Abstract
Introduction
Large-scale Indian data on the use of anti-T-lymphocyte globulin (ATLG) (Grafalon®) as induction therapy in kidney transplantation (KT) patients is lacking. The aim of this study was to determine the 1-year patient and graft survival outcomes with the use of ATLG as induction regimen in KT.
Methods
In a prospective, multicentric, observational study, adult patients who underwent ABO-compatible KT and had received ATLG as a part of induction were included in the study. The primary outcome measure was overall survival and death-censored graft survival at 12 months. The primary safety outcome was assessed by development of infectious complications and graft rejection.
Results
In total, 359 patients were included in this study. The mean age was 42.77 ± 12.30 years and 83% were male. The average ATLG dose per patient was 6.2 ± 2.2 mg/kg whereas average cumulative dose per patient was 389.6 ± 149.8 mg. The rate of graft dysfunction was 13.4% of patients and 6.7% had biopsy-proven acute rejection (BPAR). There were a total of 12 (3.3%) deaths and one graft loss. Overall survival and death-censored graft survival at 12 months were 96.65% and 99.44%, respectively. The rate of infections was 13.6% with urinary tract infections being most common.
Conclusion
ATLG at an average dose of 6 mg/kg is an effective and safe induction regimen immunosuppressant for ABO-compatible KT with favourable impact on survival and graft function in Indian patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Anti-T-lymphocyte globulin (ATLG) (Grafalon®) has been approved in India for use in kidney transplantation. However, there has been no large-scale real-world studies from India |
This multicentric study explored the 1-year outcomes of patient and graft survival as well as the rates of complications such as graft rejection and infections when ATLG was used as induction agent |
What was learned from the study? |
ATLG (Grafalon®) is associated with 1-year patient and graft survival comparable with the published literature from similar cohorts |
The rate of biopsy-proven acute rejection with the use of ATLG (Grafalon®) remains acceptable compared to previous reports |
We observed limited incidence of infective and other complications with the average mean ATLG (Grafalon®) dose of approx. 6 mg/kg body weight |
Introduction
Chronic kidney disease (CKD) is a potentially life-threatening disease associated with significant morbidity and mortality and poor quality of life [1]. CKD ultimately results in end-stage kidney disease (ESKD). In India, the approximate prevalence of CKD and ESKD is 800 and 150–200 per million population [2]. The prevalence of CKD varies from 3% to 4% [3]. With increasing prevalence of risk factors such as diabetes and hypertension in India, the prevalence of CKD is expected to rise substantially [4]. This impacts the use of renal replacement therapy (RRT). Kidney transplantation (KT) is the ultimate modality of RRT that significantly improves the quality of life (QoL) [5]. The objective of immunosuppression in KT is to prevent acute graft rejection and improve overall graft survival [6,7,8]. A tailored induction regimen has been shown to be effective with good outcomes at 1 year [9].
In India, T-lymphocyte-depleting antibody—rabbit anti-thymocyte globulin (rATG) and interleukin-2 receptor antagonist (IL2RA) are commonly utilized induction agents [10]. The choice between these induction agents varies. Anti-T-lymphocyte globulin (ATLG) (Grafalon®, formerly ATG Fresenius) is produced differently than ATG (Thymoglobulin®). ATLG is prepared by immunizing rabbits with the T cell leukemia line Jurkat while ATG is produced by immunizing with the human thymocytes. ATLG has greater antigen specificity for activated T cells [11, 12] and it also depletes CD4+CD28− T cells [13, 14]. ATLG is widely used in India as part of induction regimes for KT. Initial studies from India have shown equivalent patient survival and death-censored graft survival with ATLG and ATG [12]. Compared to basiliximab, ATLG has similar rates of biopsy-proven acute rejection (BPAR) and acute graft rejection [15]. However, rates of delayed graft function and need for dialysis were lower with ATLG than basiliximab [16]. In different studies from India, rates of BPAR varied from 13% to 32% [12, 17, 18]. As there is no multicentric, large-scale study from India assessing the utility of ATLG in KT, we conducted this study to determine the outcomes with use of ATLG and assess the impact of dose on the outcomes of KT.
Methods
Design and Setting
This was a prospective, multicentric, observational study conducted across 11 centres in India. At each centre, an institutional ethical review board approved the study protocol. Informed consent was obtained from all the participants before enrolment into the study. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments, good clinical practices and applicable local regulatory guidelines.
Participants
We enrolled adult patients aged 18 years or over, of either gender, with ESKD, undergoing KT and who had been prescribed ATLG as a part of induction. We excluded patients who had ABO-incompatible transplant, cadaveric donor transplant, patients who received ATG in the induction regime, pregnant and lactating women and patients with any other illness which in the opinion of the treating physician was hazardous in terms of patient participation in the study.
Immunosuppressive Protocol
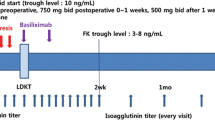
ATLG was administered on day 0 before transplant and day 1 and day 2 after transplant. ATLG was diluted in 0.9% normal saline (NS) at dilution ratio of 1:7. Over 4 h, ATLG was infused intravenously. First dose was given 6–8 h before the clamp release. Premedication with systemic antihistamine and steroid was done 1 h before ATLG administration. ATLG dose was decided by the treating physician. Mean ATLG dose was 6.2 ± 2.2 mg/kg and average cumulative dose per patient was 389.6 ± 149.8 mg. Cumulative dose varied from 100 to 900 mg. ATLG was administered either as a single dose or in divided doses. Standard regimens of prednisone (30 mg once daily), tacrolimus (0.1 mg/kg/day) and mycophenolate mofetil (500 mg thrice daily) were followed. Tacrolimus dose was adjusted on the basis of the trough levels with target levels of 8–12 ng/ml for first 3 months of transplant and 5–8 ng/ml after 3 months. All patients had received cytomegalovirus prophylaxis (valganciclovir 450 mg once a day for 3 months) and cotrimoxazole as prophylaxis for urinary infections and pneumocystis infection for 6 months.
Outcome Assessment
Patients were followed for 12 months post-transplant. The primary outcome measure was overall survival and death-censored graft survival. The primary safety outcome was assessed by development of infectious complications such as urinary tract infections (UTI), cytomegalovirus (CMV) infections, pneumonia; and development of graft rejection. Acute graft dysfunction was defined as rise of serum creatinine by 20–25% compared to baseline. In acute rejection cases, tissue biopsy from the transplanted graft was taken under standard procedures. Histological diagnosis was made as cell-mediated or antibody-mediated rejection. The diagnostic criteria for T cell-mediated rejection was based on finding of lymphocytic infiltrate of tubules (tubulitis) and larger vessels (vasculitis). Severity of these lesions depends on the degree of lymphocytic infiltrate per high-powered field. Antibody-mediated rejection was diagnosed with Banff classification based on the findings of active tissue injury, immunohistologic evidence of peritubular capillary complement split-product C4d deposition, and circulating donor-specific antibodies (DSA).
Statistical Analysis
Data from all the centres was collated and entered in Microsoft Excel 2016. Data was analysed with SPSS version 15. Data were analysed with descriptive statistics. Continuous data were presented as mean values ± standard deviation for normally distributed variables and as median and interquartile range for non-normally distributed variables. Categorical data was described with frequency and percentages.
Results
In this study, we included a total of 359 patients from different centres. Table 1 provides data on the baseline characteristics of the patients. The mean age was 42.77 ± 12.30 years and 83% of patients were male. In the native kidney disease, CKD of unknown etiology was most common (46.78%), followed by diabetes mellitus (20.8%) and chronic glomerulonephritis (11.9%). 47.6% patients had Human leucocyte antigen(HLA) mismatch greater than 3. Median dialysis vintage was 6 months (IQR25–75 3–12). Transplant was pre-emptive in 7.2% of patients whereas 0.9% had prior transplantation. The donor mean age was 46.2 ± 11.4 years. Parents, spouse and sibling constituted 31.8%, 22.3% and 11.4% of total donors, respectively.
Table 2 shows the transplant outcomes. The graft function was well preserved over a 12-month period as indicated by serum creatinine levels (Fig. 1). Graft dysfunction was seen in 13.4% of patients whereas 6.7% had BPAR. Acute cellular rejection (ACR) and antibody-mediated rejection (ABMR) were noted in 5.3% and 1.1% of patients respectively whereas one patient had both ACR and ABMR. Calcineurin inhibitor toxicity was responsible for graft dysfunction in 1.4% of cases. Asymptomatic rise in creatinine was seen in 1.4% of patients and 13.6% of patients developed one or more infections. UTI was the most common infection that developed in 7% of patients followed by sepsis (3.3%). Cytomegalovirus (CMV) disease and varicella-zoster virus (VZV) disease were observed in one patient each. Only one patient had a graft loss, which was following ABMR. All-cause mortality was 3.3%. Case of death included acute coronary syndrome (n = 5), sepsis (n = 6) and fulminant hepatic failure (n = 1). Overall survival and death-censored graft survival at 12 months were 96.65% and 99.44% respectively.
Discussion
In this large, prospective study with ATLG used as induction regimen, the achieved overall survival and the death-censored graft survival rate at 12 months post-transplant along with comparable rates of infections and graft rejection indicate that ATLG is useful for induction therapy in KT. Multiple studies reported similar rates of overall and death-censored graft survival at 1 year post-transplant [19, 20]. Even beyond 1 year (median 22 months), comparable patient survival and death-censored graft survival have been reported in India [12]. At 5 years post-transplant in patients who had steroid avoidance, the efficacy and safety of ATLG remained similar to those who received steroids for the initial 6 months [20]. These data indicate a substantial efficacy of ATLG in ‘overall and graft survival’. Adequate dose of ATLG is essential to induce better immunosuppression without increasing the risk of complications. Though the average ATLG dose in our study was 6.2 ± 2.2 mg/kg, it varied from 5.1 ± 2.7 mg/kg to 21 mg/kg in previous studies [19, 20]. A study from India using a relatively lower ATLG dose (4 mg/kg) had observed a comparatively lower rates of patient survival (91%) and graft survival (82%) at a median follow-up of 103 days [21]. However, contrasting these observations, another study from India reported comparable 12-month graft survival even with a low dose of ATLG (1.7 ± 0.15 mg/kg) [22]. Therefore, optimal dose that provides best outcomes still needs to be clearer. In our experience, an average ATLG dose of 6 mg/kg provides effective survival rates without affecting other complications and has been proved effective and safe in a previous similar study from India [12].
Acute rejections are associated with significant impact on graft survival [8]. ATLG is used as induction regime primarily to prevent the graft dysfunction and graft rejection. BPAR was relatively lower (6.7%) when compared to the rates of 12.8–36.3% among other studies from India [12, 21]. Using a regimen of 9 mg/kg prior to reperfusion of the allograft, followed by 3 mg/kg/day on day 1 to 4, a randomized study from Switzerland reported ABMR and ACR rate of 35% and 11% within 1 year of transplantation [23]. With similar dosing regime, Kamar et al. observed BPAR and graft loss rate of 3.6% and 9.6% at 1 year [15]. These data indicate that ATLG is effective in reducing acute rejection and improving graft survival. Avoiding the graft rejection is the primary objective of immunosuppression in KT. We observed well-maintained graft function over the 12 months. Persistently good graft function can be seen even after 1 year of transplant [23]. Thus, the use of ATLG in KT patients can effectively prevent rejection and help in maintaining good graft function in the post-transplant period.
Infections are common with immunosuppressive treatments. Infection rate was 13.6% in our study which is comparable to previous studies (approx. 13%) [12, 17]. Compared to ATLG, a North Indian study reported higher rates of infections with ATG [12]. That result was further substantiated by a metanalysis reporting that the rate of bacterial and viral infections is usually lower with ATLG compared to ATG (odds ratio 1.49, 95% confidence interval 0.43–5.23) [24]. Among the infective complications, reactivation of viral disease, especially CMV, is worrisome. We had very limited viral disease reactivation with once case each of CMV and VZV. Yilmaz et al. [19] observed 12.7% of patients diagnosed with CMV. A study from Thailand reported 43% incidence of CMV reactivation with use of ATG as induction regime [25]. A comparable rate of infections and lower viral disease reactivation in our study perhaps suggests that ATLG is a safe induction agent for KT in the Indian setting.
Though we did not compare the ATLG with other induction therapies, it is essential to understand the differences in the outcomes. A systematic review of randomized controlled trials (RCTs) identified no significant differences in 1-year patient survival or graft loss or BPAR when basiliximab was compared to ATG or OKT3 (eight RCTs) [26]. At 6-year follow-up, Ulrich et al. did not observe significant difference between basiliximab and ATG in the patient and graft survival outcomes, nor in the incidence of BPAR. Rates of CMV infection and haematological complications with ATG were higher than those with basiliximab [27]. Another study comparing daclizumab and ATG did not find significant difference in 1-year patient and graft survival rates. Rates of BPAR and steroid-resistant rejection were significantly lower with ATG than daclizumab [28]. However, comparing the use of single bolus dose of basiliximab or ATLG, Yang et al. [29] reported significantly higher acute rejection rates with basiliximab (35.7% vs. 15%, p = 0.032). The 1-year patient and graft survival did not differ between the two groups. Thus, the optimal induction regimen for KT that is effective and safe needs to be established in future comparative studies.
Limitations
Though the observations from this study show promising efficacy and safety of ATLG in KT recipients, the study has limitations. The proportion of male patients was substantially greater. CKD seems to be slightly more prevalent in men than women in India [30]. Previous studies have also reported gender disparity in KT with male recipient of transplants being significantly greater in number than female recipients [31]. We did not assess the outcomes based on HLA mismatch. Furthermore, our study had a shorter follow-up duration of only 1 year. Studies with longer follow-up are necessary in the Indian setting.
Conclusion
In this first large-scale Indian study of ATLG in KT, we observed better patient survival and death-censored graft survival at 12 months that were comparable to those in previous reports. With a mean dose of 6 mg/kg, we can achieve better outcomes with lower rates of infection, graft dysfunction and rejection. Thus, ATLG at median optimal dose of 6 mg/kg can be effectively and safely used in Indian patients undergoing KT.
Change history
16 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12325-022-02283-2
References
Bello AK, Alrukhaimi M, Ashuntantang GE, et al. Complications of chronic kidney disease: current state, knowledge gaps, and strategy for action. Kidney Int Suppl. 2011;2017(7):122–9.
Agarwal SK, Srivastava RK. Chronic kidney disease in India: challenges and solutions. Nephron Clin Pract. 2009;111:c197-203.
Ahlawat R, Tiwari P, D’Cruz S, Singhal R. Prevalence of chronic kidney disease in India: a systematic review and meta-analysis of observational studies. Value Health. 2015;18:A509.
Varma PP. Prevalence of chronic kidney disease in India—where are we heading? Indian J Nephrol. 2015;25:133–5.
Kostro JZ, Hellmann A, Kobiela J, et al. Quality of life after kidney transplantation: a prospective study. Transplant Proc. 2016;48:50–4.
Wiseman AC. Induction therapy in renal transplantation: why? what agent? what dose? we may never know. Clin J Am Soc Nephrol. 2015;10:923–5.
El Ters M, Grande JP, Keddis MT, et al. Kidney allograft survival after acute rejection, the value of follow-up biopsies. Am J Transplant. 2013;13:2334–41.
Koo EH, Jang HR, Lee JE, et al. The impact of early and late acute rejection on graft survival in renal transplantation. Kidney Res Clin Pract. 2015;34:160–4.
Phanish MK, Hull RP, Andrews PA, Popoola J, Kingdon EJ, MacPhee IA. Immunological risk stratification and tailored minimisation of immunosuppression in renal transplant recipients. BMC Nephrol. 2020;21:92.
Radhakrishnan RC, Basu G, Mohapatra A, et al. Utility of induction agents in living donor kidney transplantation. Indian J Transplant. 2019;13:202–9.
Popow I, Leitner J, Grabmeier-Pfistershammer K, et al. A comprehensive and quantitative analysis of the major specificities in rabbit antithymocyte globulin preparations. Am J Transplant. 2013;13:3103–13.
Jha PK, Rana A, Kher A, et al. Grafalon® vs. thymoglobulin® as an induction agent in renal transplantation—a retrospective study. Indian J Nephrol. 2021;31:336–40.
Shenton BK, White MD, Bell AE, et al. The paradox of ATG monitoring in renal transplantation. Transplant Proc. 1994;26:3177–80.
Duftner C, Dejaco C, Hengster P, et al. Apoptotic effects of antilymphocyte globulins on human pro-inflammatory CD4+CD28− T-cells. PLoS ONE. 2012;7: e33939.
Kamar N, Lepage B, Couzi L, et al. A randomized prospective study comparing anti-T-lymphocyte Igs to basiliximab in highly sensitized kidney transplant patients. Kidney Int Rep. 2020;5:1207–17.
Kyllönen LE, Eklund BH, Pesonen EJ, Salmela KT. Single bolus antithymocyte globulin versus basiliximab induction in kidney transplantation with cyclosporine triple immunosuppression: efficacy and safety. Transplantation. 2007;84:75–82.
Gulati P, Dijoo AM, Tanmay P, Kritie C, Aman G, Rajeev S. SAT-339 low dose induction immunotherapy with anti human T-lymphocyte immunoglobulin (Grafalon) in high risk renal transplantation—a real-world, single centre experience from India. Kidney Int Rep. 2020;5:S142.
Singh R, Bhalla A, Gupta A, et al. Comparative study of ATG vs ATG-F (Grafalon) as an induction agent in ABO incompatible kidney transplantation. Transplantation. 2020;104:S340.
Yilmaz MÜ, Sezer TÖ, Günay ES, et al. Efficacy and safety of ATG-Fresenius as an induction agent in living-donor kidney transplantation. Transplant Proc. 2017;49:481–5.
Cantarovich D, Rostaing L, Kamar N, et al. Early corticosteroid avoidance in kidney transplant recipients receiving ATG-F induction: 5-year actual results of a prospective and randomized study. Am J Transplant. 2014;14:2556–64.
Engineer D, Patel H, Kute V, Shah P. Initial experience with Grafalon as induction agent in kidney transplantation. J Clin Diagn Res. 2018;12:19–23.
Sharma RK, Kumar A, Kumar J, et al. Low-dose ATG is effective in treatment of acute rejection episodes. Transplant Proc. 2003;35:225–6.
Burkhalter F, Schaub S, Bucher C, et al. A comparison of two types of rabbit antithymocyte globulin induction therapy in immunological high-risk kidney recipients: a prospective randomized control study. PLoS ONE. 2016;11: e0165233.
Song T, Yin S, Li X, Jiang Y, Lin T. Thymoglobulin vs. ATG-Fresenius as induction therapy in kidney transplantation: a Bayesian network meta-analysis of randomized controlled trials. Front Immunol. 2020;11:457.
Chitasombat MN, Watcharananan SP. Burden of cytomegalovirus reactivation post kidney transplant with antithymocyte globulin use in Thailand: a retrospective cohort study. F1000Res. 2018;7:1568.
Woodroffe R, Yao GL, Meads C, et al. Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study. Health Technol Assess. 2005;9(1–179):iii–iv.
Ulrich F, Niedzwiecki S, Pascher A, et al. Long-term outcome of ATG vs. basiliximab induction. Eur J Clin Invest. 2011;41:971–8.
Noël C, Abramowicz D, Durand D, et al. Daclizumab versus antithymocyte globulin in high-immunological-risk renal transplant recipients. J Am Soc Nephrol. 2009;20:1385–92.
Yang SL, Wang D, Wu WZ, et al. Comparison of single bolus ATG and basiliximab as induction therapy in presensitized renal allograft recipients receiving tacrolimus-based immunosuppressive regimen. Transpl Immunol. 2008;18:281–5.
Singh AK, Farag YM, Mittal BV, et al. Epidemiology and risk factors of chronic kidney disease in India—results from the SEEK (Screening and Early Evaluation of Kidney Disease) study. BMC Nephrol. 2013;14:114.
Bal MM, Saikia B. Gender bias in renal transplantation: are women alone donating kidneys in India? Transplant Proc. 2007;39:2961–3.
Acknowledgements
The authors would like to thank Dr. Vijay Kher, Dr. D S Rana, Dr. Ashwani Gupta, Dr. Sundar S, Dr Manish Malik, Dr Vinant Bhargava and Dr Anurag Gupta for their valuable contribution in terms of conceptualization and data analysis for this study.
Funding
The authors are also thankful to Zydus Lifesciences, Ahmedabad, India for their unrestricted educational grant for the study.
Medical Writing Assistance
The editorial and medical writing assistance in the preparation of this article was provided by Dr. Vijay Katekhaye of Quest MedPharma Consultants, Nagpur, India. Support for this assistance was funded by Zydus Lifesciences.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Dr Sishir Gang and Dr Sanjeev Gulati conceptualized the study and contributed to study conduct, oversight and management. Dr AK Bhalla, Dr PP Varma and Dr RK Sharma helped in analysis and interpretation of the results. Dr Ravi Bansal contributed to statistical analysis. Dr DS Ray and Dr Abi Abraham contributed to drafting of the article. Critical revision of the article was done by Dr SB Bansal and Dr Vishwanath S. All the authors have contributed in final proof reading of the manuscript and approved it for publication.
Disclosures
Dr Sishir Gang, Dr Sanjeev Gulati, Dr Prem P Varma, Dr Anil K Bhalla, Dr Ravi Bansal, Dr Mammen John, Dr Abi Abraham, Dr Shyam Bansal, Dr Rajkumar Sharma and Dr Vishwanath Siddini declare to receiving research support from Zydus Lifesciences. Dr PP Varma is now affiliated to Department of Nephrology, Primus Hospital, New Delhi. Data capture and analysis of ‘One-year outcomes with use of Anti-T-lymphocyte globulin in patients undergoing kidney transplantation: Results from a prospective, multicentric, observational study from India’ was supported by Zydus Lifesciences (Previously known as Cadila Healthcare Limited), India with research support from Neovii Pharmaceuticals AG (Switzerland). Authors do not have any other conflict of interest.
Compliance with Ethics Guidelines
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from the participants for participation in the study with all the rights to withdraw anytime during the entire duration. Ethics committee approvals were obtained from individual institutional and an independent ethics committee. Ethics committee for research, Fortis Hospital (ECR/57/DL/2013/RR-2016), Ethics committee for Manipal Hospital, Bangalore Ethics committee, Sir Gangaram Hospital (EC/01/18/1313), Ethics Committee PSRI, Ethics committee, MPUH (EC/506/2018), NHRTIICS Ethics committee, Ethics Committee, Medanta Hospital and Sangini Hospital Ethics Committee (ECR/147/Inst/GJ/2013).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The original online version of this article was revised due to update in acknowledgement.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gang, S., Gulati, S., Bhalla, A.K. et al. One-Year Outcomes with Use of Anti-T-Lymphocyte Globulin in Patients Undergoing Kidney Transplantation: Results from a Prospective, Multicentric, Observational Study from India. Adv Ther 39, 4533–4541 (2022). https://doi.org/10.1007/s12325-022-02225-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02225-y