Abstract
Introduction
Current guidelines for relapsing-remitting multiple sclerosis (RRMS) call for treatment with disease-modifying therapies (DMTs) early in the disease to prevent relapses and accumulation of neurologic impairment and disability. However, patients taking certain oral DMTs may experience gastrointestinal (GI)-related adverse events (AEs), particularly at dose titration. We conducted qualitative research with healthcare professionals (HCPs) and patients in Canada to contextualize their experiences with three oral DMTs: dimethyl fumarate (Tecfidera®), fingolimod (Gilenya®), and teriflunomide (Aubagio®). The objectives of this study were to (1) gather qualitative data to better understand the patient and HCP experience of GI AEs in oral MS DMT treatment in Canada and (2) determine to what extent two patient-reported outcome (PRO) instruments used in recent oral DMT trials capture what is important to patients regarding GI AEs in oral MS DMT treatment (content validity) and to provide qualitative data to help interpret PRO scores.
Methods
This was a qualitative, non-interventional, descriptive, cross-sectional study comprising HCP and patient interviews conducted in English and French, using a 1:1 semi-structured interview approach.
Results
Patients reported 16 unique GI AE concepts related to oral DMTs. The most commonly reported symptoms were diarrhea, indigestion, and nausea. While patients acknowledged the negative impact associated with GI-related AEs, most characterized the treatment experience as positive, focusing on preference for oral administration, perceived efficacy of DMTs in terms of lack of MS relapses, slowed progression of their disease, and improvement in MS symptoms. Results supported the content validity (relevance, comprehension, and comprehensiveness) of the two PROs assessed. HCP feedback reinforced patient perspectives on both GI concepts and the two PRO instruments.
Conclusion
Outcomes of these research activities include experiential data on the symptom and impact experience of oral DMTs in MS from both patients and HCPs that contribute to the process of determining therapeutic value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Current guidelines indicate that patients with clinically definite relapsing-remitting multiple sclerosis (RRMS) should begin treatment with disease-modifying therapies (DMTs) as soon as possible. |
However, gastrointestinal (GI) side effects of oral DMT treatment for MS are an important consideration for patients and can impact adherence to treatment plans. |
This qualitative research with healthcare professionals and patients provides a more complete picture of the patient experience of RRMS and GI-related adverse events that illustrates an unmet need for an oral DMT treatment that mitigates the initial obstacle and ongoing impact of persistent GI-related adverse events on patients’ lives. |
The GI-related symptoms and impact concepts assessed by the Individual Gastrointestinal Symptom and Impact Scale (IGISIS) and Global Gastrointestinal Symptom and Impact Scale GGISIS patient-reported outcomes (PRO) questionnaires are meaningful and relevant to patients. |
Introduction
Background
Multiple sclerosis (MS) is a heterogeneous, chronic, inflammatory, neurodegenerative disease that is influenced by both environmental and genetic factors [1, 2]. The most common form of MS is relapsing-remitting MS (RRMS). Current guidelines indicate that patients with clinically definite RRMS should begin treatment with disease-modifying therapies (DMTs) as soon as possible [3,4,5]. The primary treatment goals of RRMS treatment in general, and treatment with DMTs specifically, are preventing MS relapses, reducing the accumulation of neurologic impairment and disability accumulation over time, and reducing brain inflammation and injury [2, 4, 6]. Accumulating data suggest that DMTs reduce disability progression in patients with RRMS [7,8,9].
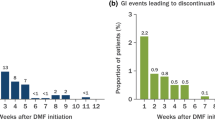
When considering which DMT to choose, clinicians and patients need to consider individual patient prognostic factors, values, and preferences as well as drug-related factors (adverse effect profile, availability, and burden of administration). Oral DMTs such as dimethyl fumarate (DMF) are an option for patients who value a self-administered oral medication over medications requiring injections and infusions [10, 11]. However, quantitative study data indicate that gastrointestinal (GI) side effects such as nausea, diarrhea, and abdominal pain may accompany oral DMT treatment, particularly during treatment initiation and dose titration [12]. These effects can impact patients’ daily lives and activities and affect tolerability and adherence to treatment [13]. The recent EVOLVE-MS-2 study investigated whether diroximel fumarate (DRF), a novel oral fumarate, had an improved (GI) tolerability profile compared with DMF [13, 15]. DRF demonstrated a significantly improved GI tolerability profile compared with DMF, with significantly fewer days where patients reported a score of ≥ 2 on the patient-reported Individual Gastrointestinal Symptom and Impact Scale (IGISIS), meeting the study’s primary endpoint. Furthermore, patient-reported Global Gastrointestinal Symptom and Impact Scale (GGISIS) responses indicated that DRF patients were less likely than DMF patients to miss work because of GI symptoms and took fewer concomitant symptomatic medications. Patients taking DRF had fewer GI adverse events (AEs) and were less likely to discontinue treatment due to GI AEs.
While these quantitative data have demonstrated differences in GI tolerability across these DMTs and the psychometric properties of IGISIS and GGISIS have been examined [14], we recognized that additional qualitative data may be useful to complement our understanding of the patient experience of GI AEs and treatment tolerability in this context and to explore what different scores on the patient-reported outcome (PRO) questionnaires used in EVOLVE-MS-2 mean to patients.
Collecting qualitative data are important; patient perspectives often provide insights and concepts that would otherwise be overlooked in the clinical context. Additionally, regulatory agencies and payers are increasingly recognizing the importance of patient-centered drug development and treatment assessment [16,17,18,19,20]. For example, in Canada, the 2018 guidance from the Institut national d’excellence en santé et en services sociaux (INESSS) highlights the need for qualitative research to complement the scientific methodology of clinical trials that are “also aimed at better understanding the patients’ experience of the disease, their expectations of…innovative therapy, and their fears” [20] when considering therapeutic value. Furthermore, collecting and examining qualitative data regarding patients’ needs, values, and preferences in this treatment context can inform efforts in clinical practice to ensure that these needs are being met through the different treatment options, supports, and services that can be provided to patients.
Therefore, we conducted a qualitative, non-interventional, cross-sectional study with seven HCPs and 12 patients with RRMS who were taking oral DMTs currently available in Canada. This research was undertaken to address two objectives: (1) gather qualitative data to better understand the patient and HCP experience of GI AEs in oral MS DMT treatment in Canada and (2) determine to what extent the IGISIS and GGISIS questionnaires used in recent oral DMT trials capture what is important to patients regarding GI AEs in oral MS DMT treatment (content validity) and to provide qualitative data to help interpret PRO scores.
Methods
Study Design
Patient and HCP interviews were conducted to gather information about the GI-related AE experience in RRMS oral DMT treatment in Canada, specifically for DMF (Tecfidera®), fingolimod (Gilenya®), and teriflunomide (Aubagio®). DMF was used as a proxy for DRF, as during the time of the study DRF was not yet available in Canada. Interviews also explored patient understanding of the IGISIS and GGISIS questionnaires used to assess GI AEs in the EVOLVE-MS-2 study. The IGISIS measures the incidence, intensity, onset, duration, and functional impact of nausea, vomiting, upper and lower abdominal pain, and diarrhea. The GGISIS measures the overall intensity of the GI symptoms mentioned above experienced during the previous 24 h, how bothersome they were to patients, and their impact on the patient’s daily activities and work [15]. (See Supplemental Material.)
Study Sample
The research team sought insight from Canadian HCPs with experience in patient-reported GI-related AEs in oral DMTs for MS. Eighteen Canadian HCPs with extensive experience in treating MS patients were invited to participate in the study.
Patient participants were selected via active sampling to reflect the diversity of MS patients taking oral DMTs in Canada, including both English and French speakers. A global agency was engaged to recruit patients through their patient database, clinician referrals, and social media advertising. Patients were considered for the study if they were living in Canada, between the ages of 19 and 65 years, had a diagnosis of RRMS, were currently taking or had previously taken an oral DMT, and had experienced GI symptoms while on the oral DMT at any time point within the past 12 weeks. Patients were excluded if they had inflammatory bowel disease, bowel cancer, or irritable bowel syndrome.
Ethics
Study documents, including the protocol, demographic and health information form, interview guide, screener, and informed consent forms, received ethical approval from Advarra IRB (IRB no. Pro00044838) prior to any contact with participants. Informed consent was obtained before proceeding with the interviews, and study participants also consented to have their responses included in this research and any resulting publication. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Interview Conduct
English- and French-speaking personnel conducted the interviews using semi-structured interview guides. English interviews were conducted by independent outcomes researchers from Modus Outcomes; French interviews were conducted by an independent qualitative interviewer from the recruiting agency. To ensure consistent data quality, all interviewers attended specific training to review the objectives of the interviews and to address any questions regarding the interview guide and general flow of the questions and probes.
HCP telephone interviews were conducted in English and lasted approximately 45–60 min per interview. Patient online interviews lasted 60–90 min and were conducted in English or French, with questionnaire items in English or French presented to patients via Research Electronic Data Capture (REDCap) [21, 22]. Interviews included two components: concept elicitation to explore patients’ GI-related symptoms, impacts, and decisions to continue or switch treatment, and cognitive interviewing to explore HCP and patient perspectives on IGISIS and GGISIS. All interviews were audio-recorded, transcribed verbatim, and anonymized. Transcripts were used for qualitative analysis.
Data Analysis
HCP Interview Data
HCP interviews were summarized, and the summaries were shared with participating HCPs to confirm the main concepts and conclusions. HCP insights informed the understanding of GI-related oral DMT issues, the assessment of the content validity of the IGISIS and GGISIS instruments, and the approach to patient interviews.
Patient Interview Data
Transcripts were coded in ATLAS.ti software using ATLAS.ti version 9.0 (ATLAS.ti GmbH, Berlin). Coders used a data-driven approach and open, inductive, thematic coding [23,24,25,26]. Independent parallel coding was used to initiate the coding and ensure consistency among coders, using the initial interview transcript. Concept elicitation codes were organized to establish a clinically meaningful catalog of GI-related AEs in MS treatment from the patient perspective. Saturation, the point at which no new relevant information emerges from additional qualitative data [27,28,29], was assessed, and results were compared to discussions with HCPs to determine whether information elicited in patient interviews aligned with the HCP perspective.
Patient feedback on the IGISIS and GGISIS format, instructions, and item content was compiled, as were descriptions of the process of providing a response and understanding the meaningfulness of patient scores.
Results
Study Sample
HCPs
Interviews with HCPs were conducted between June 26 and August 18, 2020. Of the 18 HCPs invited to participate, 7 HCPs were recruited, including 5 neurologists, 1 nurse, and 1 nurse practitioner. The providers reflected geographic diversity across Canada, with two HCPs practicing in Quebec, two in Ontario, two in British Columbia, and one in Alberta. Experience in treating patients with MS ranged from 5 to 30 years (Table 1).
Patients
Interviews with 12 patients with RRMS were conducted between August 17 and December 1, 2020. Interviews were conducted in French (n = 4) and English (n = 8). The mean age of patients was 42 years, with nine identifying as female. The entire patient sample identified as Caucasian/white. All patients were recruited from three provinces: Quebec, Ontario, and British Columbia (Table 2).
Most patients reported their MS severity as “mild disability” per the Patient Determined Disease Steps (PDDS) instrument [30]. The time since diagnosis ranged from 33 to 264 months with the average time being 129 months (11 years). Most patients were taking DMF (n = 8), with two patients on teriflunomide and fingolimod, respectively. All patients had been taking their oral DMT for a year or longer; none had discontinued their oral DMT (Table 2).
HCP and Patient Perspectives on Oral DMT Treatment for RRMS
HCP Perspectives
Health care providers perceived oral DMTs as a “major advance” in the treatment of MS, reporting that these therapies encourage patients to consider treatment earlier than they may have otherwise. Providers also observed that oral DMTs increased adherence, noting that patients valued being able to travel without needing to make special accommodations for their medication regimen. According to the HCPs, both factors contributed to patients’ willingness to try these therapies and to adhere to the DMTs once initiated. HCPs reported that both they and their patients found oral DMTs to be efficacious, and this was central to their appreciation for the treatment and their willingness to tolerate side effects to continue taking the medication.
HCPs explained that each of the oral DMTs available in Canada has positive and negative attributes to consider when determining the best treatment option for an individual patient. Five HCPs described taking patients’ GI-related comorbidities into account when prescribing DMF, noting that they would not choose this treatment for patients who had a predisposition to gastrointestinal problems. Fingolimod and teriflunomide were generally viewed as better tolerated overall, although some HCPs stated that treatment failure (more relapses) was more likely with teriflunomide than other available DMTs. HCPs reported that both they and their patients appreciated that DMF could be discontinued quickly without negative consequences such as rebound effects or the need for washout periods.
When asked what they and their patients specifically disliked about DMF, providers reported that the main patient complaints centered around GI-related symptoms, including diarrhea, nausea, vomiting, abdominal pain, and cramping. HCPs stated that diarrhea was consistently reported as the most common symptom from patients, and potentially the most noticeable and bothersome due to its impact on day-to-day functioning.
There was no HCP consensus on whether patients experienced these symptoms singly (e.g., only diarrhea) or in clusters of symptoms (e.g., nausea and abdominal cramping). Nor was there a consensus on which GI-related symptom or group of symptoms was most likely to cause patients to discontinue oral DMTs; even so, diarrhea, nausea, vomiting, and abdominal pain were all cited as likely causes. Providers stated that patient education, support, and expectation management are key to helping patients adhere to an oral DMT regimen when facing symptomatic AEs such as GI issues during dose titration.
Patient Perspectives
GI Symptomatic Adverse Events Associated with DMT Treatment
Participants reported 16 unique GI symptom-related codes, with most symptom concepts (12/16 or 75%) reported by more than one patient. The most commonly reported symptoms were diarrhea, indigestion, and nausea; all three of these symptoms were reported by nine patients. Gas was not spontaneously reported by any patients; however, once probed, nine patients endorsed this symptom. Table 3 summarizes the emergence of symptom concepts across the 12 patient interviews conducted, illustrating the achievement of conceptual saturation. All GI-related concepts reported in HCP interviews were also reported in this patient sample; no additional GI-related concepts emerged from patient interviews.
The GI-related symptoms reported were similar across all three oral DMTs. There were no clear differences in types of symptoms reported between patients in different provinces, though this is descriptive data only and these findings should be interpreted cautiously given the small sample size (See Supplemental Materials).
Positive Considerations of Oral DMTs
While patients acknowledged the negative impact of GI-related adverse events, most characterized the overall oral DMT experience as positive, focusing on the perceived efficacy of the treatment in terms of lack of MS relapse, slowed progression of disease, and improvement in MS symptoms. Participants also described aspects of oral administration that they liked, including the simplicity, a feeling of more control and a less restrictive medication schedule, and appreciation for the patient support programs run by sponsors. The most prevalent response was an expressed preference for oral medications over the alternative, injections.
Drawbacks of Oral DMTs
Drawbacks patients noted around oral DMTs reflected concerns around taking medication for MS in general. One participant expressed negative feelings about needing to be on a drug for the rest of her life, and another regarded their twice-daily DMT regimen as a constant reminder that they had MS.
Patients also described the impact that their GI-related symptoms had on their day-to-day life, including impact on functioning, activities of daily living (ADLs), and instrumental activities of daily living (IADLs), such as work, exercise and leisure, and emotions. Coping strategies for dealing with GI-related symptoms included adapting schedules based on expected symptom onset, dietary changes, taking over-the-counter medications to address specific symptoms, resting, and adapting clothing choices.
Six patients discussed the impact of GI-related symptoms on their work life. Experiences were variable, with several explaining that impact was relatively short lived during dose titration, some experiencing a low-level, ongoing impact on their work-life, and others stating that they stopped working in part due to GI symptoms related to their oral DMT regimen. Diarrhea and abdominal pain/discomfort were reported to have the most impact on patients’ work experience; nausea was also reported to interfere with work.
Several patients highlighted the impact that GI-related symptoms had on their exercise and leisure activities. For these patients, impact related specifically to the need to be near a toilet when experiencing nausea or diarrhea, which curtailed outdoor activities, and lack of motivation and desire to exercise or socialize when experiencing GI symptoms.
GI-related symptoms also had emotional impacts on this group of patients. Study participants described feelings of depression, irritability, isolation, lethargy, and worry associated with the side effects of oral DMTs.
Two different aspects of symptom duration were described by patients. Some patients described duration in terms of the length of a single episode (e.g., 3- to 4-h instances of diarrhea or stomach pain), while others referenced the persistence of the symptom over time (e.g., constipation “coming and going” over several weeks). For the patients in this sample who had been taking oral DMTs between 1 to 10 years, the experience of GI-related symptoms was not confined to the dose titration stage but instead was ongoing and persistent. Some patients described intermittent symptoms that had only an occasional impact on daily life activities. For others, GI-related symptoms and their impact on work, family life, social and leisure activities, and emotions were described as more pervasive and limiting.
Tolerability
Tolerability discussions with HCPs and patients highlighted the key role that ongoing patient education support can play in helping patients stay on these treatments. HCPs advocated for proactive patient education so that patients were prepared for potential GI-related AEs, were aware of how the treatment should be taken (i.e., with food), and knew what their options were for managing AEs when they happen. Patients and HCPs cited support during the dose titration process as a key success factor, citing regular calls with clinic staff and using support phone lines as methods helpful in navigating dose titration.
The most prevalent response was an expressed preference for oral medications over the alternative, injections. Patients described weighing these positive aspects of treatment against the impacts they experienced, particularly during dose titration, their fear that these symptoms would not resolve, the ongoing impacts of living with long-term GI-related symptoms, and their fear of long-term effects. The tolerability concepts and concerns generated in patient interviews were reflected in the interviews with HCPs, who outlined the same general picture of oral DMT tolerability considerations. Key considerations in patient experiences with oral DMTs are summarized in Table 4 below.
IGISIS and GGISIS Content Validity
HCP Feedback on PROs
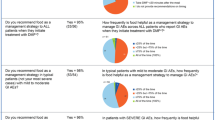
Overall, HCP feedback on the IGISIS and GGISIS questionnaires used to assess patient-reported GI AEs in DRF trials broadly supports the content validity of both instruments. HCPs thought IGISIS was a valuable instrument and easy to complete. They indicated that the five symptoms assessed by the IGISIS instrument—nausea, vomiting, upper and lower abdominal pain, and diarrhea—are among the most clinically relevant and impactful GI-related symptomatic adverse events experienced by patients. No consensus arose when HCPs were asked which symptom was the most important from the patient perspective, suggesting that all symptoms assessed by the IGIGIS are important to assess. This finding aligns with the results of the symptom concept elicitation described above, where nausea, vomiting, diarrhea, and abdominal pain/cramping were reported as the most prevalent and impactful symptoms.
All HCPs stated that the GGISIS items assessing overall symptom intensity, bothersomeness, and interference with everyday activities and work are relevant. Across the questions included in GGSIS, there were few reported issues relating to interpretation or clarity. One HCP noted that the word “bothersome” is subjective. Regarding the work questions, one HCP suggested this could be confusing for seasonal workers, and another noted that the question implies that if people are not paid, they are not employed, i.e., people doing voluntary work are excluded.
Feedback regarding both instruments as administered together was positive overall, with six HCPs specifically reporting that they complement one another and cover aspects that are important to patients. Three HCPs noted some overlap between the two scales or stated that they believe that patients would find them similar. They also noted that completing both instruments would not be burdensome to patients if the trial period is not too long.
Patient Feedback on PROs
Content validity is generally defined as the extent to which a questionnaire measures what is intended [31]. Patient debriefing of these PROs broadly supports the content validity of both the IGISIS and GGISIS questionnaires. Patients understood and endorsed the five IGISIS symptoms as relevant and comprehensive and stated that the GGISIS assessment of overall intensity, bothersomeness, daily activity interference, and work productivity is important. Patients encountered little difficulty in responding to intensity and interference questions using the response options provided. These findings align with HCP perspectives on the relevance, comprehensiveness, and acceptability of the instruments.
For each item in the scales, participants were asked what change of score on both the intensity and interference scales would constitute a meaningful change in their status. This was framed by asking the participant to explain what change in their current score (if higher than “did not have” or “not at all”) would be meaningful. Participants were also specifically asked to explore the movement from 2 to 1 or 2 to 0 on the 11-point intensity scale for both IGISIS and GGISIS. For some patients, a 1-point change was considered intrinsically meaningful. For others, a 1-point change was considered less meaningful, particularly on the lower end of the scale where some participants perceived the difference between a score of 1 or a score of 2 to be negligible. Change from 2 to 0 was universally considered meaningful by patients who were asked this question across all items and symptoms.
Discussion
While there is a growing body of qualitative and quantitative research conducted in RRMS to better understand the factors that contribute to decisions to prescribe, take, adhere to, or switch from existing and emerging DMTs in MS both internationally [34,35,36] and in Canada [37], this is the first qualitative study to our knowledge to focus specifically on GI-related AEs in oral DMTs from both the patient and HCP perspective.
Our study provides experiential data on the GI-related DMT AE symptom and impact experience from both patients and HCPs, which patients described as ranging from negligible and short term to ongoing and severe. The study builds further insight into previous qualitative research [32], adding English and French interviews with Canadian patients from three provinces with varying levels of RRMS severity. HCPs from four provinces with varying types of patient engagement (doctors, nurse practitioners) provided broad-ranging perceptions of the treatment landscape and their experiences with these oral DMTs. Both patients and HCPs characterized diarrhea, nausea, vomiting, and abdominal pain as the most important and impactful GI-related symptoms. Interestingly, while both HCPs and patients reported abdominal pain as an important symptom, patients described nausea, vomiting, and particularly diarrhea as the symptoms that cause the most impact, as they required more adaptations of their work, family, social lives, and leisure activities to accommodate the need to be near a bathroom. Importantly, though HCPs often characterized GI-related AE symptoms as most intense at treatment titration, patients described symptoms that persist longer and at severity levels that interfere with daily activities even after dose titration and substantial lifestyle adaptation.
Previous qualitative studies with people living with RRMS have suggested that deciding to take and adhere to an oral DMT regimen is a fluid process, revisited over time in the course of the disease. It depends not only on the perception of both short- and long-term treatment efficacy but also on the balance of treatment burden (e.g., side effects, route of administration) and quality of life outcomes [34, 36, 38, 39]. As in other published research [34, 40], patients in our study expressed willingness to start and stay with an oral DMT regimen after considering the burdens and benefits of treatment. Aspects of oral DMT administration flagged as positive included simplicity, feeling more in control, a less restrictive medication schedule, and avoidance of injections.
Our findings from the interviews with patients and HCPs also highlighted the importance of educating patients about their DMT options and supporting them through DMT initiation and dose titration in terms of treatment adherence. A better understanding of the characteristics and wide-ranging daily life impact of GI-related DMT AEs will enable HCPs to better discuss these potential concerns and use this information, together with other patient decision aids [41], to weigh the attributes of DMT treatment in the context of their individual circumstances, and to prepare for and navigate any GI-related DMT AE symptoms and their associated impacts.
As described above, in the EVOLVE-MS-2 trial, the IGISIS and GGISIS were used to collect patient experience data to quantify differences in GI tolerability between DMF and a novel oral fumarate DRF [13, 15]. The qualitative research conducted in our study has provided further evidence to support that IGISIS and GGISIS: (1) capture the most salient GI-related AE symptoms and impacts and are broadly relevant and acceptable to patients and (2) a change in score from 2 to 0, which is considered statistically significant, is also considered meaningful by patients who were asked this question across all items and symptoms. This is important as collecting clinically meaningful data ensures that appropriate treatment options and services are available and accessible.
Some limitations of this study should be acknowledged. The patient sample was recruited via convenience sampling; all those who met the eligibility criteria were invited to participate in the study. Of this patient sample, two-thirds were taking DMF, and the patient sample lacked racial and ethnic diversity. These factors introduce the possibility of selection bias and so may impact the generalizability of results. Furthermore, the full planned patient sample of n = 15 for the patient interview component of our qualitative study was not recruited. However, our analysis of the 12 completed interviews indicated that we reached conceptual saturation analysis on the patient experience of GI-related adverse events in oral DMT treatment for RRMS. Furthermore, concepts from patient interviews were compared to discussions with HCPs, who have collectively interacted with hundreds of patients taking the three types of oral DMTs available in Canada; this comparison indicated that patient interviews did not elicit any new GI-related symptoms not identified by HCPs. While recruitment of additional patients could have contributed to a more comprehensive understanding of the symptom, impact, and tolerability aspects of the RRMS oral DMT treatment experience in Canada, this study successfully gathered patient experience data that richly illustrate the types of considerations, challenges, and adaptations faced by patients taking these treatments. Finally, although HCP and patient participants were asked to consider their experiences outside the global pandemic context, the limitations and restrictions imposed by the COVID-19 context may have affected the types of activities considered and the broader disease and treatment context of patients and HCPs.
Conclusion
Our study sought to encompass a pan-Canadian perspective, with interviews with participants from four major provinces—British Columbia, Alberta, Ontario, and Quebec—conducted in English and French per participant preference. This study illustrates the unmet need in Canada for treatments with an improved tolerability profile that mitigate the initial obstacle and ongoing impact of persistent GI-related adverse events on patients’ lives. New medications, like DRF, can provide novel treatment for RRMS while reducing the gastrointestinal distress that both patients and HCPs reported as a significant burden and factor in medication nonadherence. This is particularly important given that a recent systematic review and meta-analysis indicated that one in five MS patients fail to adhere to daily oral DMTs and about one in four stops using a prescribed daily oral treatment within 1 year [33]. In alignment with emerging guidance [16,17,18,19,20], this qualitative research helps define and understand the patient disease experience, specifically around GI-related symptomatic AEs and tolerability, from the patient perspective, an understanding that can inform efforts in clinical practice to ensure that treatment needs are being met. Furthermore, it supports the content validity of the tools used to measure GI-related AEs in clinical trials and informs how the scores generated by these tools can be interpreted.
References
Conradsson D, et al. Changes in disability in people with multiple sclerosis: a 10-year prospective study. J Neurol. 2018;265(1):119–26.
Filippi M, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4(1):43.
Rae-Grant A, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777–88.
Montalban X, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler J. 2018;24(2):96–120.
Freedman MS, et al. Treatment optimization in multiple sclerosis. Can J Neurol Sci. 2004;31(2):157–68.
Metz LM. Clinically isolated syndrome and early relapsing multiple sclerosis. Continuum. 2019;25(3):670–88.
Amato MP, et al. Disease-modifying drugs can reduce disability progression in relapsing multiple sclerosis. Brain. 2020;143(10):3013–24.
Claflin SB, Broadley S, Taylor BV. The effect of disease modifying therapies on disability progression in multiple sclerosis: a systematic overview of meta-analyses. Front Neurol. 2019;9:1150.
Tsivgoulis G, et al. The effect of disease modifying therapies on disease progression in patients with relapsing-remitting multiple sclerosis: a systematic review and meta-analysis. PLoS ONE. 2015;10(12): e0144538.
Cohan SL, et al. Clinical outcomes in patients with relapsing-remitting multiple sclerosis who switch from natalizumab to delayed-release dimethyl fumarate: A multicenter retrospective observational study (STRATEGY). Mult Scler Relat Disord. 2018;22:27–34.
Kresa-Reahl K, et al. Effectiveness of delayed-release dimethyl fumarate on clinical and patient-reported outcomes in patients with relapsing multiple sclerosis switching from glatiramer acetate: RESPOND, a Prospective Observational Study. Clin Ther. 2018;40(12):2077–87.
Zadeh AR, et al. Mechanism and adverse effects of multiple sclerosis drugs: a review article. Part 2. Int J Physiol Pathophysiol Pharmacol. 2019;11(4):105.
Wundes A, et al. Improved gastrointestinal profile with diroximel fumarate is associated with a positive impact on quality of life compared with dimethyl fumarate: results from the randomized, double-blind, phase III EVOLVE-MS-2 study. Ther Adv Neurol Disord. 2021;14:1756286421993999.
Wundes, A., et al. Improved GI Tolerability With Diroximel Fumarate Is Associated With Clinically Meaningful Benefits on Quality of Life Compared With Dimethyl Fumarate in EVOLVE-MS-2. In: 8th Joint ACTRIMS-ECTRIMS Meeting. 2020. Multiple Scler J. 2020;26(3_suppl):118–659.
Naismith RT, et al. Diroximel fumarate demonstrates an improved gastrointestinal tolerability profile compared with dimethyl fumarate in patients with relapsing-remitting multiple sclerosis: results from the randomized, double-blind, phase III EVOLVE-MS-2 study. CNS Drugs. 2020;34(2):185–96.
US Food and Drug Administration. Patient-focused drug development: collecting comprehensive and representative input. 2018; Available from: https://www.fda.gov/files/drugs/published/Patient-Focused-Drug-Development---Collecting-Comprehensive-and-Representative-Input.pdf. Accessed 22 Jan 2022.
US Food and Drug Administration. Patient-focused drug development: methods to identify what is important to patients. 2019. https://www.fda.gov/media/131230/download. Accessed 22 Jan 2022.
US Food and Drug Administration. Patient focused drug development: select, develop, or modify fit-for-purpose clinical outcomes assessments. 2018. https://www.fda.gov/media/116277/download. Accessed 22 Jan 2022.
US Food and Drug Administration. Patient focused drug development: incorporating clinical outcome assessments into endpoints for regulatory decision-making. 2019. https://www.fda.gov/media/132505/download. Accessed 22 Jan 2022.
Institut national d'exellence en sante et services sociaux, Evaluation of drugs for listing purposes: a change of approach, INESSS, Editor. 2018: Quebec, Canada.
Harris PA, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inf. 2019;95: 103208.
Harris PA, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–81.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–90.
Bowling A. Research methods in health: investigating health and health services. 3rd ed. Maidenhead: Open University Press; 2009.
Bryman A, Burgess B. Analyzing qualitative data. New York: Routledge; 2002.
Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval. 2006;27(2):237–46.
Morse JM. The significance of saturation. Thousand Oaks: Sage publications Sage CA; 1995.
Strauss ACT. Basics of qualitative research: Grunded theory procedures and techniques. London: Sage; 1990.
Meyrick J. What is good qualitative research? A first step towards a comprehensive approach to judging rigour/quality. J Health Psychol. 2006;11(5):799–808.
Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology. 1995;45(2):251–5.
US Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. 2009. https://www.fda.gov/media/77832/download. Accessed 22 Jan 2022.
ERT and Endpoint Outcomes, GI tolerability instrument development in fumarate-based treatments for multiple sclerosis. 2018.
Nicholas JA, et al. Real-world adherence to, and persistence with, once-and twice-daily oral disease-modifying drugs in patients with multiple sclerosis: a systematic review and meta-analysis. BMC Neurol. 2020;20(1):1–15.
Sippel A, et al. Patients experiences with multiple sclerosis disease-modifying therapies in daily life - a qualitative interview study. BMC Health Serv Res. 2021;21(1):1141.
Manzano A, et al. Patient perspective on decisions to switch disease-modifying treatments in relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. 2020;46: 102507.
Lee Mortensen G, Rasmussen PV. The impact of quality of life on treatment preferences in multiple sclerosis patients. Patient Prefer Adherence. 2011;11:1789–96.
Lynd LD, et al. Quantitative analysis of multiple sclerosis patients’ preferences for drug treatment: a best-worst scaling study. Ther Adv Neurol Disord. 2016;9(4):287–96.
Van Reenen E, et al. Fear, fight, familiarize: the experiences of people living with relapsing-remitting multiple sclerosis and taking oral medication. Int J Qual Stud Health Well-Being. 2019;14(1):1648946.
Kremer IEH, et al. Comparison of preferences of healthcare professionals and MS patients for attributes of disease-modifying drugs: a best-worst scaling. Health Expect. 2018;21(1):171–80.
Jonker MF, et al. Summarizing patient preferences for the competitive landscape of multiple sclerosis treatment options. Med Decis Making. 2020;40(2):198–211.
Bansback N, et al. Development and usability testing of a patient decision aid for newly diagnosed relapsing multiple sclerosis patients. BMC Neurol. 2019;19(1):173.
Acknowledgements
We wish to thank the 12 patients and the 7 health care professionals who shared their insights on RRMS and its treatment with the research team.
Funding
This study and Advances in Therapy’s Rapid Service and Open Access fees were funded by Biogen.
Medical Writing Assistance
Medical writing support for this manuscript was provided by Lori Bacarella of Modus Outcomes; this writing support was funded by Biogen.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; were responsible for drafting the work or revising it critically for important intellectual content; provided final approval of the version to be published.
Disclosures
Sara Strzok, Emma Elliott, and Stefan Cano are employees of Modus Outcomes, which received payment from Biogen to conduct this research. Farah Jivraj, Sha Kang, and Scott Reedie are employees at and hold stock/stock options in Biogen. Marvin Rock and Shivani Kapadia were employees at and held stock/stock options in Biogen at the time this research was conducted but are no longer affiliated with Biogen. Currently, Marvin Rock is with Gilead, and Shivani Kapadia is with Otsuka.
Compliance with Ethics Guidelines
Study documents, including the protocol, demographic and health information form, interview guide, screener, and informed consent forms received ethical approval from Advarra IRB (IRB # Pro00044838) prior to any contact with participants. Informed consent was obtained before proceeding with the interviews, and study participants also consented to have their responses included in the research and any resulting publication. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
Associated data is included as electronic supplementary material.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jivraj, F., Kang, S., Reedie, S. et al. The Patient and Clinician Assessment of Gastrointestinal (GI) Related Adverse Events Associated with Oral Disease-Modifying Therapies in Multiple Sclerosis: A Qualitative Study. Adv Ther 39, 5072–5086 (2022). https://doi.org/10.1007/s12325-022-02250-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02250-x