Abstract
Introduction
To compare the mortality of hospitalized patients with COVID-19 between those that required supplemental oxygen and received dexamethasone with a comparable set of patients who did not receive dexamethasone.
Methods
We utilized the Premier Health Database to identify hospitalized adult patients with COVID-19 from July 1, 2020–January 31, 2021. Index date was when patients first initiated oxygen therapy. The primary endpoint was in-hospital mortality for patients receiving dexamethasone versus those not receiving dexamethasone 1-day pre- to 1-day post-index period. Secondary endpoints included 28-day mortality, time to in-hospital mortality, progression to invasive mechanical ventilation or death, time to discharge, and proportion discharged alive by day 28. Twenty-three models using weighting, matching, stratification, and regression were deployed through the concept of frequentist model average (FMA) to estimate the effect of dexamethasone on all-cause mortality up to the 28-day hospitalization period.
Results
A total of 1,208,881 patients with COVID-19 were screened; as an inpatient 255,216 used oxygen, and 251,536 were included in the analysis. In the dexamethasone group, odds of in-hospital mortality were higher than those of the comparator (FMA: odds ratio [OR] 1.15, 95% CI 1.08, 1.22). Using a best fit model, OR for in-hospital mortality was non-significant for the dexamethasone group compared with the comparator (OR 1.02, 95% CI 0.92, 1.14). Dexamethasone treatment was associated with poorer outcomes versus the comparator group across the majority of secondary endpoints, except for number of days in hospital, which was lower in the dexamethasone group versus the comparator group (mean difference − 2.14, 95% CI − 2.43, − 1.47).
Conclusions
Hospitalized adult patients with COVID-19 who required supplemental oxygen and received dexamethasone did not have a survival benefit versus similar patients not receiving dexamethasone. The dexamethasone group was not associated with favorable responses for outcomes such as progression to death or mechanical ventilation and time to in-hospital death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Dexamethasone has demonstrated a survival benefit in patients with COVID-19 who required supplemental oxygen |
COVID-19 is an emerging, rapidly evolving pandemic. The validity of the effectiveness of dexamethasone needs to be re-established or re-evaluated in the US population and to fit the current needs |
To our knowledge, limited real-world evidence studies to date have assessed comparative effectiveness among a vast network of private and public hospitalized patients with SARS-CoV-2 infection in the US who initiated dexamethasone versus patients who were on hospital supportive care without dexamethasone treatment, especially in the population with background mortality risk |
Therefore, our study aims to compare hospitalized patients with COVID-19 who required supplemental oxygen and were treated with dexamethasone versus patients who were not treated with dexamethasone for mortality during their in-hospital stay, 28-day all-cause mortality, and time to in-hospital death (all-cause) |
What was learned from the study? |
Our results showed that hospitalized adult patients with COVID-19 who required supplemental oxygen and received dexamethasone did not have a survival benefit compared with similar patients who did not receive dexamethasone |
The dexamethasone group was not associated with favorable responses for outcomes such as progression to death or mechanical ventilation and time to in-hospital death. Future studies are warranted to validate the findings of our study |
Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is the largest global pandemic in the past 100 years with 423.4 million confirmed cases and 5.89 million deaths worldwide as of February 2022 [1]. Approximately 77 million confirmed cases of COVID-19 and 926,287 COVID related deaths have been reported in the USA alone as of February 2022 [1]. The Center for Disease Control (CDC) and Prevention reported a total of 2,302,744 laboratory-confirmed hospitalizations in the US as of February 2022 [2]. Although reportedly hospitalization has been substantially reduced after the mass vaccination program in the US [3], a few regions continue to experience surges, therefore, increasing the burden on the healthcare system [4]. The literature suggests that among hospitalized patients with COVID-19, 14–30% developed acute respiratory distress syndrome, with an associated mortality rate of 45–75% [5, 6].
A previous study suggested that the severity of COVID-19 was reported to be positively correlated with decreased immune resilience, increasing age, male sex, and underlying comorbidities, including cardiovascular disease, chronic kidney disease, chronic lung disease, diabetes, obesity, and malignancy [7, 8]. Patients with severe COVID-19 disease require supplemental oxygen to manage hypoxemia. Most critically ill patients require mechanical ventilation or extracorporeal membrane oxygenation (ECMO) for refractory hypoxemia [9].
In the RECOVERY trial, treatment with dexamethasone demonstrated a survival benefit in patients with COVID-19 disease who required supplemental oxygen at enrollment (death in the dexamethasone arm within 28 days of enrollment: 23.3% vs. standard of care arm: 26.2%; rate ratio 0.82; 95% CI 0.72–0.94) or invasive mechanical ventilation (death in the dexamethasone arm within 28 days of enrollment: 29.3% vs. the standard of care arm: 41.4%; rate ratio 0.64; 95% CI 0.51–0.81) [10]. After the results from the RECOVERY trial, dexamethasone was recommended as standard of care for patients with COVID-19 who require oxygen or mechanical ventilation [11, 12, 13].
Multiple treatment options have been approved/authorized since the results of the RECOVERY trial were published [14]. Since COVID-19 is an emerging, rapidly evolving situation, the validity of the effectiveness of dexamethasone needs to be re-evaluated to fit the current needs. To date, there are limited real-world evidence studies assessing comparative effectiveness of dexamethasone among hospitalized patients with COVID-19 versus those receiving supportive care without dexamethasone, especially in the population with background mortality risk similar to that of the US health care system [15]. Therefore, our study aimed to compare hospitalized patients with COVID-19 who required supplemental oxygen and received dexamethasone with patients who did not receive dexamethasone for mortality during in-hospital stay (hereon referred as in-hospital mortality).
Methods
Study Design
This retrospective cohort study utilized the Premier Health Database (PHD) [16] to identify hospitalized adult patients (≥ 18 years) with COVID-19 from July 1, 2020, to January 31, 2021. This study period was selected because dexamethasone was considered the standard of care for hospitalized COVID-19 patients from July 2020 onwards. The index date was the date when patients first initiated any oxygen therapy (Supplementary Fig. S1). The baseline period starts from admission to the index date.
Data Source
The PHD is one of the most comprehensive inpatient electronic health databases in the US and represents approximately 25% of annual inpatient admissions [16]. With more than a thousand contributing hospitals or healthcare systems, PHD contains information on inpatient discharges from geographically diverse non-profit, non-governmental, community, teaching hospitals, and health systems from rural, and urban areas. The data include demographics, early diagnosis, admission and discharge diagnoses, and information on billed services, including costs at the departmental level. The PHD contains de-identified, Health Insurance Portability and Accountability Act compliant data and is exempted from Institutional Review Board oversight [16].
Compliance with Ethics Guidelines
This is an observational study that uses previously collected data and does not impose any form of intervention and was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data have been deidentified to protect subject privacy and to be fully compliant with the US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996, and did not require institutional review board waiver or approval.
Patient Population
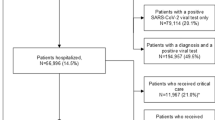
Patients were included in this study if they had at least one inpatient diagnosis of COVID-19 and initiated oxygen therapy during the study period. For the dexamethasone group, patients with the following were included: (1) ≥ 1 report of a COVID-19 diagnosis (ICD-10 CM: U07.1) from an inpatient hospital stay during the study period; (2) gender entry not missing; (3) presence of oxygen use (as defined in the Adaptive COVID-19 Treatment Trial Ordinal Scale [ACTT OS] 5, 6, or 7) [17, 18]; (4) who initiated dexamethasone between 1-day pre- to 1-day post-index period. Similar criteria were used for including patients in the comparator group except for the use of dexamethasone between 1-day pre- to 1-day post-index period. The differences in steroid usage between the dexamethasone group and the comparator group were balanced using all the baseline characteristics available in the database. Patients with death recorded before or on index date were excluded from both the groups. For the comparator group, patients with dexamethasone use within 7 days prior to index were also excluded (Fig. 1).
Analyses of Primary Endpoint
The primary endpoint was the binary outcome of in-hospital mortality (includes patients that were discharged and deceased at the hospice) for patients receiving dexamethasone versus those who did not receive dexamethasone at the time of receiving oxygen therapy. The effect of dexamethasone on in-hospital mortality was estimated using multiple methods through a frequentist model averaging framework (FMA). FMA is an ensemble approach in which multiple pre-specified candidate methods/models are entered and cross-validation is used to select or upweight the methods/models that perform best. FMA addresses model uncertainty and has been shown to produce more robust estimates of treatment effects than using a single pre-selected method [19]. Specifically, the mean squared prediction error (MSPE) for each candidate method is computed via (five fold) cross validation and weights are derived for each candidate method with higher weights given to candidate methods with smaller MSPE. For binary and continuous outcomes (including the primary outcome of in-hospital mortality) final treatment estimates are obtained by either selecting the method with the smallest MSPE (averaged across bootstrap samples) or a weighted average of the treatment effect estimates across all methods is computed. However, as there is no guidance in the literature on FMA weighting for time to event outcomes, such analyses were ranked by model fit, e.g., Akaike information criterion or Bayesian information criterion followed by the lowest average absolute standardized difference of means.
Table 1 provides the details of the pre-specified individual methods used in the FMA analysis. A total of 23 pre-specified individual models/methods were entered into the FMA analysis, including analyses based on weighting, matching, stratification, and regression. For those models that used propensity score (PS), the variables used in balancing the baseline covariates are presented in Supplementary Table S1. In brief, the following variables were used to adjust for confounding: any underlying comorbidities during in-hospital stay, baseline demographics, serious infection, background treatments (remdesivir, enoxaparin, and corticosteroid), ACTT OS scale, and hospital admission status.
Prior to conducting the analysis of treatment effects, the feasibility of the analysis was confirmed by first assessing the positivity assumption through quantifying the overlap in population characteristics between the two treatment groups. This was done both graphically through overlapping histogram plots and quantitatively through statistics summarizing differences in populations (standardized mean difference, Tipton’s index). Second, the ability of PS to produce balance in baseline patient characteristics between the two treatment groups was assessed using standardized mean differences, variance ratios, and graphical assessment of the distribution of each covariate between groups. This feasibility step was completed prior to conducting outcome analyses.
The validity of the treatment effect estimates depends on the statistical assumption of no unmeasured confounding. To quantify the strength of the findings relative to potential unmeasured confounding variables, the E-value was estimated [20]. The E-value is the minimum strength of association on the risk ratio scale that an unmeasured confounder would need to have with both the treatment and outcome to fully explain the observed outcome, conditional on the observed covariates. To aid in the interpretation of the E-value, we compared the E-value with the strength of associations of select observed covariates.
Finally, sensitivity analyses were performed to check whether the primary endpoint analysis varied according to the various choices on the patient population and modeling strategies described in Table 1. We conducted nine sensitivity analyses, categorized in five ways—(1) restricting the analysis to patients who were classified as ACTT OS 5–7 and ACTT OS 5 or 6 at index date, (2) FMA including additional models based on alternative PS models, (3) excluding patients who received dexamethasone prior to the index date or for the comparator cohort that received dexamethasone post-index, (4) including patients with an index date from April 2020, and (5) including patients with an index date from April 2020, but not including the index date as part of the PS.
All analyses were conducted using SAS 9.4 and R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Analyses of Secondary Endpoints
Secondary endpoints included 28-day mortality, time to in-hospital mortality, progress to invasive mechanical ventilation or death for patients who were not receiving invasive mechanical ventilation on the index date, time to discharge, proportion discharged alive by day 28, and length of hospital stay. The effect of dexamethasone on all the secondary endpoints was compared between patients who received dexamethasone versus those who did not, within 1 day of index, adjusting for confounders, using the same statistical approach used for the primary outcome measure (see Table 1).
Results
Of the 1,208,881 patients diagnosed with COVID-19, 389,442 (32.2%) were hospitalized (Fig. 1). A total of 255,216 patients used oxygen during their hospital stay. After excluding patients per the criteria mentioned above, a total of 251,536 patients were included in the current analysis. These patients were further categorized into dexamethasone (with dexamethasone use ± 1 day from index; N = 203,755; 81%) and comparator groups (without dexamethasone use; N = 47,781; 19%). Overall, most patients belonged to the 65–74 years age group; 46.08% of the patients were female, and 70.8% were White (Table 2). A history of diabetes was reported in 44.23% of the patients, and hypertension in 72.93%, with 72.16% having any serious co-infection post-index. The time between admission to oxygen use in all patients was 0.78 ± 1.96 days.
Baseline (before receiving oxygen support) demographic and clinical characteristics varied significantly across both the groups for the majority of variables except asthma and diabetes. These small differences in baseline characteristics were shown as being statistically significant because of a large sample size in each cohort. Mean ± SD time between admission to oxygen use was 0.72 ± 1.63 days in the dexamethasone group and 1.05 ± 2.98 days in the comparator group. Compared with the dexamethasone group (10.84%), a higher proportion of patients in the comparator arm received corticosteroids other than dexamethasone (32.97%) in the baseline period. In the dexamethasone group, 21.73% of the patients used anticoagulants and corticosteroids at baseline compared with the comparator group (28.96%). A higher proportion of patients in the dexamethasone group received remdesivir concomitantly (55.71%) versus the comparator arm (16.43%, Table 2). At each index month, a higher proportion of patients initiated dexamethasone (Fig. 2).
Treatment by index date. Dexamethasone group included patients with: a ≥ 1 report of a COVID-19 diagnosis (ICD-10 CM: U07.1) from an inpatient hospital stay during the study period; b gender entry not missing; c presence of oxygen use [as defined in the Adaptive COVID-19 Treatment Trial Ordinal Scale (ACTT OS) 5, 6, or 7 and d who initiated dexamethasone between 1-day pre- to 1-day post-index period. Similar criteria were used for including patients in the comparator group except for the use of dexamethasone between 1-day pre- to 1-day post-index period
Propensity Score Model
For those models that used a PS to balance between treatment cohorts, there was good overlap between the PS for the dexamethasone and comparator groups (Tipton index = 0.94 [acceptable range ≥ 0.9]; SMD = 0.69 [acceptable range ≤ 0.25 or < 0.1]) (Fig. 3). After using PS for matching and re-weighting, the absolute standardized differences were reduced to < 0.1 for all the covariates listed in Supplementary Figure S2.
Distribution of propensity scores among the dexamethasone and comparator groups. Best models can be identified based on the description below: SMD standardized difference of means between treatment groups (difference in means between group divided by the pooled standard deviation). Acceptable ranges are < 0.25 or < 0.1 (Austin). SMD = 0.69. Ratio of variances (treated/controls). Acceptable ranges 0.5–2.0. Preference score: A transformation of the PS representing the preference for one treatment over another for an individual based on baseline patient characteristics (Walker et al. 2013). Value reported is the percentage of patients with a preference score between 0.3 and 0.7. PS = 0.79 and 0.7. Tipton index: a single metric describing the similarity of two distributions combining the SMD and variance ratios (Tipton 2014). Should be > 0.9.T = 0.94
Primary Endpoint
In the dexamethasone group, the odds of in-hospital mortality were higher than those in the comparator group (FMA: odds ratio [OR] 1.16, 95% CI 1.09, 1.23, Fig. 4). However, in the best fit model, the OR for in-hospital mortality was non-significant for the dexamethasone group compared with the comparator group (OR 1.02, 95% CI 0.93, 1.14). Of the 23 individual methods used in the FMA analysis (including analyses based on weighting, matching, stratification, and regression), 21 models demonstrated a higher odd of in-hospital mortality for the dexamethasone group (Fig. 4 and Table 1). The two models (matching band weighting F) showed higher odds of in-hospital mortality for the comparator group.
Forest plot of models for in-hospital mortality. *See Table 1 for detailed model description. ESS explained sum of squares, MSPE mean squared prediction error, FMA frequentist model averaging, OR odds ratio
However, no single model was consistently identified as best fitting; the weights for each model did not vary greatly (from 0.029 to 0.058). The stratification, including regression-adjusted models (Fig. 4), had higher weights (0.047–0.058) than those based on matching 0.029–0.031.
To further understand whether the primary analysis result is robust to potential uncontrolled confounders, we computed the E-value for both the estimate (E-value = 1.36) and the lower confidence interval limit of the estimated risk ratio (E-value = 1.26) for in-hospital mortality. Thus, if there were an unmeasured confounder with an association with treatment selection and outcome equating to risk ratios of 1.36, such a confounder could produce sufficient bias to produce an observed RR for the primary analysis of 1.36 even when there was no true treatment effect. While there is no clear guidance on what a robust E-value is, Fig. 5 demonstrates that covariates with the similar explanatory power of ‘Age’ would be sufficient, if not accounted for in the analysis, to produce a RR higher than the observed RR even under the situation of no true treatment effect.
E-value for in-hospital mortality. CI confidence interval, RR relative risk. E-value was calculated from the FMA OR (converted to RR) for the primary objective, and a separate E-value (CI) was calculated from the lower limit of the FMA OR for the primary objective. The y-axis characterizes the strength of association between the unmeasured confounder and the outcome. The x-axis characterizes the extent to which the prevalence of the unmeasured confounder is unbalanced between the two treatment cohorts
The results of the sensitivity analyses were similar to the data obtained in the primary analysis and remained robust. Except for the sensitivity analysis 6 in Table 3 excluding patients in the dexamethasone group who received dexamethasone prior to index date or for the comparator cohort that received dexamethasone post-index with minor difference (FMA OR 1.47) when compared to the in-hospital mortality estimate of 1.157, the FMA OR for all other analyses were in the range of 1.07–1.25.
Secondary Endpoints
Dexamethasone treatment was associated with poorer outcomes compared with the comparator group across the majority of endpoints, except for number of days in hospital, which was lower in the dexamethasone group versus the comparator group (mean difference: − 2.14, 95% CI − 2.43, − 1.48) (Table 4). However, it also included patients who died while estimating the average hospital stay. Patients receiving dexamethasone were 7% less likely to be discharged within 28 days from the hospital (FMA: OR 0.93, 95% CI 0.86, 0.99) and 3% less likely to have reduced number of days between index and date of hospital discharge (average hazard ratio 0.97, 95% CI 0.93, 1.00) compared with the comparator group. Patients in the dexamethasone group had a higher likelihood of time to in-hospital death versus the comparator group (hazard ratio 1.10, 95% CI 1.02, 1.15). E-values for all secondary endpoints were low, which suggests an unmeasured confounder could influence the results from our analysis (Table 5).
Discussion
This is the first real-world evidence study that observed a lack of survival benefit with dexamethasone versus comparators in 251,536 hospitalized patients with COVID-19 in the US who received supplemental oxygen. The demographic and clinical characteristics of the patients analyzed in this study are consistent with the previous reports on hospitalized patients with COVID-19 from the PHD [16–, 21, 22, 23]. Most of the patients in our study received dexamethasone, and the reasons for patients who did not receive dexamethasone were most likely due to their characteristics, drug access, or treatment protocols of the health system. Due to reported drug shortages of dexamethasone and hospital policies that may restrict its use in a subset of critically ill patients with COVID-19 [24], clinicians may be reluctant to use corticosteroids because of the conflicting evidence for steroids in other forms of acute respiratory distress syndrome [25, 26]. In our study, the comparative effectiveness of in-hospital mortality and time to all-cause in-hospital death were assessed through the concept of model averaging. We found that except for the number of days in the hospital, the dexamethasone group showed unfavorable outcomes for all other endpoints compared with the comparator group, which could be due to a survival enrichment/bias for those who did not receive dexamethasone; however, the cause-effect relationship cannot be addressed using the PHD database. The E-values were low and could be influenced by an unmeasured confounder showing similar confounding to variables in our database (such as age). The uncertainty in the impact of unmeasured confounders could have impacted the results; therefore, the results should be interpreted with caution. Results of sensitivity analyses are robust across all models and were similar to the data in the primary analysis.
Overall, dexamethasone showed higher odds of in-hospital mortality contrary to previous results from the RECOVERY trial [10]. While the RECOVERY study was an open-label clinical trial executed in the UK, our study used PHD claims database, and use of remdesivir was reported to be high in the US. The RECOVERY trial evaluated dexamethasone use and found a significant benefit in survival in hospitalized patients with oxygen use or mechanical ventilation and a 1-day shorter length of hospital stay [10]. Also, our results were in contrast with an early terminated trial, REMAP-CAP (Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia), which suggested benefit for hydrocortisone in patients with severe COVID-19 [27]. Despite a rigorously designed study and a multiple analysis approach, our results did not demonstrate the benefits of dexamethasone in hospitalized COVID-19 patients with oxygen use in a real-world setting. However, we observed a decrease in the hospital stay, which was consistent with that observed in the RECOVERY trial. The effectiveness of corticosteroid or dexamethasone in the treatment of COVID-19 remains to be explored further in detail; the findings from a meta-analysis by Cheng et al. suggest that corticosteroid therapy was associated with the clinical recovery and a significantly shortened length of ICU hospitalization, but it did not affect the mortality, the utilization of mechanical ventilation, and the virus clearance time in COVID-19 patients. Although corticosteroids appeared to improve prognosis and promote clinical recovery in patients with severe COVID-19, these may cause easily managed adverse outcomes such as transient hyperglycemia, hypokalemia, skin eruption, and increased blood pressure in some patients [28, 29]. Hence, corticosteroid therapy might not demonstrate similar efficacy in all patients with COVID-19.
Earlier studies related to other distinct coronavirus-mediated pandemics such as MERS also provide a similar finding when retrospectively analyzed for the mortality benefit of corticosteroids, particularly dexamethasone. Arabi et al. have used a marginal structure modeling in MERS patients and concluded that corticosteroid therapy in patients with MERS was not associated with a difference in mortality (adjusted odds ratio 0.75; 95% CI 0.52–1.07; P = 0.12) but was associated with a delay in MERS coronavirus RNA clearance (adjusted hazard ratio 0.35; 95% CI 0.17–0.72; P = 0.005) after adjustment for time-varying confounders [30]. A meta-analysis that included 16 studies comparing the use of corticosteroids between severe and non-severe patients with COVID-19 observed that there is no statistical difference between survivors and non-survivors regarding the use of corticosteroids (risk ratio = 1.38, 95% CI = 0.87–2.18, P = 0.17) [31]. A recent retrospective cohort study reported that corticosteroid treatment in non-severe patients with COVID-19 was significantly associated with worse clinical outcomes [32].
Another meta-analysis by Ma et al. with 7 randomized clinical trials (RCTs) including 6250 patients suggested that corticosteroids were associated with a decreased all-cause mortality (27.3 vs. 31.1%; risk ratio 0.85; 95% CI 0.73–0.99; P = 0.04; low-certainty evidence). However, the survival benefit diminished after excluding the RECOVERY trial in their sequential analysis (risk ratio 0.83; 95% CI 0.65–1.06; P = 0.13) [33]. Results from another meta-analysis by Kow and Hasan, 34, observed no significant difference in the risk of developing a fatal course of COVID-19 with preadmission use of inhaled corticosteroids in patients with COVID-19 relative to non-use of inhaled corticosteroids (pooled OR = 1.28; 95% confidence interval 0.73–2.26).
Another meta-analysis of peer-reviewed RCTs showed no difference in survival in critically ill hospitalized COVID-19 patients who received systemic corticosteroid therapy compared to usual care or placebo (OR 0.82, 95% CI 0.64–1.05, P = 0.09). However, a subgroup analysis of the 1967 critically ill patients showed improved survival in patients who received systemic corticosteroid therapy (OR 0.67, 95% CI 0.51–0.87, P = 0.01) [35]. The results of the meta-analysis by Robinson et al. [35] are likely to be influenced by the early termination of three of the included RCTs (CAPE COVID [36], CODEX [37], and REMAP-CAP [27] trials) because of the preliminary results of the RECOVERY trial. It is often challenging to replicate RCT results in a real-world setting because of multiple confounders, such as differences in healthcare settings, study populations, endpoint measurement, effect measures, statistical analyses, and bias or confounding. The lack of agreement in findings between our study and the RECOVERY trial could be attributed to the aforementioned challenges. However, combined RCTs and observational studies can provide valuable and complementary information about patient outcomes and facilitate regulatory and clinical decision-making [38]. Therefore, the usefulness of our study is not just limited to current treatment of COVID-19, but potentially for future pandemics as well. Future prospective studies that can reflect practices in the US may be needed to confirm the findings from our study.
Given the aforementioned conflicting evidence, our study also provides an improved understanding of the effect of dexamethasone in hospitalized COVID-19 patients in the US [35]. From the public health standpoint, the current guidance for using dexamethasone in COVID-19 could benefit from the addition of a cautionary note that it may not be effective in all hospitalized patients requiring supplemental oxygen. Therefore, this study adds value to the literature regarding clinical treatment of hospitalized COVID-19 patients and future management of the disease.
Strengths and Limitations
This analysis has various strengths: a large sample size, robust analyses, and the use of the FMA method. The FMA framework encompasses any estimation strategy and accounts for model uncertainty by computing a cross-validated estimate of MSPE. In this approach, the performance of each modeling approach is assessed, and a weighted average of these models is reported, with the better fitting models receiving the highest weights. The advantage of using FMA over an individual model approach is that the choice of modeling approach (for example, matching vs, reweighting) minimizes the influence one model has on the conclusions. A total of 23 candidate models were included in the FMA in this study, and the differences observed through the choice of model, and the levels of uncertainty with different model choices, were reported. Other strategies, such as linear regression, are reported to perform well in simple scenarios but are inferior to the FMA in a scenario with complex confounding [19]. The study derived data from the PHD, a comprehensive and diverse inpatient electronic health database in the US that includes both rural and urban areas [16].
However, there are some limitations of this study. First, our study results are not from a clinical database or a registry and are rather from a medical claims database, which lacks data on acute physiology such as oxygen saturation and other laboratory data that may be important predictors of mortality; hence, these results may not be generalizable to other populations beyond those identified in the PHD. The PHD also does not include clinical events and encounters outside of the hospital [16]. Any of these unmeasured confounders could be strong enough to affect the results observed in our analysis. Second, this analysis was conducted prior to the introduction of the vaccines, and since the course of COVID-19 is continuously evolving, changes in clinical practice and clinical strategies along with the available vaccination protocols must be accounted for while assessing the effectiveness of existing therapies. Third, this analysis may reflect that time to discharge is shorter for the dexamethasone group, which may be biased because nearly 20% of patients died in this group, which may be erroneously imputed as a shorter time to discharge. Additionally, since ER physicians may be more aggressive with the hospitalized COVID-19 patients, it is possible that this intrinsic treatment bias could be due to the dexamethasone group being sicker than the comparator group. Furthermore, since the designated hospitals were providing care to COVID-19 patients during the pandemic, most of the patients received healthcare services at hospitals that they had not visited before. Thus, most of the patients did not have an encounter with the hospital system prior to their first hospital admission. This limits the analytical approaches to adjust for risk factors of COVID-19 severity prior to hospital admission.
We attempted to balance these differences between the groups using all the baseline characteristics available in the database. As stated earlier, in June 2020, dexamethasone shortages were reported, and hospital policies may also have restricted its use to subsets of patients critically ill with COVID-19 [24]. In addition, clinicians may be unwilling to use corticosteroids in critically ill patients with COVID-19 because of the conflicting evidence for steroids in other forms of acute respiratory distress syndrome [25, 26]. The fact that other trials of steroids in COVID-19 were stopped early and did not uniformly show the same mortality benefit may also contribute to the lack of universal use of steroids in mechanically ventilated patients with COVID-19 [39].
Conclusions
In summary, our results showed that hospitalized adult patients with COVID-19 who required supplemental oxygen, including mechanical ventilation, and received dexamethasone did not have a survival benefit compared with patients who did not receive dexamethasone. Dexamethasone use was not associated with favorable responses for outcomes such as progression to death or mechanical ventilation and time to in-hospital death. The size of the treatment effect observed in our study could possibly be explained by unmeasured confounders. Future studies are warranted to validate the findings of this study.
References
WHO: WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ (2021). Accessed 27 Oct 2021.
Laboratory-Confirmed COVID-19 Associated Hospitalizations. https://gis.cdc.gov/grasp/COVIDNet/COVID19_5.html (2021). Accessed 27 Oct 2021.
Moghadas SM, Vilches TN, Zhang K, Wells CR, Shoukat A, Singer BH, et al. The impact of vaccination on COVID-19 outbreaks in the United States. medRxiv. 2021. https://doi.org/10.1101/2020.11.27.20240051.
Miller IF, Becker AD, Grenfell BT, Metcalf CJE. Disease and healthcare burden of COVID-19 in the United States. Nat Med. 2020;26(8):1212–7. https://doi.org/10.1038/s41591-020-0952-y.
Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24(1):516. https://doi.org/10.1186/s13054-020-03240-7.
Potere N, Valeriani E, Candeloro M, Tana M, Porreca E, Abbate A, et al. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Crit Care. 2020;24(1):389. https://doi.org/10.1186/s13054-020-03022-1.
Best JH, Mohan SV, Kong AM, Patel K, Pagel JM, Ivanov B, et al. Baseline demographics and clinical characteristics among 3471 US patients hospitalized with COVID-19 and pulmonary involvement: a retrospective study. Adv Ther. 2020;37(12):4981–95. https://doi.org/10.1007/s12325-020-01510-y.
Marconi VC, Krishnan V, Ely EW, Montano M. Immune health grades: finding resilience in the COVID-19 pandemic and beyond. J Allergy Clin Immunol. 2022;149(2):565–8. https://doi.org/10.1016/j.jaci.2021.10.025.
Berlin DA, Gulick RM, Martinez FJ. Severe COVID-19. N Engl J Med. 2020;383(25):2451–60. https://doi.org/10.1056/NEJMcp2009575.
Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704. https://doi.org/10.1056/NEJMoa2021436.
Bhimraj AMR, Shumaker AH, Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Gallagher J, Muller WJ, O'Horo JC, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y. Infectious diseases Society of America Guidelines on the Treatment and management of patients with COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. (2021). Accessed 27 Oct 2021.
COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/ (2021). Accessed 27 Oct 2021.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
Coronavirus disease 2019 (COVID-19). https://www.fda.gov/emergency-preparedness-and-response/counterterrorism-and-emerging-threats/coronavirus-disease-2019-covid-19 (2021). Accessed 02 Dec 2021.
Crothers K, DeFaccio R, Tate J, Alba PR, Goetz MB, Jones B, et al. Dexamethasone in hospitalised coronavirus-19 patients not on intensive respiratory support. Eur Respir J. 2021. https://doi.org/10.1183/13993003.02532-2021.
Premier Healthcare Database White Paper. Data that informs and performs. Premier Applied Sciences®, Premier Inc. 2020.
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383(19):1813–26. https://doi.org/10.1056/NEJMoa2007764.
Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384(9):795–807. https://doi.org/10.1056/NEJMoa2031994.
Zagar A, Kadziola Z, Lipkovich I, Madigan D, Faries D. Evaluating bias control strategies in observational studies using frequentist model averaging. J Biopharm Stat. 2022;32(2):247–76. https://doi.org/10.1080/10543406.2021.1998095.
VanderWeele TJ, Mathur MB. Commentary: developing best-practice guidelines for the reporting of E-values. Int J Epidemiol. 2020;49(5):1495–7. https://doi.org/10.1093/ije/dyaa094.
Fan X, Johnson BH, Johnston SS, Elangovanraaj N, Coplan P, Khanna R. Evolving treatment patterns for hospitalized COVID-19 patients in the United States in April 2020–July 2020. Int J Gen Med. 2021;14:267–71. https://doi.org/10.2147/IJGM.S290118.
Lavery AM, Preston LE, Ko JY, Chevinsky JR, DeSisto CL, Pennington AF, et al. Characteristics of hospitalized COVID-19 patients discharged and experiencing same-hospital readmission—United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(45):1695–9. https://doi.org/10.15585/mmwr.mm6945e2.
Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. 2020;3(12):e2029058. https://doi.org/10.1001/jamanetworkopen.2020.29058.
Silverman E. Hospitals see shortages of a cheap steroid that one study says helps COVID-19 patients. https://www.statnews.com/pharmalot/2020/06/25/covid19-coronavirus-dexamethasone-shortages/ (2020). Accessed 13 Apr 2022.
Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378(9):797–808. https://doi.org/10.1056/NEJMoa1705835.
Villar J, Ferrando C, Martinez D, Ambros A, Munoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–76. https://doi.org/10.1016/S2213-2600(19)30417-5.
Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–29. https://doi.org/10.1001/jama.2020.17022.
Cheng W, Li Y, Cui L, Chen Y, Shan S, Xiao D, et al. Efficacy and safety of corticosteroid treatment in patients with COVID-19: a systematic review and meta-analysis. Front Pharmacol. 2020;11:571156. https://doi.org/10.3389/fphar.2020.571156.
Ni Q, Ding C, Li Y, Zhao H, Liu J, Zhang X, et al. Effect of low-to-moderate dose glucocorticoids on viral clearance in COVID-19: a retrospective study. Chin J Clin Infect Dis. 2020:21–4.
Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–67. https://doi.org/10.1164/rccm.201706-1172OC.
Wang Y, Ao G, Qi X, Zeng J. The influence of corticosteroid on patients with COVID-19 infection: a meta-analysis. Am J Emerg Med. 2021;43:267–9. https://doi.org/10.1016/j.ajem.2020.06.040.
Chen Z, Yin X, Tan X, Wang J, Jiang N, Tian M, et al. Effectiveness of systemic corticosteroids therapy for nonsevere patients with COVID-19: a multicenter, retrospective longitudinal cohort study. Value Health. 2022;25(5):709–16. https://doi.org/10.1016/j.jval.2021.12.013.
Ma S, Xu C, Liu S, Sun X, Li R, Mao M, et al. Efficacy and safety of systematic corticosteroids among severe COVID-19 patients: a systematic review and meta-analysis of randomized controlled trials. Signal Transduct Target Ther. 2021;6(1):83. https://doi.org/10.1038/s41392-021-00521-7.
Kow CS, Hasan SS. Preadmission use of inhaled corticosteroids and risk of fatal or severe COVID-19: a meta-analysis. J. Asthma. 2022 59(4):787-90.
Robinson R, Prakash V, Tamimi RA, Albast N, Al-Bast B. Impact of systemic corticosteroids on hospitalized patients with COVID-19: January 2021. Meta-analysis of randomized controlled trials. medRxiv. 2021. https://doi.org/10.1101/2021.02.03.21251065.
Dequin PF, Heming N, Meziani F, Plantefeve G, Voiriot G, Badie J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(13):1298–306. https://doi.org/10.1001/jama.2020.16761.
Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–16. https://doi.org/10.1001/jama.2020.17021.
Sheffield KM, Dreyer NA, Murray JF, Faries DE, Klopchin MN. Replication of randomized clinical trial results using real-world data: paving the way for effectiveness decisions. J Comp Eff Res. 2020;9(15):1043–50. https://doi.org/10.2217/cer-2020-0161.
Carlet J, Payen D, Opal SM. Steroids for sepsis and ARDS: this eternal controversy remains with COVID-19. Lancet. 2020;396(10259):e61–2. https://doi.org/10.1016/s0140-6736(20)32132-2.
Acknowledgements
The authors acknowledge the contributions of Alan James Michael Brnabic and Stephanie de Bono for their inputs on this manuscript.
Funding
The study and journal’s Rapid Service and Open Access fees was funded by Eli Lilly and Company.
Medical Writing Support
Priyanka Bannikoppa and Suchita Dubey, employees of Eli Lilly Services India Pvt., Ltd., provided medical writing support.
Author Contributions
Casey Kar-Chan Choong, Mark Belger and Venkatesh Krishnan were responsible for the study concept and contributed to the initial draft and analysis of the data. Hamed Abedtash, Douglas Faries, Alisa E. Koch, and Kristin J. Meyers provided critical review and analysis of the data. Vincent C. Marconi provided critical review of the manuscript and aided in the analysis of the data.
Disclosures
Vincent C. Marconi reports research grants from the CDC, Gilead Sciences, NIH, Veterans Affairs, and ViiV Healthcare; honoraria from Eli Lilly and Company; has served as an advisory board member for Eli Lilly and Company and Novartis; and has participated as a study section chair for the NIH. Casey Kar-Chan Choong, Mark Belger, Alisa E. Koch, Kristin J. Meyers, Hamed Abedtash, Douglas Faries and Venkatesh Krishnan are employees of Eli Lilly and Company.
Compliance with Ethics Guidelines
This is an observational study that uses previously collected data and does not impose any form of intervention and was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data have been deidentified to protect subject privacy and to be fully compliant with the US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996, and did not require institutional review board waiver or approval.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available due to individual data privacy but may be available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Choong, C.KC., Belger, M., Koch, A.E. et al. Comparative Effectiveness of Dexamethasone in Hospitalized COVID-19 Patients in the United States. Adv Ther 39, 4723–4741 (2022). https://doi.org/10.1007/s12325-022-02267-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02267-2