Abstract
The surgical management of glaucoma has been revolutionized by the introduction of minimally invasive glaucoma surgery (MIGS). The various MIGS options aim to meaningfully lower intraocular pressure with a better safety profile than traditional glaucoma surgery. The key clinical attributes and the emerging potential of an ab externo MicroShunt (PreserFlo™) are reviewed in the context of published evidence and clinical experience. This novel MicroShunt consists of an 8.5-mm-long tube that is implanted in the eye via an ab externo approach enabling aqueous humor drainage into the sub-Tenon’s space through the formation of a bleb, similar in appearance to that created by trabeculectomy. The efficacy and safety of this procedure, the concomitant use of antimetabolites, the impact of tube positioning, and its future value in clinical practice are critically reviewed. Recent evidence has demonstrated the MicroShunt to be less effective than traditional filtration surgery, but with a significant improvement in safety. Cumulative data suggest that the new implant provides tangible clinical benefits to selected patients with glaucoma in need of further intraocular pressure (IOP) lowering. Future research should delineate the precise role of this and other MIGS options in the rapidly evolving glaucoma treatment algorithm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
PreserFlo MicroShunt is a novel option developed with the aim of providing meaningful intraocular pressure reduction with improved safety than gold-standard glaucoma filtration surgery. |
A significant body of evidence has confirmed that MicroShunt offers good efficacy with an encouraging safety profile in the majority of patients operated on for open-angle glaucoma. |
One-year results of a prospective, randomized, multicenter, noninferiority study have demonstrated that the probability of success is lower with MicroShunt compared with trabeculectomy. The trabeculectomy group exhibited lower mean IOP on fewer medications. |
Despite promising results to date, there are several issues concerning the technique, efficacy, and safety of MicroShunt that merit further investigation. The efficacy and safety of this device in other glaucoma forms (e.g., exfoliative glaucoma) and its precise role and timing in glaucoma stepwise therapy require further elucidation. |
Introduction
Over the last decade, surgical options for glaucoma management have been augmented with the introduction of minimally invasive glaucoma surgery (MIGS) and minimally penetrating glaucoma surgery (MPEGS) [1]. The aim of the latter is to effectively lower intraocular pressure (IOP) with greater safety than that offered by traditional glaucoma filtering surgery [2, 3].
MIGS options can enhance aqueous humor outflow through various pathways: increasing trabecular outflow by bypassing the trabecular meshwork, increasing uveoscleral outflow via suprachoroidal routes, or by reducing aqueous synthesis by the ciliary body. Surgical options creating a sub-Tenon’s drainage route for aqueous humor cannot be considered MIGS; for this approach the alternative term MPEGS or bleb-forming MicroShunts is preferred (Table 1) [4, 5]. Commercially available MIGS targeting the trabecular meshwork or Schlemm’s canal have been shown to modestly reduce IOP, thus providing a viable option in patients with mild-to-moderate glaucoma needing further IOP lowering, or desiring a reduction in medical therapy burden. However, for those patients with more advanced disease in need of substantial IOP lowering to the low teens, bleb-forming devices with sub-Tenon’s filtration appear a better choice.
The PreserFlo™ MicroShunt (Santen, Osaka, Japan), formerly known as the InnFocus MicroShunt, is a glaucoma device that facilitates aqueous humor drainage to a sub-Tenon’s bleb. It has been approved in Europe since 2012 and is awaiting Food and Drug Administration (FDA) approval in the USA. The present review deals with the historical development, design, surgical technique, results and complications reported by published studies with this novel drainage device. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Material and Historical Development
Poly(styrene-block-isobutylene-block-styrene) (SIBS) is a biostable thermoplastic elastomer with physical properties that overlap silicon rubber and polyurethane [6]. This material was first employed in the design of medical devices in 2002, when coronary stents made with this material were first introduced. Its value in medicine relies on its biocompatibility and long-term stability in the human body, which result in negligible degradation and minimal inflammatory and fibrotic reactions [6]. Indeed, when SIBS devices were employed in the cornea and sub-Tenon’s space, they proved to be effective options causing less irritation and inflammatory reaction than other materials. Furthermore, encapsulation of SIBS implants in rabbits’ eyes was uncommon, suggesting its possible value in the development of glaucoma drainage devices [6].
The Miami InnFocus drainage implant (MIDI-Tube) was first described in 2006 by Acosta and coworkers [7] and represents the first application of SIBS in a glaucoma drainage device. This has subsequently been modified to the current design. Its concept is based on the assumption that, in a similar fashion to trabeculectomy and Express valve surgery, the trabecular meshwork, Schlemm’s canal, and episcleral veins should be bypassed in order to obtain a desirable IOP level below 14 mmHg. Therefore, this implant was designed as a bleb-forming device with ab externo implantation into the anterior chamber [8]. The MIDI-Tube design was an 11-mm-long, 0.25-mm-diameter SIBS microtube with a 1.1×1 mm fin halfway along the tube in order to prevent migration of the tube into the anterior chamber. The tube’s lumen (70 μm) was thought optimal in preventing hypotony according to the Hagen–Poiseuille equation, without the need for a valved mechanism [9, 10]. Moreover, it was conceived to create a bleb under Tenon’s capsule without the need to fashion a scleral flap, a step requiring significant surgical skill [11]. This initial design was tested successfully in experimental studies in rabbits, with no visible tube migration, tube obstruction, or significant inflammatory reaction at 6 months, while a low and diffuse bleb became visible in all cases. Encouragingly, no cases with pronounced hypotony or flat anterior chamber were observed on the first postoperative day [6, 7, 12].
Following this device, another design called the MIDI-Ray was tested. It consisted of a 350-μm-diameter SIBS tube with a 100-μm lumen along with a 7-mm-diameter SIBS plate, similar to current tube shunts, which required suture ligation also, to restrict early flow. Studies performed at the Bascom Palmer Eye Institute Optical Biophysics Center elicited encouraging results and supported further clinical testing of this device [12]. Subsequently, human pilot studies were carried out investigating both SIBS devices. Bordeaux I (24 eyes) and Bordeaux II (16 eyes) trials focused on the MIDI-tube design with and without mitomycin C (MMC) (0.2 mg/ml applied for 2–3 min). As expected, higher success rates were obtained with the use of the antimetabolite (67% vs 42%) whilst at the same time no cases of significant hypotony were observed. The Dominican Republic I study employing the MIDI-Ray design involved 12 cases without concomitant MMC use and revealed a 58% success rate, but was associated with cystic-type blebs and a high rate of acute hypotony [12].
Current PreserFlo Design
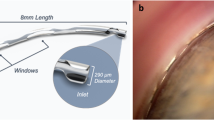
The current design known as the PreserFlo™ MicroShunt consists of an 8.5-mm-long tube with a 350-μm outer diameter and a 70-μm lumen [7]. The diameter of the internal lumen is designed to act as a flow restrictor, with a calculated flow rate of approximately 2.5 μL/min and a consequent IOP level of 5–10 mmHg in the absence of any distal resistance to outflow [7]. Located halfway along the tube there is a 1.1×1 mm fin similar to an arrow which serves multiple purposes: it seals the device in the scleral pocket, preventing leakage around the tube; it prevents the MicroShunt from migrating into the anterior chamber and it orientates the device so that the bevel in the anterior chamber faces the cornea [11] (Fig. 1).
Surgical Technique
As previously stated, the MicroShunt device is implanted via an ab externo approach using MMC and bypasses the trabecular meshwork to create a filtering bleb (Fig. 2) . The procedure is performed under topical anaesthesia. A conjunctival incision is created at the limbus and Tenon’s capsule is dissected from the sclera with scissors, to at least 8–10 mm posterior to the limbus. Hemostasis is performed with a bipolar diathermy in a humid environment. Three sponges saturated with MMC are placed under the flap for 2–3 min followed by thorough irrigation with sterile saline solution. Mitomycin C concentrations of 0.02 and 0.04 mg/ml have been employed to date. The site of entry is marked 3 mm from the surgical limbus. Using the provided 1-mm triangular knife, a scleral pocket 1 mm wide and 2 mm long is created from the previously marked point. Then, a 25-G needle is advanced through the scleral pocket to create a transscleral tunnel from the apex of the scleral pocket into the anterior chamber, through which the device is introduced in such a way that the fins of the MicroShunt are located into the scleral pocket. The proximal end of the MicroShunt should ideally extends 2–3 mm into the anterior chamber. Flow is confirmed by observing drop formation at the distal end of the tube prior to placing the device on the sclera beneath Tenon’s capsule. As a final step the conjunctiva is sutured in watertight fashion to the limbus as with other filtration procedures, employing either Vicryl 9/0, or Nylon 10/0 sutures [12].
It should be noted that gonioscopy or iridectomy is not required when performing this procedure. If no flow through the tube is visible the following corrective steps can be carried out: (1) ensure that the device’s bevel is not occluded by debris, the iris, or the cornea; (2) increase the IOP level by injecting saline in the anterior chamber; (3) use a 30-G cannula to inject saline through the lumen; (4) check for possible leaks around the tube; (5) withdraw the MicroShunt slightly and reposition the fins correctly in the scleral pocket, as they can potentially constrict the lumen; (6) remove the device and create a new transscleral tunnel. If none of these steps are successful in creating visible aqueous flow, the MicroShunt should be removed and replaced with a new one [12].
Efficacy
In most studies to date, the MicroShunt has been shown to effectively lower IOP by 40–55% and to maintain postoperative IOP levels below 14 mmHg in at least 80% of patients undergoing surgery for open-angle glaucoma (Table 2). In addition, this device significantly reduces the mean number of concomitant antiglaucoma medications used typically from two to three medications preoperatively, to at or below one medication postoperatively. Table 2 outlines the results of the larger studies published to date. In accordance with traditional filtration surgery, when patients receiving the MicroShunt are stratified according to baseline IOP, a higher baseline IOP predicts a greater IOP reduction over the following 2 years according to a pooled analysis [13].
In the most comprehensive comparison versus trabeculectomy to date, the efficacy and safety of MicroShunt surgery performed in 395 eyes were compared with that of trabeculectomy performed in 132 eyes [14]. This was a prospective, randomized, multicenter, noninferiority, masked study conducted in the USA and Europe in patients with mild-to-severe POAG insufficiently controlled on maximum tolerated medical therapy. In both surgical groups MMC was concomitantly applied at a concentration of 0.2 mg/ml. At 12 months, the probability of success was lower in the MicroShunt cohort compared with the trabeculectomy group (54% vs 73% respectively); (P < 0.01). In the MicroShunt group mean IOP was reduced from 21.1 ± 4.9 mmHg to 14.3 ± 4.3 mmHg (− 29%; P < 0.01) whereas in the trabeculectomy group baseline IOP decreased from 21.1 ± 5.0 mmHg to 11.1 ± 4.3 mmHg (− 45%; P < 0.01). Mean glaucoma medication use was significantly reduced from 3.1 ± 1.0 to 0.6 ± 1.1 in the MicroShunt group and from 3.0 ± 0.9 to 0.3 ± 0.9 in the trabeculectomy group. In both surgical cohorts vision-threatening complications were uncommon: 1.0% in the MicroShunt group versus 0.8% in the trabeculectomy group. However, the reported rate of transient hypotony was higher with trabeculectomy compared with MicroShunt surgery (50% vs 29%); (P < 0.01). Moreover, postoperative interventions, including laser suture lysis, were significantly less common (41%) in the MicroShunt group as opposed to the trabeculectomy group (67%); (P < 0.01). At 1-year follow-up, 72% of MicroShunt patients were medication-free compared to 85% of trabeculectomy patients. Endothelial cell density loss was − 5.2% with the new device compared with − 6.9% after trabeculectomy. The authors concluded that the probability of success was lower with the MicroShunt compared with standard filtration surgery, whereas meaningful reductions in mean IOP and glaucoma medications over 1 year were observed in both groups. Nevertheless, the trabeculectomy group achieved a lower mean IOP on fewer medications [14].
A comparison of the MicroShunt versus trabeculectomy as primary surgery was also reported in a smaller, non-randomized, prospective, 6-month German study with uniform inclusion and exclusion criteria, which analyzed 52 cases with open-angle glaucoma [15]. Twenty-six cases which underwent MicroShunt surgery were compared with 26 cases which underwent trabeculectomy with both options augmented with MMC. The study analyzed the mean diurnal IOP characteristics before and after surgery as determined with five sitting Goldmann tonometry IOP measurements (performed at 13:00, 16:00, 19:00, 22:00, and 07:00) and one supine IOP reading performed at midnight. At 6 months both procedures were found to be equally effective and safe in lowering mean diurnal IOP (10.8 mmHg with MicroShunt versus 10.3 mmHg with trabeculectomy). Further, no differences were observed between the procedures in mean diurnal peak IOP (13.0 vs 12.5 mmHg) and mean diurnal IOP fluctuation (5.0 vs 4.0 mmHg) [15]. None of the trial patients experienced severe adverse events, but the rate of postoperative interventions was significantly more common in the trabeculectomy group (p = 0.004). The authors suggested that the more intensive postoperative management needed after trabeculectomy might balance the higher cost and time of surgery required with MicroShunt surgery.
Wagner and coworkers [16] reported a retrospective case–control 6-month study including 105 patients with refractory POAG, or exfoliative glaucoma who underwent either trabeculectomy, XEN45 gelstent insertion, or MicroShunt implant surgery all augmented with 0.2 mg/ml MMC for 3 min [16]. Of note, the trabeculectomy group (n = 35) exhibited higher baseline IOP (21.0 mmHg) and required more medications (3.0) than the XEN45 group (n = 35), or the MicroShunt group (n = 35). The primary outcome was the proportion of complete surgical success after 6 months (defined as an IOP level between 5 and 18 mmHg, no revision surgery, no loss of light perception, and no postoperative glaucoma therapy). After 6 months follow-up complete success was statistically similar: 74% in the trabeculectomy group, 51% in the XEN45 group, and 74% in the MicroShunt group; (p = 0.08). Nevertheless, in this comparative study IOP reduction in the trabeculectomy group (12.1 ± 7.9 mmHg) was significantly greater (by 5.8 mmHg) compared to the XEN45 group (p < 0.001) and 4.8 mmHg greater than MicroShunt surgery (p = 0.01). Interestingly, there was no difference in IOP reduction between the XEN45 and the MicroShunt groups (p = 0.81). Trabeculectomy demonstrated a statistically higher rate of strict success compared with XEN45 surgery (p = 0.006), while it did not exhibit statistical difference with the MicroShunt group (p = 0.42). Postoperative medication use was comparable in the three surgical groups.
The results to date of MicroShunt efficacy are heterogeneous, with retrospective studies leading to selection bias. With the exception of the controlled study by Baker and coworkers [14], MicroShunt published studies have insufficient power and relatively small samples to document differences in efficiency and safety. Different criteria and definitions of success may account for the considerable differences in the reported success rates of MicroShunt compared with trabeculectomy and other MIGS. For example, the surgical success criterion employed by Baker and coworkers [14] was IOP lowering of at least 20% from baseline without an increase in medical therapy. This was different from the criterion employed by Wagner and coworkers [16]. Varying definitions of success in the study protocols investigating novel IOP-lowering surgical interventions, especially MIGS, are a persistent obstacle limiting the comparability of these studies [2, 3, 16]. Further, differences in MMC concentration and duration of application, as well as history of previous surgeries (cataract, glaucoma, and laser), induce biases in the reported findings. A review of pertinent studies suggests that the precise criteria of exposure of tissues to MMC play a key role in the reported success in all forms of MIGS surgery including the novel MicroShunt discussed here. Indeed, Beckers and coworkers [17] reported a trend towards lower IOP and greater reduction in medication use when a higher concentration of 0.4 mg/ml MMC was employed compared to the standard 0.2 mg/ml.
Combining MicroShunt implantation with cataract surgery in patients with glaucoma open-angle glaucoma has yielded satisfactory IOP lowering with similar success to standalone MicroShunt surgery [18]. In a 12-month, retrospective, open-label study Martinez-de-la-Casa and coworkers [18] compared the effect of the new device augmented with MMC with and without cataract surgery in 58 cases with open-angle glaucoma. No significant difference in IOP lowering or medication use was detected between the two groups. The authors suggested that combined surgery is a viable option for patients with insufficiently controlled open-angle glaucoma [18]. The concomitant use of Ologen collagen matrix has not demonstrated an additional benefit at 6 months, although no longer follow-up is available as yet [19].
Available evidence to date suggests that the need for bleb revision or needling as well as a second surgery is low with this procedure. In a series of 125 patients, Aptel and coworkers [13] reported needling in 12 patients over a follow-up period of 2 years (three required two needling procedures and two required at least three) with an overall 50% response rate and the IOP being reduced to 13.8 ± 2.0 mmHg. Of the six initial non-responders, two met the responder criteria after a second needling procedure, three had no change, or an increase in IOP and one had a secondary needling within 3 months. Complications associated with needling included one case of hypotony. Unfortunately, to date most studies do not provide sufficient details about bleb needling following MicroShunt surgery.
Safety
Adverse events following MicroShunt surgery have been described in 10–25% of patients operated on [1, 13, 14, 17]. The most common complications reported include early hypotony and hyphema, although they usually resolve spontaneously or with medical treatment (Table 3). Therefore, the presumed advantage in safety profile with MicroShunt compared with gold-standard filtration surgery appears to be confirmed in most studies given that the vast majority of patients do not demonstrate major hypotony-related complications that require surgical intervention. However, hemorrhagic choroidal detachment has been described with this device in a patient on anticoagulants, thus discontinuation of these medications prior to implantation of the device may be necessary [20].
Other reported adverse effects include tube extrusion, although few cases have been reported in the literature [21, 22]. Bunod and coworkers [23] presented two cases, which shared ocular surface inflammation prior to surgery and a deficiency in Tenon’s capsule as a common risk factor. Despite amniotic membrane graft, tube repositioning, and conjunctival suturing, early recurrences of tube exposure were noted in both cases and the device was removed.
On the other hand, it is known that corneal endothelial cell density progressively decreases over time and that intraocular surgery, such as cataract surgery, and a glaucoma implant inserted in the anterior chamber can lead to endothelial cell damage. This was the reason for the withdrawal of the Cypass microstent from the market [24]. It is worth noting that in the COMPASS-XT analysis of Cypass results at 5 years, the device’s position in the anterior chamber angle was the only factor in the analysis that correlated with endothelial cell loss, with similar rates to controls when no ring was visible, and rates which increased as rings became visible [24]. The iStent inject (Glaukos) and the Hydrus Microstent have not shown as yet statistically significant differences in endothelial cell loss at 2 years when compared with controls.
With regard to the MicroShunt, Baker and coworkers [14] reported similar rates of endothelial cell loss between the MicroShunt (− 5.2%) and trabeculectomy (− 6.9%) in a large series of surgical patients at 12 months consistent with other reports [15, 25]. In a similar fashion, Ibarz-Barberá and coworkers [26] evaluated the changes in corneal endothelial cell density during the first year following insertion of the implant and reported a 7% loss, which correlated well with the tube–endothelium distance. Despite a generally low rate of reported endothelial cell loss in published studies, individual cases demonstrating significant endothelial cell damage where the implant had to be removed have also been reported [27]. Further long-term studies investigating the rate of endothelial cell loss with the MicroShunt are therefore desirable in view of the negative experience with Cypass.
MicroShunt Tube Location
Literature to date has mostly described placing the MicroShunt superiorly as primary surgical procedure in open-angle glaucoma. However, recent reports have reported reasonably good results in patients who had undergone previous glaucoma surgeries. Therefore, the question arises as to whether an inferior location could also be a successful choice. Durr and coworkers [28] identified no differences between superior and inferior MicroShunt implantation in 83 and 35 eyes, respectively. All eyes had previous glaucoma subconjunctival filtering surgery or atypical forms of glaucoma. Although disease severity was not significantly different between groups, the percentage of patients with high-risk glaucoma was lower in the inferior placement group, along with a higher rate of previous surgery. The multivariate analysis revealed only high preoperative IOP as a risk factor for failure.
Use of Antimetabolites
Antifibrotic agents such as 5-fluorouracil (5-FU) and MMC have been extensively used in glaucoma surgery since the 1980s. It is well documented that antifibrotics significantly increase the overall surgical success of trabeculectomy, albeit with a commensurate increase in the risk of complications. Similar to trabeculectomy, the success of bleb-forming MIGS is reduced by subconjunctival fibrosis and hence the concomitant use of MMC in nearly all cases is a necessity. Initial studies with previous versions of the MicroShunt (Bordeaux I) reported lower success rates of 40–60%. Subsequent studies employing MMC (0.2 mg/ml) documented higher success rates, supporting its wider use [12]. This was further encouraged when even better results were obtained with a higher MMC concentration (0.4 mg/ml). In fact, higher MMC concentrations can achieve a greater than 80% surgical success even 2 years after the surgery [11, 29].
In the first retrospective, two-center, two-surgeon study (France and Dominican Republic) comparing MMC doses and locations Riss and coworkers [30] reported the surgical outcome of 87 cases divided into three groups: (1) 0.4 mg/ml MMC applied near the limbus (23; 26%); (2) 0.2 mg/ml MMC applied near the limbus (31; 36%); and (3) 0.4 mg/ml MMC applied deep in the conjunctival pocket (33; 38%), all for a duration of 2–3 min. Results obtained at 1 year showed a 55%, 52%, and 38% IOP reduction, respectively. Although the authors observed that applying 0.04 mg/ml MMC near the limbus was associated with greatest IOP reduction, no statistical analysis was performed. In addition, baseline characteristics were not detailed enough and the complications described did not specify the study group of each patient. Hence, it cannot be stated whether the greater IOP reduction was consistent with a corresponding increase in complications [30]. Further reports described similar IOP reductions in both the 0.2 and the 0.4 mg/mL MMC groups [31, 32]. However, García-Feijóo and coworkers [33] reported a greater reduction in IOP and medication use with the 0.4 mg/mL MMC concentration compared with the 0.2 mg/mL MMC concentration in 124 patients, although this was a pooled analysis of two cohorts of patients from two different studies. Thus, available data suggest that higher MMC dosage may increase success rate, but at the possible cost of more device-related adverse events and a greater need for postoperative surgical interventions [34]. Hence although it is clear that the use of MMC improves MicroShunt’s outcome, the ideal MMC dosing needs to be elucidated. It should also be stated that, although adjunct antifibrotic agents are widely used in bleb-forming MIGS surgery, their use in antiglaucoma surgery remains as yet off-label.
Emerging Role of MicroShunt in Clinical Practice
As previously discussed, bleb-forming MicroShunt augmented with MMC provides meaningful and consistent IOP lowering, allowing its adoption in patients with higher baseline IOP and more advanced glaucoma than with other MIGS. At the same time, overall hypotony rates appear lower than with standard trabeculectomy, allowing the device to serve as an alternative to trabeculectomy in many patients. As with other MIGS, and bearing in mind the lifelong nature of glaucoma, it is desirable to gather more long-term evidence with this device.
In addition to the management of POAG, this device may be used to treat XFG, angle-closure glaucoma, and normal-tension glaucoma. To date there is insufficient evidence for the role of MIGS in these forms of glaucoma, which are often refractory to medical therapy, and thus represent important, growing indications for all novel surgical options [8, 35]. Results in XFG were similar to those in patients with POAG, although higher rates of transient hypotony and choroidal detachment were observed in the former [35]. The MicroShunt, like other tubes, should be used with caution in cases with narrow angles, since proximity and contact with the corneal endothelium should be avoided.
Further, MicroShunt may have a role in pediatric glaucoma surgery or in other atypical forms of glaucoma, including neovascular and uveitic glaucoma. In a series of uveitic glaucoma cases, baseline IOP reduced from 27.0 ± 9.6 mmHg to 15.6 ± 5.8 mmHg at 12 months with 0.9 ± 1.1 medications. Importantly, only 4 of 24 patients presented with recurrence of anterior uveitis, 2 of which were associated with cystoid macular edema [28, 36]. A comparison of trabeculectomy with XEN, Cypass, and MicroShunt demonstrated that all these procedures appeared as effective as standard trabeculectomy for the management of patients with medically uncontrolled uveitic glaucoma, at least for the first year [37].
As highlighted above, implantation of MicroShunt can be successfully performed in combination with cataract surgery. Although most studies investigate the effect of the procedure alone, the MicroShunt can be used in both phakic and pseudophakic patients, as well as in combined surgery. However, there is some emerging evidence [38] identifying combination procedures as a risk factor for surgical failure (4.3; 1.7–10.8) increasing the need for postoperative needling (4.5; 2.1–9.6). Others have only detected differences in the need for needling [39], whereas other investigators described no differences at all [18, 40]. Moreover, the device is small enough so that multiple devices could be placed in the same eye in the event one fails or if a lower target IOP is subsequently desired [11, 12].
Comparison with Other MIGS and MPEGS
Several MIGS procedures utilize different mechanisms for IOP lowering, mainly by increasing the trabecular outflow, bypassing the trabecular meshwork, suprachoroidal drainage, or by promoting sub-Tenon’s filtration. Our current understanding of trabecular MIGS implies they offer additional safety and diminish certain problems associated with standard filtration surgery augmented with MMC. On the other hand, they provide less IOP lowering possibly due to episcleral venous pressure. In contrast, suprachoroidal MIGS have the potential to reduce IOP significantly, but have been associated with sight-threatening complications including severe and prolonged hypotony [41].
It is worth noting that the only suprachoroidal MIGS device with significant time in the market, the CyPass, had to be withdrawn because of corneal adverse events with progressive endothelial cell loss due to its anterior placement facilitating contact with the corneal endothelium [24]. This implant provided a substantial IOP reduction in the mid-teens when CyPass was employed as an alternative to trabeculectomy [42]. Towards the end of 2021, another suprachoroidal MIGS device called the MINIjet was approved for clinical use in the European Union. Preliminary results with this device suggest it lowers postoperative IOP into the mid-teens. More long-term controlled evidence is required; however, it appears that the best indication for these implants would be moderate glaucoma, or patients whose conjunctival status does not commend standard bleb-forming surgery.
Given the different mechanisms through which they lower IOP and their safety profile, the indications for MIGS and MPEGS differ significantly. In general terms, MIGS may be considered in patients with high-risk ocular hypertension and early-to-moderate glaucoma, which require a postoperative target IOP range in mid to high teens. Moreover, in many cases these surgical options can be considered in conjunction with cataract surgery.
There is one real alternative to MicroShunt within the MPEGS group: the XEN implant. Wagner and coworkers [16] reported XEN to reduce IOP from 19.2 ± 4.4 mmHg at baseline to 13.8 ± 3.8 mmHg (− 28%) at 24 months, whereas MicroShunt further lowered IOP from 20.1 ± 5.0 mmHg at baseline to 12.1 ± 3.5 mmHg (− 39%) at 24 months. Efficacy differences, however, were not statistically significant after 3 months of follow-up. Both techniques showed a qualified success rate of around 80% [16]. Similarly, in a comparative case series [43] there was no significant difference: with XEN mean IOP was lowered from 19.2 ± 4.4 mmHg at baseline to 13.8 ± 3.8 mmHg; with MicroShunt the IOP was reduced from 20.1 ± 5.0 to 12.1 ± 3.5 mmHg after 24 months of follow-up (p = 0.19) [43]. Nevertheless, more controlled studies with sufficient power are needed to confirm the comparative efficacy of these devices. Of note another non-comparative study reported a lower success rate and higher postoperative IOP with the MicroShunt despite a higher dose of MMC (0.4 mg/mL) [44]. Furthermore, it is clinically relevant that the number of postoperative interventions needed appears higher with XEN [43]. Overall, results in the literature demonstrate that both MPEGS are effective: they lead to substantial IOP lowering and a meaningful reduction in the number of topical medications used in the majority of surgical cases.
To date, reported complication rates with these two devices compare favorably with gold-standard filtration surgery. Overall, both MicroShunt and XEN devices attain a similar or better safety profile than classic filtration surgery, although there is limited controlled evidence and few studies compare available techniques [16, 43, 45, 46]. As with efficacy evidence there is limited comparative controlled evidence with sufficient power between the various devices and standard glaucoma surgery. There is also a pressing need for long-term assessment of scarring development with these novel devices. As previously discussed, longer-term safety data are needed, especially regarding the impact of these devices on the corneal endothelium.
Future Research
Cumulative evidence suggests that PreserFlo MicroShunt is a promising alternative to gold-standard filtration surgery and could therefore find a tentative place in the stepwise management of glaucoma. It should be noted, however, that as yet, published studies are limited in scope and stem only from a few groups. On the other hand, there are several studies underway that may broaden our understanding and help the wider adoption on MicroShunt into our practice. When evidence from these trials becomes available the role and value of this device will be appreciated better. Ideally, future research should also evaluate the long-term efficacy of this device in POAG and investigate its use in other refractory glaucoma forms (e.g., XFG).
Despite promising results to date, there are several unanswered issues concerning the technique, efficacy, and safety of MicroShunt, which merit further investigation. Firstly, more comparative controlled evidence is needed before this MicroShunt can be considered as a true alternative to conventional gold-standard filtration surgery in patients with refractory and advanced glaucoma. Secondly, there is large variability in the dosing and timing of MMC used in this procedure. Controlled trials investigating the optimal MMC dosing in primary surgery with MicroShunt are needed. These ought to better delineate the risk of subsequent scarring and cumulative surgical failure. The only sufficiently large controlled trial to date [14] has employed a 0.02 mg/ml MMC concentration and reported lower efficacy compared to trabeculectomy. In contrast, other smaller trials have reported greater efficacy and a lower rate of postoperative interventions (needling and open bleb revision) when a higher MMC concentration is employed with MicroShunt [29, 44]. It would therefore be desirable to document in a long-term controlled trial whether a higher MMC concentration increases the success of this procedure against standard filtration surgery (trabeculectomy), and at the same time clarify how this affects the long-term safety profile.
Since glaucoma is a 24-h disease it is essential to monitor the diurnal and 24-h efficacy of MicroShunt in patients with open-angle glaucoma versus stepwise medical therapy regimens, trabeculectomy, XEN, and other available MIGS. Controlled 24-h evidence could strengthen the popularity of this device if it demonstrates 24-h efficacy superiority in comparison with alternative options. To the best of our knowledge there have been no studies assessing the precise impact of MicroShunt on ocular surface metrics, rate of glaucoma therapy-related ocular surface disease, and long-term adherence. Since this device substantially reduces the need for combined medical therapy it should, in theory, enhance ocular surface health, tolerability, and adherence. These will be promising lines of future research, although their outcome is uncertain. Another area of interest would be establishing the cost of this procedure versus the cost of other medical and surgical therapy options in long-term glaucoma care.
Although there are small case series exploring the potential role of MicroShunt in patients with advanced glaucoma, or after previous unsuccessful filtration surgery, the role of this implant in this clinical scenario remains speculative [47]. Further, to date no differences in endothelial cell have been detected compared to trabeculectomy. It would be desirable to further document long-term corneal endothelial changes and to evaluate the effect the positioning of the implant has and its relationship with anterior chamber depth, since these parameters may impact the safety profile of this procedure in various patients with glaucoma [14]. Similarly, the role of postoperative needling to improve MicroShunt success rate and overall bleb survival is limited with this form of surgery and thus requires further elucidation.
In the event of surgical failure due to fibrosis of the filtration bleb, surgical intervention with bleb revision can be an option. However, data on the efficacy and safety of this approach is scarce. Likewise, the possible indications for surgical revision and the guidelines for stepwise management after surgical failure have not been adequately documented. Specifically, which form of additional glaucoma surgery should be recommended (trabeculectomy or tube surgery) when MicroShunt fails and the precise impact of MicroShunt failure upon the success of subsequent glaucoma interventions require further elucidation.
References
García-Feijóo J, Larrosa JM, Martínez-de-la-Casa JM, Polo V, Julvez LP. Redefining minimally invasive glaucoma surgery. Minimally penetrating glaucoma surgery. Arch Soc Esp Oftalmol. 2018;93:157–9.
Francis BA, Singh K, Lin SC, et al. Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118:1466–80.
Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96–104.
Gillmann K, Mansouri K. Minimally invasive surgery, implantable sensors, and personalized therapies. J Ophthalmic Vis Res. 2020;15:531–46.
Pillunat LE, Erb C, Jünemann AG, Kimmich F. Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents. Clin Ophthalmol. 2017;11:1583–600.
Pinchuk L, Wilson GJ, Barry JJ, et al. Medical applications of poly(styrene-block-isobutylene-block-styrene) (“SIBS”). Biomaterials. 2008;29:448–60.
Acosta AC, Espana EM, Yamamoto H, et al. A newly designed glaucoma drainage implant made of poly(styrene-b-isobutylene-b-styrene): biocompatibility and function in normal rabbit eyes. Arch Ophthalmol. 2006;124:1742–9.
Condon GP, Moster MR. Minimizing the invasiveness of traditional trabeculectomy surgery. J Cataract Refract Surg. 2014;40:1307–12.
Arrieta EA, Aly M, Parrish R, et al. Clinicopathologic correlations of poly-(styrene-b-isobutylene-b-styrene) glaucoma drainage devices of different internal diameters in rabbits. Ophthalmic Surg Lasers Imaging. 2011;42:338–45.
Lim KS. Control and optimisation of fluid flow in glaucoma drainage device surgery. Eye (Lond). 2018;32:230–4.
Pinchuk L, Riss I, Batlle JF, et al. The use of poly(styrene-block-isobutyleneblock- styrene) as a MicroShunt to treat glaucoma. Regen Biomater. 2016;3:137–42.
Sadruddin O, Pinchuk L, Angeles R, Palmberg P. Ab externo implantation of the MicroShunt, a poly(styrene-block-isobutylene-block-styrene) surgical device for the treatment of primary open-angle glaucoma: a review. Eye Vis (Lond). 2019;6:36.
Aptel F, Batlle JF, Beckers H, et al. Effect of baseline intraocular pressure on MicroShunt outcomes in primary open-angle glaucoma: a 2-year pooled analysis. AAO Meeting 2019.
Baker ND, Barnebey HS, Moster MR, et al. Ab-externo MicroShunt versus trabeculectomy in primary open-angle glaucoma: one-year results from a 2-year randomized, multicenter study. Ophthalmology. 2021;128:1710–21.
Pillunat KR, Herber R, Haase MA, et al. PreserFlo™ MicroShunt versus trabeculectomy: first results on efficacy and safety. Acta Ophthalmol. 2021. https://doi.org/10.1111/aos.14968.
Wagner FM, Schuster AK, Munder A, et al. Comparison of subconjunctival microinvasive glaucoma surgery and trabeculectomy. Acta Ophthalmol. 2021. https://doi.org/10.1111/aos.15042.
Beckers HJM, Aptel F, Webers CAB, et al. Safety and effectiveness of the PreserFlo® MicroShunt in primary open-angle glaucoma: results from a 2-year multicenter study. Ophthalmol Glaucoma. 2022;5(2):195–209.
Martínez-de-la-Casa JM, Saenz-Francés F, Morales-Fernandez L, et al. Clinical outcomes of combined PreserFlo MicroShunt implantation and cataract surgery in open-angle glaucoma patients. Sci Rep. 2021;11:15600.
Vastardis I, Fili S, Perdikakis G, et al. Preliminary results of PreserFlo MicroShunt versus PreserFlo MicroShunt and Ologen implantation. Eye Vis (Lond). 2021;8:33.
Micheletti E, Riva I, Bruttini C, Quaranta L. A case of delayed-onset hemorrhagic choroidal detachment after PreserFlo MicroShunt implantation in a glaucoma patient under anticoagulant therapy. J Glaucoma. 2020;29:e87–90.
Stangos A, Sunaric-Megevand G. Sub-conjunctival MicroShunt (PRESERFLO™) vs canaloplasty for open-angle glaucoma: comparison of short-term complication and revision rates. Invest Ophthalmol Vis Sci. 2020;61(7):3130.
Durr GM, Schlenker MB, Samet S, et al. One-year outcomes of stand-alone ab externo SIBS MicroShunt implantation in refractory glaucoma. Br J Ophthalmol. 2022;106(1):71–9.
Bunod R, Robin M, Buffault J, et al. PreserFlo MicroShunt® exposure: a case series. BMC Ophthalmol. 2021;21(1):273.
Lass JH, Benetz BA, He J, et al. Corneal endothelial cell loss and morphometric changes 5 years after phacoemulsification with or without CyPass Micro-Stent. Am J Ophthalmol. 2019;208:211–8.
Sng CCA, Barton K. Minimally invasive glaucoma surgery–coming of age. Br J Ophthalmol. 2018;102:1315–6.
Ibarz-Barberá M, Morales-Fernández L, Corroto-Cuadrado A, et al. Corneal Endothelial Cell Loss After PRESERFLO™ MicroShunt implantation in the anterior chamber: anterior segment OCT tube location as a risk factor. Ophthalmol Ther. 2022;11:293–310.
Chamard C, Hammoud S, Bluwol E, Lachkar Y. Endothelial cell loss 5 years after PreserFlo MicroShunt implantation: about two cases. Am J Ophthalmol Case Rep. 2021;25: 101238.
Durr G, Schlenker M, Michaelov E, Ahmed IIK. Intermediate term outcomes of superior or inferior ab externo SIBS MicroShunt in refractory eyes. AGS 2019 Meeting Abstract. San Francisco, March 14–17 2019.
Batlle JF, Fantes F, Riss I, et al. Three-year follow-up of a novel aqueous humor MicroShunt. J Glaucoma. 2016;25:e58-65.
Riss I, Batlle J, Pinchuk L, et al. Résultats à un an de l’efficacité et de l’innocuité du MicroShunt InnFocus selon l’emplacement et la concentration de MMC [One-year results on the safety and efficacy of the InnFocus MicroShunt™ depending on placement and concentration of mitomycin C]. J Fr Ophtalmol. 2015;38:855–60.
Riss I. Intraocular pressure outcomes after InnFocus MicroShunt glaucoma drainage system implantation with 0.2 or 0.4 mg/mL Mitomycin C: results of a single-centre study. Société Française d'Ophtalmologie Meeting. 2018.
Palmberg P, Riss I, Batlle J. Comparison of intraocular pressure (IOP) outcomes after aqueous humor MicroShunt procedure with 0.2 or 0.4 mg/ml mitomycin C (MMC). World Glaucoma Congress, 2017.
Garcia-Feijoo J, Batlle J, Riss I, et al. A 2-year pooled analysis of the MicroShunt in patients with primary open-angle glaucoma (POAG): 0.2 versus 0.4 mg/mL mitomycin C (MMC) outcomes. World Glaucoma Congress, Melbourne, Australia 2019.
Schlenker MB, Durr GM, Michaelov E, Ahmed IIK. Intermediate outcomes of a novel standalone ab externo SIBS MicroShunt with mitomycin C. Am J Ophthalmol. 2020;215:141–53.
Nobl M, Freissinger S, Kassumeh S, Priglinger S, Mackert MJ. One-year outcomes of MicroShunt implantation in pseudoexfoliation glaucoma. PLoS One. 2021;16:e0256670.
Wang J, Triolo G, Jayaram H, Barton K. PreserFlo MicroShunt in the surgical management of glaucoma secondary to uveitis. Washington: American Glaucoma Society Meeting; 2020.
Perucho L, Triolo G, Saenz-Frances F, Garcia-Feijoo J, Barton K. Analysis of one-year surgical outcomes of minimaly invasive glaucoma surgeries (MIGS) in medically uncontrolled uveitis glaucoma patients. Melbourne: World Glaucoma Congress; 2019.
Schlenker M, Michaelov E, Durr G, Ahmed II. Intermediate term outcomes of stand-alone SIBS MicroShunt or in combination with phacoemulsification. American Glaucoma Congress San Francisco; 2019.
Ahmed I, Michaelov E, Schlenker M. Intermediate term outcomes of Sibs MicroShunt alone or in combination with phacoemulsification. ASCRS Meeting. 2019.
Batlle J, Pinchuk L, Kato Y, Weber B, Parel J. One and two year results comparing phakic, pseudophakic and combined with cataract surgery cases for the InnFocus MicroShunt®. European Society of Cataract and Refractive Surgeons (ESCRS) Meeting, 2016.
Sheheitli H, Tirpack AR, Parrish RK. Which patients would most likely to benefit: MIGs or MeGs, which one is it? Asia-Pacific J Ophthalmol. 2019;8:436–40.
García-Feijoo J, Rau M, Grisanti S, et al. Supraciliary micro-stent implantation for open-angle glaucoma failing topical therapy: 1-year results of a multicenter study. Am J Ophthalmol. 2015;159:1075–81 (e1).
Scheres LMJ, Kujovic-Aleksov S, Ramdas WD, et al. XEN ® Gel Stent compared to PreserFlo™ MicroShunt implantation for primary open-angle glaucoma: two-year results. Acta Ophthalmol. 2021;99:e433–40.
Batlle JF, Corona A, Albuquerque R. Long-term results of the PRESERFLO® MicroShunt in patients with primary open-angle glaucoma from a single-center non-randomized study. J Glaucoma. 2021;30:281–6.
Gillmann K, Mansouri K. Minimally invasive glaucoma surgery: where is the evidence? Asia-Pacific J Ophthalmol. 2020;9:203–14.
Do AT, Parikh H, Panarelli JF. Subconjunctival microinvasive glaucoma surgeries: an update on the Xen gel stent and the PreserFlo MicroShunt. Curr Opin Ophthalmol. 2020;31:132–8.
Quaranta L, Micheletti E, Carassa R, et al. Efficacy and safety of PreserFlo ® MicroShunt after a failed trabeculectomy in eyes with primary open-angle glaucoma: a retrospective study. Adv Ther. 2021;38:4403–12.
Fea AM, Laffi GL, Martini E, et al. Effectiveness of MicroShunt in patients with primary open-angle and pseudoexfoliative glaucoma: a retrospective European multicenter study. Ophthalmol Glaucoma. 2022;5(2):210–8.
Scheres LMJ, Kujovic-Aleksov S, Ramdas WD, et al. XEN® Gel Stent compared to PreserFlo™ MicroShunt implantation for primary open-angle glaucoma: two-year results. Acta Ophthalmol. 2021;99:e433–40.
Triolo G, Jayaram H, Gazzard G, et al. InnFocus MicroShunt for the treatment of glaucoma: safety and efficacy outcomes at 24 months. AAO Meeting 2019.
Acknowledgements
Funding
No funding or sponsorship was received for the publication of this article. No Open Access Fee was received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Barbara Burgos-Blasco: drafting the manuscript. Julián García-Feijóo: concept and design, drafting and review the manuscript. Lucia Perucho-Gonzalez: drafting the manuscript. Noemi Güemes-Villahoz: drafting the manuscript, references search and review. Laura Morales-Fernandez: drafting the manuscript, references search. Carmen D. Mendez-Hernández: concept and design. Jose M. Martinez de la Casa: concept and design. Anastasios G. Konstas: concept and design, review of manuscript.
Disclosures
J. Garcia-Feijoo. Research funding from Santen, Omni-Vision, Allergan, Glaukos, Alcon, AJL, Thea, Pfizer, Novartis, Ivantis, iStar; Advisory Board of Santen, Alcon, Allergan, Alimera and iStar. Anastasios G. Konstas: Editorial board member for Advances in Therapy; research funding from Allergan, Bayer, Omni Vision and Santen; travel support and congress expenses from Vianex; honoraria from Allergan, Esteve Pharmaceuticals, Santen and Vianex.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Burgos-Blasco, B., García-Feijóo, J., Perucho-Gonzalez, L. et al. Evaluation of a Novel Αb Εxterno MicroShunt for the Treatment of Glaucoma. Adv Ther 39, 3916–3932 (2022). https://doi.org/10.1007/s12325-022-02230-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02230-1