Abstract
Introduction
To demonstrate efficacy and safety of an ophthalmic hydrogel formulation of netilmicin/dexamethasone, containing xanthan gum twice a day (b.i.d.) versus netilmicin/dexamethasone eye drops four times a day (q.i.d) to treat inflammation and prevention of infection after cataract surgery.
Methods
Patients undergoing phacoemulsification with intraocular lens implantation (IOL) were randomised in two groups: group 1, twice daily (b.i.d.) dexamethasone 0.1%/netilmicin 0.3% (Netildex) ophthalmic gel; group 2, four times daily (q.i.d.) dexamethasone 0.1%/netilmicin 0.3% (Netildex) eye drops. Both treatments were administered for 14 days after surgery. Patients were evaluated before surgery, on the day of surgery and at 1, 7, 15 and 60 postoperative days. The primary efficacy endpoint was evaluation of cellularity and flare in the anterior chamber through slit-lamp biomicroscopy 7 days after surgery. Secondary endpoints included: presence of signs/symptoms of postoperative ocular inflammation and incidence of infection.
Results
One hundred seventy-three patients were randomised and 168 were evaluable. Flare and cellularity were resolved at day 7 in 92.5% of patients and almost completely by day 15. In both intent to treat (ITT) and per-protocol (PP) populations, the efficacy analysis demonstrated that the gel formulation administered twice a day was non-inferior to the eye drops administered four times a day. For ITT analysis, the lower limit of the 97.5% confidence interval (− 0.0535) was greater than the non-inferiority limit of -0.10. For the PP analysis, the lower limit of the 97.5% confidence interval (− 0.0526) was greater than the non-inferiority limit of − 0.10. The patient's global tolerability and reported symptoms were similar between treatment groups. No microbial load and no safety events were observed.
Conclusions
Efficacy of the gel reduced posology (twice a day) is not inferior to four times a day eye drops. Both treatments were well tolerated and efficacious. The new reduced posology hydrogel formulation may improve patient compliance and quality of life.
Trial Registration
Eudract: 2016-0021138-63; ClinicalTrial.gov: NCT029738880.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Advances in surgical instruments and techniques have significantly improved post-surgery outcomes reducing postoperative complications. However, some degree of ocular pain and inflammation may still occur after surgery, even in uneventful procedures, leading to possible complications. |
A new preservative-free ophthalmic hydrogel formulation of dexamethasone 0.1%/netilmicin 0.3% fixed combination (Netildex® Gel, SIFI SpA, Catania Italy) has been developed to reduce the high-frequency instillation of corticosteroids required with the eye drop formulation. The addition of xanthan gum in this ophthalmic formulation may increase the pseudo-plastic characteristics of the product, prolonging ocular retention time and giving a beneficial hydration to the ocular surface. |
What was learned from the study? |
The administration of dexamethasone 0.1%/netilmicin 0.3% (Netildex) ophthalmic gel b.i.d. is sufficient to obtain a non-inferior efficacy in the prevention and treatment of post-cataract surgery ocular inflammation compared with dexamethasone 0.1%/netilmicin 0.3% (Netildex) eye drop solution administered q.i.d. for the same period. No infections were reported. |
The new ophthalmic gel formulation, therefore, allows a reduced administration frequency while maintaining the same efficacy and simultaneously favouring patients' convenience and comfort, with possible better compliance to treatment and patients' quality of life. |
Introduction
Cataract is a common ophthalmic disease, especially among the elderly [1,2,3]. Currently, the most popular procedure for cataract removal is phacoemulsification, followed by the implantation of an artificial intraocular lens (IOL) [1, 2, 4, 5].
Recent advances in surgical instruments and techniques have significantly improved post-surgery outcomes reducing postoperative complications. However, some degree of ocular pain and inflammation may still occur after surgery, even in uneventful procedures [6, 7], leading to possible complications, such as corneal oedema, intraocular pressure (IOP) spikes, posterior capsule opacification and cystoid macular oedema [8,9,10,11].
The two main topical treatments in the management of post-surgical ocular inflammation and pain are non-steroidal anti-inflammatory drugs (NSAIDs) and topical corticosteroids, with the latter being considered the gold standard treatment. Corticosteroids are usually administered through topical eye drop instillation [10, 12,13,14,15,16].
Besides inflammation, one of the main risks of ocular surgery is the occurrence of ocular infection, which can lead to rare but devastating effects, such as postoperative endophthalmitis [16,17,18]. The use of antibiotics in cataract surgery includes a variety of regimens and protocols, including intracameral and postoperative topical antibiotics. Evidence that injecting intracameral antibiotics (cefuroxime, moxifloxacin) at the end of cataract surgery is effective for endophthalmitis prophylaxis is growing [20]. Among postoperative topical antibiotics, aminoglycosides and fluoroquinolones are the most prescribed agents and provide an excellent wide-spectrum coverage [17, 19,20,21,22]. In this respect, it has been established that a patient’s ocular flora is the source of microbes responsible for most cases of intraocular infections [23]. Thus, reducing the number of microbes on the ocular surface or eliminating the organisms that may have reached the eye can decrease the risk of endophthalmitis [19]. Recently, particular attention has been given to avoiding the administration of cytotoxic antibiotics in pre- and post-cataract surgery to ensure good visual and refractive outcomes after cataract surgery [24].
According to these considerations, a new preservative-free ophthalmic hydrogel formulation of dexamethasone 0.1%/netilmicin 0.3% fixed combination (Netildex® Gel, SIFI SpA, Catania Italy) has been developed to reduce the high-frequency instillation of corticosteroids required with the eye drop formulation. This new formulation contains xanthan gum, a high-molecular-weight polysaccharide used in ophthalmic preparations as a viscosity enhancer. The addition of xanthan gum in ophthalmic formulations has been shown to increase the pseudo-plastic characteristics of the product, prolonging ocular retention time [25,26,27] and with beneficial effect on the ocular surface [28, 29]. Furthermore, xanthan gum is a polymer with a well-known lubricant with pseudoplastic behaviour and antioxidant action on the ocular surface [30, 31] and could prevent the exacerbation of low-grade and/or non-symptomatic dry eye disease that often happens after cataract surgery, especially in elderly patients [24].
The aim of this study was to investigate whether a reduced frequency of administration of an innovative hydrogel formulation containing dexamethasone 0.1%/netilmicin 0.3%, instilled twice daily (b.i.d.), was non-inferior to dexamethasone 0.1%/netilmicin 0.3% fixed combination formulated in eye drop solution administered four-times daily (q.i.d.), in the treatment and prevention of ocular inflammation after phacoemulsification and IOL implantation.
Methods
Study Design and Subjects
This was an international phase III, randomised, double-blind study to evaluate the non-inferiority of a hydrogel formulation administered b.i.d. (Netildex gel) as compared to the eye drops formulation q.i.d. (Netildex® Eye drops, SIFI SpA, Catania, Italy) in patients undergoing phacoemulsification and IOL implantation. Both formulations are a fixed combination of netilmicin sulphate 3 mg/ml and dexamethasone phosphate 1 mg/ml. The preservative-free ophthalmic gel single-dose formulation contains xanthan gum, which acts as a viscosity enhancer by increasing the retention of the product on the ocular surface [24,25,26,27].
The study adhered to the tenets of the Declaration of Helsinki and was approved by the independent Ethics Committees of the participating institutions. Written informed consent was obtained from all patients before randomisation.
The study was conducted in five centres located in Italy (3 centres) and Germany (2 centres). Patients were included if they were aged ≥ 40 years and undergoing phacoemulsification with IOL implantation, with a grade 2 or 3 cataract according to the lens opacity classification system III (LOCS III) and an endothelial cell count within limits for age but not < 1200 cell/mm2. The main exclusion criteria included patients with a medical history of ocular inflammatory disease, trauma, herpes infections, uveitis or Sjogren’s syndrome and those with at least one of the following concomitant ocular diseases: ocular infections, glaucoma, diabetic retinopathy, uncontrolled diabetes, maculopathy, shallow anterior chamber, pseudo-exfoliation syndrome, poor mydriasis and IOP > 24 mmHg; patients who had been treated for external ocular infections within 1 month before the study enrollment and those receiving any ocular treatment apart from artificial tears were also excluded.
Patients were randomised to receive either single-dose ophthalmic hydrogel b.i.d. (group 1) or single-dose eye drops solution (group 2) q.i.d., administered topically in the conjunctival sac of the operated eye starting on the day of cataract surgery (day 0) until day 14 after surgery. To ensure masking, patients treated with the ophthalmic hydrogel also received two daily doses of placebo (single-dose ophthalmic hydrogel, containing sodium hyaluronate and xanthan gum) to reach four daily administrations. In both treatment groups, almost all patients (99.4%) received bromfenac (a non-steroidal anti-inflammatory drug) for a maximum of one drop twice daily (b.i.d.) from day − 3 to day − 1 (the day before surgery). Concomitant medications included intracameral injection of cefuroxime 1 mg/0.1 ml in the anterior chamber at the end of the surgery according to participating centres preference.
Measurements
Patients were evaluated 4–7 days before cataract surgery (screening visit), on the day of surgery and at 1, 7 and 15 days after surgery; an additional follow-up visit was performed 60 days after surgery.
The primary efficacy endpoint was the evaluation of inflammation (cellularity and flare) in the anterior chamber using slit-lamp biomicroscopy on day 7 after surgery. The scoring system is shown in Table 1, adapted from Hogan, 1964, and Jobs, 2005 [32, 33]. Patients were classified as full responders if both cellularity and flare were zero, partial responders if at least one of the 2 measurements were zero and non-responders if both cellularity and flare were greater than zero. The same evaluation was repeated 15 days after surgery.
The secondary endpoints included: (1) the presence of clinical signs/symptoms of ocular inflammation (anterior chamber flare, cellularity, conjunctival hyperaemia, lid oedema, corneal oedema, ocular pain, photophobia, tearing) assessed at days 1, 7 and 15 after surgery; (2) incidence of infection, assessed by clinical evaluation, and in the case of suspected infection ocular swabs were performed.
Global tolerability was measured at days 1, 7 and 15 after surgery through a standard questionnaire, classified on a categorical scale from 0 to 3: (0) none; (1) mild (present but not distressing); (2) moderate (distressing but not interfering with daily life); (3) severe (very distressing and interfering with daily life). Subjective tolerability was also assessed at days 1, 7 and 15 after surgery by interviewing patients about their symptoms, such as burning, stinging and blurred vision, and it was scored using the above-mentioned scale.
Safety was assessed by measuring frequency and severity of adverse events (AEs), changes in IOP (assessed by the Goldman applanation tonometry, CSO, Florence, Italy) and visual acuity, clinically significant laboratory abnormalities and physical examination findings.
Compliance was evaluated through direct interviews with the participating patients.
Statistical Analysis
A drop-out rate of 28% was considered. The sample size was calculated considering the detection of a true difference in favour of the reference treatment of 5% and required 130 patients to be 90% sure that the upper limit of a one-sided 97.5% confidence interval (CI) excluded a difference in favour of the reference group of > 10%. The difference in the primary efficacy endpoint, namely the proportion of full responders, was analysed by a chi-square test for proportion along with a one-sided 97.5% CI of the difference based on Student’s t distribution.
Secondary endpoints were tabulated with frequency and percentages at each time point during the study. The two treatment groups were compared by means of logistic regression techniques in univariate and multivariate models, adjusted for demographic and clinical factors. A per-protocol analysis was performed, which excluded ten patients in group 1 for protocol violations.
The non-inferiority analysis to obtain the difference in the proportion was performed using the Proc Freq in SAS v. 9.4, with one-sided 97.5% confidence intervals and a non-inferiority margin of 0.10.
Results
Patient Demographics and Treatment Adherence
The enrolment at the investigational sites was competitive to reach the target of 180 patients. Moreover, the study was stopped after 173 had been randomised patients since the number of statistically evaluable patients had already been reached (168 patients in ITT population patients versus the 130 requested in the protocol). The mean age was 72.6 (range 38–89) years, although this resulted in a protocol minor violation for patients aged 30–39 years. Age and gender were well matched among treatment groups. Patient disposition, according to the CONSORT diagram, is displayed in Fig. 1.
Almost all randomised patients received the first dose of treatment on the day of surgery (168/173, 97.1%). Five patients were randomised but had not received any study treatments (3 to hydrogel, 2 to eye drops formulation); thus, the number of patients reported in the baseline period is 168 (85 in group 1 and 83 in group 2).
After 7 days from surgery the treatment compliance was high for both treatments: 96.4% in group 1 and 100% in group 2. Demographic and baseline characteristics (age, gender and race) of the intention-to-treat (ITT) and safety population were similar across treatment groups, with no statistically significant differences (p > 0.05) (Table 2). Almost all patients (99.4%) received bromfenac 0.1% eye drops for a maximum of one drop twice daily (b.i.d.) from day − 3 to day − 1 (the day before surgery), and most of the patients received an intracameral injection of cefuroxime at the end of surgery in accordance with the ESCRS Guidelines [34].
Primary Endpoint (Cellularity and Flare Responder Rate)
Based on the ITT dataset, which included 168 patients receiving the treatment at day 7 post-surgery (85 in group 1 and 83 in group 2), 75 patients (88.2%) in group 1 and 78 patients (93.9%) in group 2 showed no signs of ocular inflammation (no flare and no cellularity in the anterior chamber) and were classified as full responders (Table 3).
The non-inferiority analysis (non-inferiority margin of − 0.10 and 97.5% CI) showed that the hydrogel administered b.i.d. was non-inferior to the eye drops formulation administered q.i.d. since the lower limit of the 97.5% CI (− 0.0535) was greater than the non-inferiority limit of − 0.10. The non-inferiority was also confirmed by the multivariate analysis, in which patients' sex and/or age were considered as cofactors (Fig. 2).
The per-protocol analysis, which excluded two patients in group 1 for protocol violations (inclusion criteria), showed similar results, with 89.7% and 95% of the patients classified as full responders 7 days post-cataract surgery, respectively, in the ophthalmic gel and eye drops treatment arms. The results confirmed the non-inferiority of the hydrogel compared with eye drop formulation, with an estimated risk difference at the univariate analysis of -0.0526 (95% CI − 0.0562 to 0.1613) and a lower limit of 97.5% CI equal to − 0.0562, therefore greater than the non-inferiority limit of − 0.10.
Secondary Endpoints
Clinical Signs and Symptoms of Ocular Inflammation
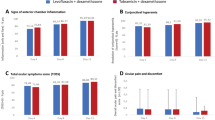
No significant difference was detected between the two groups in terms of ocular inflammation signs and symptoms (anterior chamber flare, cellularity, conjunctival hyperaemia, lid oedema, corneal oedema, ocular pain, photophobia, tearing) from day 1 up to 15 days post-surgery. All patients recovered by day 15 post-surgery, reporting no signs of ocular inflammation, apart from one patient in group 2 who had a few cells (score 0.5) on day 15 post-surgery. Furthermore, the ophthalmoscopy evaluation indicated no relevant differences throughout the study between the two groups.
Tolerability
In group 1 at day 1, 81 patients (97.6%) reported no discomfort at all, and 2 patients (2.4%) reported mild discomfort (present but not distressing); at day 7, all patients reported no discomfort; at day 15, 79 patients (98.8%) had no discomfort, and only 1 patient (1.3%) reported moderate discomfort, which was distressing but did not interfere with daily life.
In group 2 at day 1, 81 patients (98.8%) reported no discomfort, and 1 patient (1.2%) reported severe discomfort; at day 7, all patients reported no discomfort; at day 15, 80 patients (98.8%) had no discomfort, and only one patient (1.2%) reported mild discomfort.
Regarding symptoms of burning, stinging or blurred vision, there was no difference between the groups.
Safety Results
Ten out of 168 patients experienced at least one AE, 4 (4.7%) in group 1 and 6 (7.2%) in group 2. All AEs were classified as expected and of mild or moderate grade. Only two patients in group 2 experienced serious AEs (2.4%), vascular occlusion and macular oedema, which were assessed as not related to the study arm, and they completely recovered after appropriate treatment. Only one patient in group 1 reported a treatment-related AE, namely drug hypersensitivity. No patient showed signs of infection or endophthalmitis.
No between-group difference was found for visual acuity and IOP. IOP for the treated and non-treated eye was within the normal range (12–22 mmHg) and did not change during treatment in both arms, except for one patient in the group 1 arm who had increased IOP on day 1 only, which decreased by day 7 and remained stable and within the normal range at the following visits (Table 4).
Discussion
The management of ocular inflammation and prevention of infection after cataract surgery are of capital importance to reduce postoperative complications and to improve patients' subjective comfort. Among different anti-inflammatory and antibiotic perioperative protocols, postoperative antibiotic and steroid eye drops (in either fixed combinations or in separated formulations) are the most commonly prescribed [20].
Regarding the prophylaxis of infections, it has been established that a patient's ocular flora is the source of microbes responsible for most cases of intraocular infections [23]. Thus, reducing the number of microbes on the ocular surface or eliminating the organisms that may have reached the eye can decrease the risk for endophthalmitis. [20].
Both formulations used in this study, hydrogel and eyedrops, contain dexamethasone 0.1%/netilmicin 0.3%. Netilmicin is an antibiotic with an effective wide-spectrum antimicrobial activity, including methicillin-resistant (MR) strains (such as MR Staphylococcus aureus and MR coagulase-negative Staphylococci) [35, 36]. In an in vitro evaluation has been demonstrated that netilmicin has better tolerability and is less cytotoxic regarding the antimicrobial agents commonly used in clinical ophthalmological practice as fluoroquinolones [37]. Marino et al., in an in vivo study on rabbits, demonstrated that netilmicin, in respect to ofloxacin, may offer a superior toxicological profile in both normal eyes and clinical situations where the integrity of the ocular epithelium is altered [38, 39].
The second main component is dexamethasone phosphate, a potent steroid, well absorbed, especially in the presence of ocular inflammation [32]. The efficacy of this formulation in controlling ocular inflammation after cataract surgery has been proved in previous studies [40, 41]. Recently, a new single-dose dexamethasone 0.1%/netilmicin 0.3% ophthalmic hydrogel containing xanthan gum has been marketed. This hydrogel formulation has shown a longer retention time on the ocular surface than the eye drop solution [27], possibly increasing the interval between instillations and reducing the frequency of administration. Moreover, the presence of xanthan gum fosters the healing process after the preoperative disinfection [28, 29].
In this context, the purpose of our study was to assess whether the dexamethasone/netilmicin ophthalmic gel administered b.i.d. is non-inferior to the ophthalmic solution instilled q.i.d. in controlling post-cataract surgery inflammation and in preventing infection.
In this study and considering the ITT population of 168 patients, 7 days of treatment were sufficient to control the post-surgical inflammation in both groups: most patients (88.2% in group 1 and 93.9% in group 2) showed no signs of inflammation of the anterior chamber after 7 days of treatment, and all patients, except one who received the eye drop formulation, completely recovered within 15 days post-surgery. The non-inferiority of the hydrogel in terms of reduction of ocular flare and cellularity reported in the univariate analysis (lower 97.5% CI − 0.0535, greater than the non-inferiority limit of -0.10) was confirmed by both the multivariate analysis and the per-protocol analysis, which excluded seven patients in the hydrogel arm and three in the eye drops arm for deviations to the protocol (lower 97.5% CI − 0.0526).
The results obtained for secondary variables (clinical signs and symptoms of ocular inflammation, visual acuity) confirmed no differences between the two arms.
Moreover, the hydrogel proved to be equal to the Netildex eye drops in terms of global patient tolerability. Indeed, both treatments were well tolerated with very few mild and reversible AEs, which were not considered as related to the study drug, and only one case of drug hypersensitivity was reported in the hydrogel arm.
In the ophthalmic gel formulation, the presence of xanthan gum which acts as a viscosity enhancer increases the retention of the product on the ocular surface and reduces the number of instillations necessary to maintain an adequate concentration of corticosteroids [25, 26]. Moreover, it keeps the ocular surface hydrated and creates a coating that fosters the healing process. One of the main disadvantages of the eye drop formulation is that after topical administration, the concentration of corticosteroids in the anterior chamber increases and declines within hours [42], necessitating frequent daily instillations, with a consequential negative effect on patient compliance and a potential decrease of therapeutic efficacy. It is important to notice that, for double masking reasons, patients in the hydrogel arm also received two placebo administrations. Therefore, it was not possible to evaluate the favourable impact of a reduced number of administrations per day provided by the hydrogel formulation regarding patient compliance and quality of life. The reduction of the number of daily instillations may improve patient adherence to treatment, especially in the case of concomitant use of other topical medications (e.g., anti-glaucoma drugs, non-steroidal anti-inflammatory drugs or artificial tears). The formulation may also act as artificial tears.
As a potential limitation of the study, anterior chamber inflammation was measured, for practical reasons, by slit-lamp examination rather than by a laser flare and cell metre. Even if the scoring system used to measure flare and cells by slit-lamp examination is subjective and semiquantitative, it still corresponds to the actual daily routine of practice [43].
Conclusions
In conclusion, this large, international clinical trial shows that the administration of Netildex ophthalmic gel b.i.d. is sufficient to obtain a non-inferior efficacy in the prevention and treatment of post-cataract surgery ocular inflammation compared with Netildex eye drop solution administered q.i.d. for the same period. The new ophthalmic gel formulation, therefore, allows a reduced administration frequency while maintaining the same efficacy and simultaneously favouring patients' convenience and comfort, with possible better compliance to treatment and patients' quality of life.
References
Klein BE, Klein R, Lee KE, Gangnon RE. Incidence of age-related cataract over a 15-year interval the Beaver Dam Eye Study. Ophthalmology. 2008;115(3):477–82. https://doi.org/10.1016/j.ophtha.2007.11.024 (Epub 2008 Jan 2 PMID: 18171585).
Liu YC, Wilkins M, Kim T, Malyugin B, Mehta JS. Cataracts. Lancet. 2017;390(10094):600–12. https://doi.org/10.1016/S0140-6736(17)30544-5 (Epub 2017 Feb 25 PMID: 28242111).
Davis G. The evolution of cataract surgery. Mo Med. 2016;113(1):58–62 (PMID: 27039493; PMCID: PMC6139750).
Petrash JM. Aging and age-related diseases of the ocular lens and vitreous body. Investig Ophthalmol Vis Sci. 2013;54(14):ORSF54-9. https://doi.org/10.1167/iovs.13-12940 (PMID: 24335070; PMCID: PMC3864378).
Kelman CD. Phaco-emulsification and aspiration: a new technique of cataract removal: a preliminary report. Am J Ophthalmol. 2018;191:xxx–xl. https://doi.org/10.1016/j.ajo.2018.04.014 (PMID: 29929630).
Dick HB, Schwenn O, Krummenauer F, Krist R, Pfeiffer N. Inflammation after sclerocorneal versus clear corneal tunnel phacoemulsification. Ophthalmology. 2000;107(2):241–7. https://doi.org/10.1016/s0161-6420(99)00082-2 (PMID: 10690818).
Rajpal RK, Roel L, Siou-Mermet R, Erb T. Efficacy and safety of loteprednol etabonate 0.5% gel in the treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2013;39(2):158–67. https://doi.org/10.1016/j.jcrs.2012.09.013 (Epub 2012 Dec 3 PMID: 23218817).
El-Harazi SM, Feldman RM. Control of intra-ocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. 2001;12(1):4–8. https://doi.org/10.1097/00055735-200102000-00002 (PMID: 11150074).
Rotsos TG, Moschos MM. Cystoid macular edema. Clin Ophthalmol. 2008;2(4):919–30. https://doi.org/10.2147/opth.s4033.PMID:19668445;PMCID:PMC2699812.
McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25(1):33–55. https://doi.org/10.2165/00002018-200225010-00004 (PMID: 11820911).
Chu CJ, Johnston RL, Buscombe C, Sallam AB, Mohamed Q, Yang YC, United Kingdom Pseudophakic Macular Edema Study Group. Risk factors and incidence of macular edema after cataract surgery: a database study of 81984 eyes. Ophthalmology. 2016;123(2):316–23. https://doi.org/10.1016/j.ophtha.2015.10.001 (Epub 2015 Dec 8, PMID: 26681390).
Korenfeld MS, Silverstein SM, Cooke DL, Vogel R, Crockett RS, Difluprednate Ophthalmic Emulsion 0.05% (Durezol) Study Group. Difluprednate ophthalmic emulsion 0.05% for postoperative inflammation and pain. J Cataract Refract Surg. 2009;35(1):26–34. https://doi.org/10.1016/j.jcrs.2008.09.024 (PMID: 19101421).
Lorenz K, Dick B, Jehkul A, Auffahrt GU. Inflammatory response after phacoemulsification treated with 0.5% prednisolone acetate or vehicle. Graefes Arch Clin Exp Ophthalmol. 2008;246(11):1617–22. https://doi.org/10.1007/s00417-008-0908-2 (Epub 2008 Aug 26, PMID: 18726610).
Comstock TL, Paterno MR, Singh A, Erb T, Davis E. Safety and efficacy of loteprednol etabonate ophthalmic ointment 0.5% for the treatment of inflammation and pain following cataract surgery. Clin Ophthalmol. 2011;5:177–86. https://doi.org/10.2147/OPTH.S16832 (Epub 2011 Feb 10, PMID: 21383946; PMCID: PMC3045067).
Sanders DR, Kraff M. Steroidal and nonsteroidal anti-inflammatory agents. Effect on postsurgical inflammation and blood-aqueous humor barrier breakdown. Arch Ophthalmol. 1984;102(10):1453–6. https://doi.org/10.1001/archopht.1984.01040031173012 (PMID: 6385931).
Walters T, Endl M, Elmer TR, Levenson J, Majmudar P, Masket S. Sustained-release dexamethasone for the treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2015;41(10):2049–59. https://doi.org/10.1016/j.jcrs.2015.11.005 (Erratum in: J Cataract Refract Surg. 2016 Mar;42(3):512. PMID: 26703279).
Liesegang TJ. Use of antimicrobials to prevent postoperative infection in patients with cataracts. Curr Opin Ophthalmol. 2001;12(1):68–74. https://doi.org/10.1097/00055735-200102000-00012 (PMID: 11150084).
Soriano ES, Nishi M. Endophthalmitis: incidence and prevention. Curr Opin Ophthalmol. 2005;16(1):65–70. https://doi.org/10.1097/00055735-200502000-00012 (PMID: 15650583).
Friling E, Lundström M, Stenevi U, Montan P. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg. 2013;39(1):15–21. https://doi.org/10.1016/j.jcrs.2012.10.037 (PMID: 23245359).
Olson RJ, Braga-Mele R, Chen SH, Miller KM, Pineda R 2nd, Tweeten JP, Musch DC. Cataract in the adult eye preferred Practice Pattern®. Ophthalmology. 2017;124(2):P1–119. https://doi.org/10.1016/j.ophtha.2016.09.027 (Epub 2016 Oct 13, PMID: 27745902).
Kessel L, Flesner P, Andresen J, Erngaard D, Tendal B, Hjortdal J. Antibiotic prevention of postcataract endophthalmitis: a systematic review and meta-analysis. Acta Ophthalmol. 2015;93(4):303–17. https://doi.org/10.1111/aos.12684 (Epub 2015 Mar 16, PMID: 25779209; PMCID: PMC6680152).
Bowen RC, Zhou AX, Bondalapati S, Lawyer TW, Snow KB, Evans PR, Bardsley T, McFarland M, Kliethermes M, Shi D, Mamalis CA, Greene T, Rudnisky CJ, Ambati BK. Comparative analysis of the safety and efficacy of intracameral cefuroxime, moxifloxacin and vancomycin at the end of cataract surgery: a meta-analysis. Br J Ophthalmol. 2018;102(9):1268–76. https://doi.org/10.1136/bjophthalmol-2017-311051 (Epub 2018 Jan 11, PMID: 29326317; PMCID: PMC6041193).
Speaker MG, Milch FA, Shah MK, Eisner W, Kreiswirth BN. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991;98(5):639–49. https://doi.org/10.1016/s0161-6420(91)32239-5 (discussion 650, PMID: 2062496).
Labetoulle M, Rousseau A, Baudouin C. Management of dry eye disease to optimize cataract surgery outcomes: two tables for a daily clinical practice. J Fr Ophtalmol. 2019;42(8):907–12. https://doi.org/10.1016/j.jfo.2019.03.032 (Epub 2019 Jul 24, PMID: 31351686).
Ceulemans J, Vinckier I, Ludwig A. The use of xanthan gum in an ophthalmic liquid dosage form: rheological characterization of the interaction with mucin. J Pharm Sci. 2002;91(4):1117–27. https://doi.org/10.1002/jps.10106 (PMID: 11948550).
Mazzone M Sr, Peri O, Moschetti V. Evaluation of mucoadhesive properties of xanthan gum hydrogels versus marketed ophthalmic gel formulations using a tensile strength method. Investig Ophthalmol Vis Sci. 2006;47:1966.
Gagliano C, Papa V, Amato R, Malaguarnera G, Avitabile T. Measurement of the retention time of different ophthalmic formulations with ultrahigh-resolution optical coherence tomography. Curr Eye Res. 2018;43(4):499–502. https://doi.org/10.1080/02713683.2017.1418893 (Epub 2017 Dec 28, PMID: 29283672).
Kocatürk T, Gençgönül A, Balica F, Özbağcivan M, Çakmak H. Combined eye gel containing sodium hyaluronate and xanthan gum for the treatment of the corneal epithelial defect after pterygium surgery. Clin Ophthalmol. 2015;13(9):1463–6. https://doi.org/10.2147/OPTH.S85638 (PMID: 26316686; PMCID: PMC4540174).
Faraldi F, Papa V, Santoro D, Rasà D, Mazza AL, Rabbione MM, Russo S. A new eye gel containing sodium hyaluronate and xanthan gum for the management of post-traumatic corneal abrasions. Clin Ophthalmol. 2012;6:727–31. https://doi.org/10.2147/OPTH.S31776 (Epub 2012 May 9, PMID: 22654499; PMCID: PMC3363309).
Postorino EI, Aragona P, Rania L, Spinella R, Puzzolo D, Micali A, Mazza AML, Papa V. Changes in conjunctival epithelial cells after treatment with 0.2% xanthan gum eye drops in mild-moderate dry eye. Eur J Ophthalmol. 2020;30(3):439–45. https://doi.org/10.1177/1120672119833278 (Epub 2019 Mar 11, PMID: 30852915).
Naderi K, Gormley J, O’Brart D. Cataract surgery and dry eye disease: a review. Eur J Ophthalmol. 2020;30(5):840–55. https://doi.org/10.1177/1120672120929958 (Epub 2020 Jun 9, PMID: 32515220; PMCID: PMC7549290).
Hogan MJ, Kimura SJ, Thygeson P. Signs and symptoms of uveitis. I Anterior uveitis Am J Ophthalmol. 1959;47(5 Pt 2):155–70. https://doi.org/10.1016/s0002-9394(14)78239-x (PMID: 13649855).
Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–16. https://doi.org/10.1016/j.ajo.2005.03.057 (PMID: 16196117; PMCID: PMC8935739).
Barry P, Cordovés L, Gardner S. ESCRS guidelines for prevention and treatment of endophthalmitis following cataract surgery: data, dilemmas and conclusions. In: Paper presented at the European Society of Cataract and Refractive Surgeons. Dublin, Ireland: 2013.
Blanco AR, Sudano Roccaro A, Spoto CG, Papa V. Susceptibility of methicillin-resistant Staphylococci clinical isolates to netilmicin and other antibiotics commonly used in ophthalmic therapy. Curr Eye Res. 2013;38(8):811–6. https://doi.org/10.3109/02713683.2013.780624 (Epub 2013 Mar 27, PMID: 23534928).
Sanfilippo CM, Morrissey I, Janes R, Morris TW. Surveillance of the activity of aminoglycosides and fluoroquinolones against ophthalmic pathogens from Europe in 2010–2011. Curr Eye Res. 2016;41(5):581–9. https://doi.org/10.3109/02713683.2015.1045084 (Epub 2015 Jul 22, PMID: 26200173).
Viola S, De Pasquale G, Zappulla C, Curatolo MC, Mazzone MG, Giuliano F. In vitro evaluation of cytotoxic potential associated to antimicrobial agents commonly used in clinical ophthalmological practice, vol 97(S263). Special issue: abstracts from the 2019 European Association for vision and eye research conference. https://doi.org/10.1111/j.1755-3768.2019.5259.
Marino C, Paladino GM, Scuderi AC, Trombetta F, Mugridge K, Enea V. In vivo toxicity of netilmicin and ofloxacin on intact and mechanically damaged eyes of rabbit. Cornea. 2005;24(6):710–6. https://doi.org/10.1097/01.ico.0000154233.56736.08.
Leibowitz HM, Kupferman A. Antiinflammatory medications. Int Ophthalmol Clin. 1980;20(3):117–34. https://doi.org/10.1097/00004397-198002030-00012 (PMID: 6998894).
Rapisarda A, Arpa P, Fantaguzzi PM, Faraldi F, Ratiglia R, Rizzo S, Vaona P, Iannacone C, Papa V. Dexamethasone/netilmicin eye drops and eye gel for the treatment of ocular inflammation after micro-incisional vitreoretinal surgery. Clin Ophthalmol. 2020;14(14):3297–303. https://doi.org/10.2147/OPTH.S257541 (PMID: 33116381; PMCID: PMC7569042).
Russo S, Papa V, Di Bella A, Favero A, Radulescu C, Gafencu O, Carstocea B, Milazzo G. Dexamethasone-netilmicin: a new ophthalmic steroid-antibiotic combination. Efficacy and safety after cataract surgery. Eye (London). 2007;21(1):58–64. https://doi.org/10.1038/sj.eye.6702123 (Epub 2005 Nov 4, PMID: 16273088).
Watson D, Noble MJ, Dutton GN, Midgley JM, Healey TM. Penetration of topically applied dexamethasone alcohol into human aqueous humor. Arch Ophthalmol. 1988;106(5):686–7. https://doi.org/10.1001/archopht.1988.01060130748037 (PMID: 3358736).
Saccà S, Marletta A, Pascotto A, Barabino S, Rolando M, Giannetti R, Calabria G. Daily tonometric curves after cataract surgery. Br J Ophthalmol. 2001;85(1):24–9. https://doi.org/10.1136/bjo.85.1.24 (PMID:11133707; PMCID:PMC1723696).
Acknowledgements
Funding
Sponsorship for this study and the journal’s Rapid Service and Open Access Fees were funded by SIFI S.p.A., Catania (Italy).
Medical Writing and Editorial Assistance
Editorial and medical writing assistance in the preparation of this article was provided by Laura C Collada Ali (Medical Writing Consultant). Support for this assistance was funded by SIFI S.p.A., Catania (Italy).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Author Contributions
Conceptualisation: Rita Mencucci, Aldo Caporossi, Maria Grazia Mazzone, Claudine Civiale; Methodology: Rita Mencucci, Aldo Caporossi, Thomas Ach, Liekfeld Anja, Antonio Scialdone, Maria Grazia Mazzone, Claudine Civiale; Writing—original draft preparation and review: Rita Mencucci, Maria Grazia Mazzone, Claudine Civiale; Supervision: Aldo Caporossi, Rita Mencucci, Maria Grazia Mazzone, Claudine Civiale.
Disclosures
Rita Mencucci received honoraria for medical meetings and advisory board from SIFI S.p.A. Tomas Ach, Anja Liekfeld, Antonio Scialdone, Claudine Civiale, Maria Grazia Mazzone and Aldo Caporossi have nothing to disclose.
Compliance with Ethics Guidelines
The study protocol and related documentation were reviewed and approved by the Ethics Committees of the main coordinating centre (Catholic University of the Sacred Heart, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy) and of all other participating centres.
Informed consent was obtained from all study participants.
The study was conducted in accordance with this approval and adhered to the tenets of the Declaration of Helsinki revised in 2013. The study was registered in the European Union Drug Regulating Authorities Clinical Trials (EUDRACT) database with code number 2016-0021138-63 and on ClinicalTrial.gov (NCT029738880).
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Study Participants
The study investigators thank study participants for their involvement in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mencucci, R., Ach, T., Liekfeld, A. et al. Reduced Posology of an Ophthalmic Hydrogel Containing Dexamethasone/Netilmicin to Prevent and Treat Ocular Inflammation After Cataract Surgery: Efficacy and Tolerability. Adv Ther 39, 5474–5486 (2022). https://doi.org/10.1007/s12325-022-02295-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02295-y