. 2022;239:83–91) [46]
Concerning the assessment of safety and tolerability in the subjects of the PRISMA-1, PRISMA-2 and PRISMA-3 (both double-blind and OLE phases) studies, in general, both doses of risperidone ISM (75 mg and 100 mg) were well tolerated. The AEs observed were as expected and there was homogeneity between the trials. In the double-blind phase of the PRISMA-3 study, all AEs were mild or moderate in most patients. In addition, no patients died from AEs during the study [36]. The most frequently reported AEs for 75 mg and 100 mg of risperidone ISM were increased blood prolactin (9.0% and 14.4%), headache (6.3% and 3.4%), hyperprolactinaemia (5.6% and 8.9%) and weight gain (3.5% and 4.1%). Despite the events related to the prolactin increase being among the more usually reported treatment-related AEs (TEAEs) in the study, their incidence was comparable to that described by others [44, 47]. Although the frequency of AEs was lower with placebo (0%, 2.7%, 0.7% and 1.4%, respectively), the rate of patients leaving the study as a result of undesirable effects was lower among those treated with risperidone ISM (7.5% with placebo, 4.2% with risperidone 75 mg and 6.2% with risperidone 100 mg). Tolerability at the injection site was adequate. No relevant differences between treatment groups were seen in the 0–10 Visual Analog Scale (VAS) score, with a median value of 2.0 in all treatment groups; redness was the most frequent event. Similarly, for the three scales used to assess extrapyramidal symptoms (AIMS, BARS and SAS), treatment groups were comparable and no relevant changes from baseline to end of treatment were observed in any treatment group.
For the laboratory parameters, there were no notable differences between treatment arms from baseline through end of treatment and no notable changes in either treatment arm, except for prolactin, which increased in both risperidone ISM groups, with mean (SD) endpoint prolactin levels of 875.4 (1080.7) mIU/L with risperidone ISM 75 mg and 904.8 (810.6) mIU/L with risperidone ISM 100 mg. Of note, an increase in prolactin plasma levels was considered as an AE (either for hyperprolactinaemia or blood prolactin increased) when any of the following criteria were present: values above 1000 mIU/L for three consecutive determinations after randomisation, although no clinical symptoms were present, or values above 530 mIU/L if clinical symptoms of hyperprolactinaemia were present (e.g. headache, decreased libido, oligomenorrhoea) [36]. Nevertheless, the incidence of hyperprolactinaemia was similar to that described with other risperidone formulations [48].
In the OLE phase of the PRISMA-3 trial, at least one treatment-related AE was reported in 39.1% of patients; the most frequent AEs were headache (12.1%), hyperprolactinaemia (9.8%) and asthenia (5.1%). Injection site reactions were reported in eight patients (0.3%) and the injection site pain score was low in all 2355 doses evaluated. Risperidone ISM was considered an effective, safe and well-tolerated long-term treatment of schizophrenia in adults, regardless of the initial severity of illness or whether patients were previously treated with risperidone ISM during an acute exacerbation or switched from stable doses of orally administered risperidone [46].
Finally, DMSO is an excipient of the risperidone ISM formulation which is used as the solvent for the reconstitution of the product. In the nonclinical toxicology programme, no systemic toxicity attributed to DMSO was observed and only transient pain following injection was observed in dogs. During the clinical development programme harmful effects were not reported [49].
Discussion and Expert Opinion
Antipsychotics are essential drugs in the management of acute symptoms of schizophrenia, and maintenance treatment to reduce the risk of relapse, improve functional capacity and quality of life, and reduce hospitalisations [6,7,8]. However, non-adherence to oral antipsychotics is very common in daily clinical practice. LAIs have improved adherence and thus efficacy. However, there remain some unmet clinical needs such as the necessity for initial concomitant oral antipsychotic treatment for 2–3 weeks or parenteral loading doses to reach therapeutic levels and the frequency of administration. To address this need, a new formulation of long-acting risperidone using ISM® technology has been developed. Risperidone ISM removes the need for initial supplementation existing with other second-generation LAIs and allows for four-weekly administration instead of every 2 weeks (as is the case with risperidone in microspheres) [35, 36]. This initiation regimen can directly address some of the barriers reported by psychiatrist towards the use of LAIs. Specifically, the aversion that patients have towards needles can be mitigated since only one injection is needed when initiating treatment with risperidone ISM, allowing them to accept treatment more easily with an LAI when offered by the psychiatrist. By virtue of addressing the concerns and preferences of the patient, this can in turn contribute to the improvement of the therapeutic alliance between patient and psychiatrist.
From a pharmacokinetic point of view, after a single administration in the gluteal or deltoid, the pharmaceutical technology of risperidone ISM allows therapeutic plasma concentrations to be reached within the first 2 h, without the need for any previous loading dose or orally administered risperidone supplementation (as is required with risperidone in microspheres, aripiprazole monohydrate or the necessary injectable supplementation with paliperidone palmitate) [32]. In addition, risperidone ISM LAI formulation provides sustained release of risperidone from day 1 to day 28. These two distinguishing features of risperidone ISM would improve adherence to treatment [36, 37].
D2 dopaminergic receptor occupancy greater than 60% is considered to be related to the antipsychotic activity of risperidone, while D2 receptor occupancy greater than 80% may result in the occurrence of extrapyramidal effects [50]. Since steady-state administration of risperidone ISM (75 mg and 100 mg) produces D2 receptor occupancy between 69% and 75%, it would be, with minimal fluctuations, within the therapeutic range [35, 37].
Risperidone ISM 75 mg has shown pharmacokinetic parameters comparable to those achieved with repeated oral administration of risperidone at 3 mg/day doses [37]. However, orally administered risperidone has a wide inter-peak fluctuation rate (Cmax) of 3.30 between peaks (potentially responsible for AEs) [51], and troughs (Cmin) (related to subtherapeutic concentrations that may promote relapse) [52]. In contrast, LAIs show very little fluctuation owing to their constant release, ensuring stable D2 receptor occupancy and resulting in better tolerability and adherence to treatment [53].
On the other hand, the results of the PRISMA-3 study demonstrate the efficacy, safety and tolerability of four-weekly risperidone ISM (75 mg and 100 mg) in the treatment of patients with schizophrenia, even in those with the highest severity (PANSS ≥ 95), who may require hospitalisation due to the exacerbation of symptoms. Moreover, this drug agent can act rapidly, not only on positive symptoms from 8 days after administration but also on negative symptoms from 15 days after administration [36]. Furthermore, long term-efficacy of risperidone ISM has recently been confirmed by the OLE phase of PRIMA-3 clinical trial [46]. Notably, the 1-year relapse rate was low (10.7%) in comparison with other recently published results (24%) [54].
Indirect comparisons between clinical studies should be interpreted with caution because of methodological differences and the particular characteristics of the patients recruited. None of studies described above involved direct comparison with standard risperidone LAI. More head-to-head comparisons among different LAIs would make meta-analytic approaches further feasible [55], and the results more robust as has been the case with oral antipsychotics [48]. Nevertheless, in methodologically similar studies with other second-generation LAIs, the mean placebo-adjusted PANSS total score reduction with risperidone ISM (75 mg and 100 mg) was almost twice as large as those observed with a subcutaneous extended-release risperidone formulation (RBP-7000; 90 mg and 120 mg), paliperidone palmitate (25 mg, 100 mg, 150 mg) and aripiprazole lauroxil (441 mg/882 mg with 3-week oral supplementation), and similar to the differences obtained with monthly aripiprazole (400 mg with 2-week oral supplementation) [23]. In addition, risperidone ISM demonstrated efficacy in treating of negative symptomatology, which was not observed with the monthly subcutaneous injectable formulation of risperidone (RBP-7000) and did not result in significant changes in the PANSS negative symptom subscale [36, 47]. With the methodological limitations discussed above, these data argue in favour of the efficacy of risperidone ISM over other second-generation LAIs, without the need for supplementation or initial loading dose.
Risperidone ISM has shown good tolerability and the AEs observed were those expected for the active substance at therapeutic doses. In the phase 3 study, all AEs were mild or moderate in most patients, with a lower dropout rate due to AEs with risperidone ISM than with placebo. Also, extrapyramidal effects did not exceed those observed with placebo, which could be due to peak plasma levels of the active moiety not reaching the threshold for extrapyramidal effects. Hyperprolactinaemia was observed in 5.6% and 8.9% of patients with risperidone ISM 75 mg and 100 mg respectively. Of note, these figures are similar to those described with other risperidone presentations [33]. According to Filts et al. [46], a kinetic hypothesis has also been proposed to explain the safety profile of atypical antipsychotics in which both association and dissociation rates of the drug for dopamine D2 receptors are considered, as well as the potential for dissociated ligands to rebind to dopamine receptors leading to increased competition with the local dopamine at receptors on the synapse [56]. Using this model, Sykes et al. correlated the incidence of extrapyramidal symptoms with atypical antipsychotics and the reversal rate of dopamine D2 receptor blockade [56]. Thus, the favourable tolerability profile observed in the maintenance treatment of adult patients with schizophrenia may be explained by the unique pharmacokinetic profile of risperidone ISM, which could be linked to its optimized binding kinetics at the D2 receptor [35]. Interestingly, during the OLE phase also, there was a very low rate (3.2%) of adverse reactions leading to treatment discontinuation; besides, only 4.2% of patients reported treatment-related extrapyramidal events after 1 year of treatment with risperidone ISM [50].
Conclusions
Risperidone ISM is a new four-weekly LAI that provides immediate and sustained plasma levels without a loading dose or oral supplementation and was recently authorized by the European Union for the treatment of schizophrenia in adults for whom tolerability and effectiveness have been established with orally administered risperidone. Four-weekly IM risperidone ISM 75 mg or 100 mg shows good tolerability and significantly improved the symptomatology and severity of schizophrenia in acutely exacerbated patients, and in the long term. The statistically significant improvement in efficacy outcomes was observed as early as 8 days after the first dose and was maintained throughout the treatment period. Risperidone ISM therefore meets the need for an antipsychotic with rapid onset of action and sustained efficacy, and is safe and well tolerated. Thus, it is a drug to consider for schizophrenia both in the short term when an exacerbation has recently occurred and for long-term maintenance.
References
Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3–10.
Fazel S, Khosla V, Doll H, Geddes J. The prevalence of mental disorders among the homeless in western countries: systematic review and meta-regression analysis. PLoS Med. 2008;5(12): e225.
Hunt GE, Large MM, Cleary M, Lai HMX, Saunders JB. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990–2017: systematic review and meta-analysis. Drug Alcohol Depend. 2018;191:234–58.
Plana-Ripoll O, Pedersen CB, Agerbo E, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. 2019;394(10211):1827–35.
Simon GE, Stewart C, Yarborough BJ, et al. Mortality rates after the first diagnosis of psychotic disorder in adolescents and young adults. JAMA Psychiat. 2018;75(3):254–60.
Arango C, Baeza I, Bernardo M, et al. Long-acting injectable antipsychotics for the treatment of schizophrenia in Spain. Rev Psiquiatr Salud Ment (Engl Ed). 2019;12(2):92–105.
Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–71.
Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388(10039):86–97.
Kishimoto T, Hagi K, Kurokawa S, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021;8(5):387–404.
Suzuki T. A further consideration on long-acting injectable versus oral antipsychotics in the treatment of schizophrenia: a narrative review and critical appraisal. Expert Opin Drug Deliv. 2016;13(2):253–64.
Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892–909.
Novick D, Haro JM, Suárez D, Pérez V, Dittmann RW, Haddad PM. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 2010;176(2–3):109–13.
Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10(4):315–33.
Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31–41.
Tifratene K, Robert P, Metelkina A, Pradier C, Dartigues JF. Progression of mild cognitive impairment to dementia due to AD in clinical settings. Neurology. 2015;85(4):331–8.
Rognoni C, Bertolani A, Jommi C. Second-generation antipsychotic drugs for patients with schizophrenia: systematic literature review and meta-analysis of metabolic and cardiovascular side effects. Clin Drug Investig. 2021;41(4):303–19.
Najarian D, Sanga P, Wang S, et al. A randomized, double-blind, multicenter, noninferiority study comparing paliperidone palmitate 6-month versus the 3-month long-acting injectable in patients with schizophrenia. Int J Neuropsychopharmacol. 2022;25(3):238–51.
Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2–44.
Kane JM, García-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry Suppl. 2009;52:S63–7.
Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29823 patients with schizophrenia. JAMA Psychiat. 2017;74(7):686–93.
Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(Suppl 3):1–24.
Taipale H, Mehtala J, Tanskanen A, Tiihonen J. Comparative effectiveness of antipsychotic drugs for rehospitalization in schizophrenia-a nationwide study with 20-year follow-up. Schizophr Bull. 2018;44(6):1381–7.
Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274–80.
Citrome L, Belcher E, Stacy S, Suett M, Mychaskiw M, Salinas GD. Management of schizophrenia with long-acting injectable antipsychotic medications: an assessment of the educational needs of clinicians. Neuropsychiatr Dis Treat. 2022;18:111–23.
Biagi E, Capuzzi E, Colmegna F, et al. Long-acting injectable antipsychotics in schizophrenia: literature review and practical perspective, with a focus on aripiprazole once-monthly. Adv Ther. 2017;34(5):1036–48.
Correll CU, Lauriello J. Using long-acting injectable antipsychotics to enhance the potential for recovery in schizophrenia. J Clin Psychiatry. 2020;81(4):MS19053AH5C.
Llorca PM, Abbar M, Courtet P, Guillaume S, Lancrenon S, Samalin L. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry. 2013;13:340.
Roca M, Cañas F, Olivares J, Rodríguez A, Giner J. Treatment adherence in schizophrenia. Spanish clinical consensus. Actas Esp Psiquiatr. 2007;35(1 Suppl):1–6.
National Institute for Health and Care Excellence (NICE). Psychosis and schizophrenia in adults: prevention and management. https://www.nice.org.uk/guidance/cg178. Accessed 6 Jun 2022.
Lehman AF, Libermen JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia. 2nd ed: American Psychiatric Association; 2010.
Ramos PS. Shared decision making in mental health: myths, barriers, and benefits. Rev Psiquiatr Salud Ment. 2016;9(3):175–6.
Clark I, Taylor D. Newer formulations of risperidone: role in the management of psychotic disorders. CNS Drugs. 2020;34(8):841–52.
Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39–59.
Knox ED, Stimmel GL. Clinical review of a long-acting, injectable formulation of risperidone. Clin Ther. 2004;26(12):1994–2002.
Llaudó J, Anta L, Ayani I, et al. Phase I, open-label, randomized, parallel study to evaluate the pharmacokinetics, safety, and tolerability of one intramuscular injection of risperidone ISM at different dose strengths in patients with schizophrenia or schizoaffective disorder (PRISMA-1). Int Clin Psychopharmacol. 2016;31(6):323–31.
Correll CU, Litman RE, Filts Y, et al. Efficacy and safety of once-monthly Risperidone ISM® in schizophrenic patients with an acute exacerbation. NPJ Schizophr. 2020;6(1):37.
Anta L, Llaudó J, Ayani I, Martínez J, Litman RE, Gutierro I. A phase II study to evaluate the pharmacokinetics, safety, and tolerability of Risperidone ISM multiple intramuscular injections once every 4 weeks in patients with schizophrenia. Int Clin Psychopharmacol. 2018;33(2):79–87.
Walling DP, Hassman HA, Anta L, et al. The steady-state comparative bioavailability of intramuscular risperidone ISM and oral risperidone: an open-label, one-sequence study. Drug Des Devel Ther. 2021;15:4371–82.
Anta L, Mata E, Ochoa Díaz de Monasterioguren L. Newer formulations of risperidone: remarks about risperidone ISM®. CNS Drugs. 2020;34(10):1087–8.
ClinicalTrials.gov. Evaluation of IM letrozole ISM® pharmacokinetics, safety, and tolerability in healthy post-menopausal women (LISA-1). https://clinicaltrials.gov/ct2/show/NCT03401320. Accessed 6 Jun 2022.
Farré M, Matínez-González J, Cordero P, et al. A clinical trial to evaluate the pharmacokinetics, safety and tolerability of single doses of risperidone with the novel long-acting injectable technology ISM® in healthy volunteers. Eur Arch Psychiatry Clin Neurosci. 2011;261(Suppl 1):S57 (Abstract O-04–005).
Winkler J, Snoeck E, Llaudó Garin J, Ayani I, Martínez-González J, Gutierro AI. Population pharmacokinetic modeling and simulations of long-acting intramuscular risperidone ISM®. Clin Ther. 2015;37(8 Suppl):e3–4.
Litman RE, Filts Y, Pata M, et al., editors. P.0839 Risperidone ISM® effect size evaluation: post-hoc findings from the prisma-3 phase III study. 34th European College of Neuropsychopharmacology (ECNP); 2021 October 2–5th; Lisbon, Portugal.
Litman R, Anta L, Martínez J, Martínez-González J, Litman RE, Correll C, editors. Personal and social functioning in patients with schizophrenia treated with once-monthly Risperidone ISM®. 8th European Conference on Schizophrenia Research (ECSR); 2021 September 23–25th; Berlin, Germany.
Correll C, Pata M, Litman RE, Naber D, editors. Risperidone ISM® efficacy in schizophrenia patients with severe psychotic symptoms during an acute exacerbation patients with severe psychotic symptoms. 8th European Conference on Schizophrenia Research (ECSR); 2021 September 23–25th; Berlin, Germany.
Filts Y, Litman RE, Martínez J, Anta L, Naber D, Correll CU. Long-term efficacy and safety of once-monthly Risperidone ISM® in the treatment of schizophrenia: results from a 12-month open-label extension study. Schizophr Res. 2022;239:83–91.
Nasser AF, Henderson DC, Fava M, et al. Efficacy, safety, and tolerability of RBP-7000 once-monthly risperidone for the treatment of acute schizophrenia: An 8-week, randomized, double-blind, placebo-controlled, multicenter phase 3 study. J Clin Psychopharmacol. 2016;36(2):130–40.
Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–51.
European Medicine Angency. Committee for Medicinal Products for Human Use. Okedi Assessment report. EMA/CHMP/11233/2022 rev.1, 16 December 2021. https://www.ema.europa.eu/en/documents/assessment-report/okedi-epar-public-assessment-report_en.pdf. Accessed 6 Jun 2022.
Medori R, Mannaert E, Grunder G. Plasma antipsychotic concentration and receptor occupancy, with special focus on risperidone long-acting injectable. Eur Neuropsychopharmacol. 2006;16(4):233–40.
Sheehan JJ, Reilly KR, Fu DJ, Alphs L. Comparison of the peak-to-trough fluctuation in plasma concentration of long-acting injectable antipsychotics and their oral equivalents. Innov Clin Neurosci. 2012;9(7–8):17–23.
Wakamatsu A, Aoki K, Sakiyama Y, Ohnishi T, Sugita M. Predicting pharmacokinetic stability by multiple oral administration of atypical antipsychotics. Innov Clin Neurosci. 2013;10(3):23–30.
Schoretsanitis G, de Leon J, Haen E, Stegmann B, Hiemke C, Grunder G, et al. Pharmacokinetics of risperidone in different application forms—comparing long-acting injectable and oral formulations. Eur Neuropsychopharmacol. 2018;28(1):130–7.
Ceraso A, Lin JJ, Schneider-Thoma J, et al. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2020;8(8):CD008016.
Ostuzzi G, Bertolini F, Del Giovane C, et al. Maintenance treatment with long-acting injectable antipsychotics for people with nonaffective psychoses: a network meta-analysis. Am J Psychiatry. 2021;178(5):424–36.
Sykes DA, Moore H, Stott L, et al. Extrapyramidal side effects of antipsychotics are linked to their association kinetics at dopamine D2 receptors. Nat Commun. 2017;8(1):763.
Acknowledgements
Funding
The development and writing assistance of this manuscript and the journal’s Rapid Service and Open Access Fees were funded by Laboratorios Rovi, S.A. (Madrid, Spain).
Medical Writing and Editorial Assistance
I would like to thank María Romero and Fernando Sánchez Barbero of Springer Healthcare Communications for writing and editorial assistance and Matt Weitz of Springer Healthcare Communications for English language editing, funded by Laboratorios Rovi, S.A. (Madrid, Spain).
Authorship
The named author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, takes responsibility for the integrity of the work as a whole, and has given his approval for this version to be published.
Author Contributions
Cecilio Álamo revised and corrected the entire manuscript. The author performed the literature search and data analysis and drafted the work.
Disclosures
Cecilio Álamo, in the last 5 years, has participated as a consultant and/or speaker in training activities related to Neuropsychopharmacology organized by the following companies: Adamed, Angelini, Casen-Recordati, Exeltis, Ferrer, Grunenthal, Indivior, Italdrug, Janssen-Cilag, Juste SAQF, Kyowa Kiry, Lundbeck, Mundipharma Neuraxpharm, Normon, Novartis, Otsuka, Pfizer, Roche, Rovi, Rubió Servier and Takeda-Shire.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by the author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Álamo, C. Risperidone ISM as a New Option in the Clinical Management of Schizophrenia: A Narrative Review. Adv Ther 39, 4875–4891 (2022). https://doi.org/10.1007/s12325-022-02299-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02299-8