Abstract
Introduction
Multiple studies have demonstrated the effectiveness of pharmacokinetic (PK)-guided individualized prophylaxis with human coagulation factor VIII (FVIII) compared with standard prophylaxis, but no studies have evaluated the economics of PK-guided prophylaxis in China. Hence, we conducted this study to assess the cost-effectiveness of PK-guided prophylaxis with recombinant FVIII (rFVIII) versus standard prophylaxis in Chinese adult patients with severe hemophilia A.
Methods
A discrete event simulation model was developed to simulate 10,000 patients with hemophilia A who received rFVIII treatment over a 1-year time horizon. The standard prophylaxis rFVIII dose was 30 IU/kg by intravenous injection. The PK-guided prophylaxis dosage was adjusted for each patient to maintain FVIII trough level at 1–5 IU/dL. Dosing interval for both approaches was kept fixed at 48 h. The health outcomes included annual joint bleed rate (AJBR) and quality-adjusted life years (QALYs). The model considered the costs of drug. Incremental cost-effectiveness ratio (ICER) was estimated and scenario analysis was performed.
Results
A total of 94.3% of patients receiving PK-guided individualized prophylaxis achieved the goal of maintaining the trough concentration at 1–5 IU/dL compared with 62.7% on standard prophylaxis. AJBR and QALYs gained in PK-guided and standard prophylaxis were 1.527 vs 1.601, and 0.8384 vs 0.8383, respectively. Costs of drug prophylaxis and costs of treatment for bleeding events in PK-guided prophylaxis (148,641.47 USD; 4546.43 USD) were lower than those in standard prophylaxis (159,620.93 USD; 4753.39 USD). An average saving of USD 11,186.47 was obtained by the PK-guided approach. The prophylaxis treatment scenarios were the most influential factors.
Conclusion
PK-guided individualized prophylaxis appeared to be a dominant treatment compared with standard prophylaxis, with slightly higher QALYs but lower total costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Multiple studies have demonstrated the effectiveness of pharmacokinetic (PK)-guided individualized prophylaxis with human coagulation factor VIII (FVIII) compared with standard prophylaxis, but no studies have evaluated the economics of PK-guided prophylaxis in China. |
This study assessed the cost-effectiveness of PK-guided prophylaxis with recombinant FVIII (rFVIII) versus standard prophylaxis in Chinese adult patients with severe hemophilia A. |
PK-guided individualized prophylaxis appeared to be a dominant treatment compared with standard prophylaxis, with slightly higher QALYs but lower total costs. |
This study provided an initial support for PK-guided dosing regimen using myPKFiT® in Chinese patients with severe hemophilia A. |
Introduction
Hemophilia A is an X-linked recessive, hereditary bleeding disease caused by the deficiency of coagulation factor VIII (FVIII) [1]. Based on the levels of circulating FVIII, it can be categorized as mild (5–40 IU/dL of FVIII level), moderate (1–5 IU/dL of FVIII level), and severe (< 1 IU/dL of FVIII level) hemophilia A [2]. The current incidence of hemophilia A as per the World Federation of Hemophilia (WFH) global survey report 2019 is 24.6 cases per 100,000 male individuals [2] and China currently has a total of 16,158 patients with hemophilia A as per the WFH global survey 2018 [3]. However, it is estimated that the total number of patients with hemophilia could be approximately 130,000 in China [4, 5].
Patients with severe hemophilia A require lifelong replacement therapy with exogenous FVIII to prevent bleeding and associated complications in muscles and joints [6]. FVIII replacement therapy can either be administered on demand during episodes of bleeding or prophylactically [7]. It has been documented that repeated episodes of joint bleeding would cause arthropathy [8]. Prophylactic FVIII regimens are effective in reducing bleeding frequency and arthropathy in patients with severe hemophilia A [9,10,11]. Standard prophylaxis with 20–40 IU/kg FVIII concentrates every other day or at least three times a week helps in reducing bleeding episodes, decreases hospitalization, and improves long-term joint function [12,13,14]. Despite the effectiveness of standard prophylaxis in reducing bleeding episodes, a subpopulation of patients still experience bleeding, probably because of insufficient plasma FVIII levels during dosing intervals [15, 16]. Although an increase in the FVIII dose may provide universally higher FVIII levels [17], it is not feasible because of the high costs of FVIII. As per the WFH global survey 2018, the global mean per capita use of FVIII is 2.40 IU, whereas the per capita FVIII usage in China is 0.026 [3]. The lower per capita consumption of FVIII in China could be attributed to the lower level of reimbursement for the treatment of hemophilia in China, which is far lower than that in the developed countries, leading to higher out-of-pocket treatment cost [18]. High economic burden leads to insufficient treatment, with only 6.2% of patients receiving adequate FVIII replacement treatment, which further leads to significantly higher annual joint bleeding rate (AJBR) and hemophilia-related arthritis [19].
Contrary to standard prophylaxis, individualized pharmacokinetic-guided (PK-guided) prophylaxis could provide significant clinical benefits with lower consumption of FVIII, thereby reducing the economic burden [20]. Multiple studies have proven the effectiveness of PK-guided individualized prophylaxis compared with standard prophylaxis [21,22,23]. Hence, the latest hemophilia guidelines, including WFH Guidelines for the Management of Hemophilia [2], Chinese Hemophilia Treatment Guidelines (2020 Version) [24], and British Hemophilia Association Guidelines (2020) [25], recommend individualized prophylaxis. A pharmacoeconomic analysis conducted in Italy through the development of a microsimulation model showed that PK-driven prophylaxis was more effective and less costly than standard prophylaxis in patients with severe hemophilia A [20]. PK-guided prophylaxis using myPKFiT®, a medical device that calculates the prophylactic dose for ADVATE® (Recombinant Human Coagulation Factor VIII, Takeda Pharmaceutical Company Ltd.) according to individual PK parameters based on selected trough levels and infusion intervals, was approved by the National Medical Products Administration (NMPA) in 2020 in China. However, there are no studies in China to evaluate the economics of PK-guided individualized prophylactic treatment in patients with severe hemophilia A. Hence, we conducted this study to evaluate the cost-effectiveness of PK-guided prophylaxis with recombinant human coagulation factor VIII versus standard prophylaxis in the treatment of Chinese adult patients with severe hemophilia.
Methods
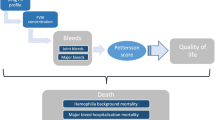
A discrete event simulation (DES) model was developed to assess the cost-effectiveness of PK-guided prophylaxis compared with the standard prophylaxis in the treatment of severe hemophilia A. The model structure is presented in Fig. 1. The simulated health states were “bleeding” and “non-bleeding.”
According to the instruction of ADVATE®, for long-term prophylaxis of patients with severe hemophilia A, the routine recombinant FVIII (rFVIII) dose is 20–40 IU/kg, and the injection interval is 2–3 days. The model simulated a hypothetical cohort of 10,000 adult patients with severe hemophilia A. Each patient was assumed to undergo 1 year of standard prophylaxis at a fixed regimen of 30 IU/kg. The simulated population of 10,000 with the same characteristics as standard prophylaxis were treated with PK-guided individualized prophylaxis using myPKFiT®, and the injection dosage was adjusted depending on the PK parameters and target trough concentration. The dosing interval for both prophylaxis regimen was kept fixed to 48 h.
The FVIII pharmacokinetic curve (concentration/time curve) for each simulated patient was obtained by implementing the Björkman equation [26]. The standard dose of FVIII was adjusted to maintain a trough concentration of 1–5 IU/dL. This is based on the reported increased risk of bleeding when the FVIII trough concentration is less than 1 IU/dL and the risk of joint bleeding decreases to less than 1 per year when the trough concentration is greater than 5 IU/dL [27]. Accordingly, the FVIII dose was increased when the trough concentration was less than 1 IU/dL and decreased when the trough concentration was greater than 5 IU/dL.
The health outcomes included AJBR and quality-adjusted life years (QALYs). For cost-effectiveness analysis, only the direct medical cost of treatment regimens from the perspective of China’s healthcare system was taken into consideration. The unit of cost measurement was United States dollars (USD). An incremental cost-effectiveness ratio (ICER) was measured to support the result of cost-effectiveness analysis. Three times the per capita gross domestic product (GDP) of China in 2020 (32,337.12 USD) was used as a willingness to pay (WTP) threshold for ICER obtained in different scenarios. Since this article is based on previously conducted studies and does not contain any new studies with human participants or animals, ethics committee approval was not required.
Clinical Data
The standard prophylaxis regimen was considered as the control treatment and the clinical and economic outcomes were compared against PK-guided individualized prophylaxis using myPKFiT®. The baseline characteristics of the patients were based on a previous Chinese study on individualized prophylaxis in patients with severe hemophilia A [28]. The patients age ranged from 19 to 44 years with a mean age of 29.3 and the body weight ranged from 45 to 82 kg, with a mean weight of 59.1 kg (Table 1). The distribution of age and weight was in accordance with the characteristics of myPKFiT® population approved by NMPA. As a result of the lack of PK parameters of FVIII in the Chinese population, the PK parameters in this model were derived from Iannazzo et al., and were based on the parameters of American patients with hemophilia and a median body weight of 56 kg and a median age of 22 years (Table 1) [20]. The DES model simulated the clinical pathway of individual patients under different treatment methods. The PK parameters included, as per the pharmacokinetic two-compartment model, were clearance (CL), distribution volume (V), clearance between compartments (Q), and second compartment distribution volume (V2).
Because the WFH (2020) guidelines and the expert survey suggested that 70–80% of all the bleeding events were joint bleeding events [2], and as a result of the lack of other bleeding data, the model only considered joint bleeding events. AJBR was considered as the metric for comparing the efficacy of standard and PK-guided prophylaxis. The AJBR values for the simulated patients were based on the baseline FVIII levels and associated risk of bleeding as reported by den Uijl et al. (Supplementary Fig. 1) [27], and the model was developed on the basis of the data extracted from Iannazzo et al. (Supplementary Table 1) [20]. In case of standard prophylaxis, patients were given regular and fixed-dose injection of 30 IU/kg, whereas in the PK-guided individualized prophylaxis, the injection dose was adjusted according to the individual PK parameters. After injection, plasma FVIII concentration of the patients gradually decreases over time, and the change in trend is determined according to the individual FVIII injection dose, PK parameters, and administration frequency (the model assumed that the administration frequency of all patients is the same and fixed at 48 h). The PK distribution curve was divided into 24 different time–concentration ranges that correspond to plasma FVIII concentration ranging from peak to trough concentrations. The risk of AJBR at each of these time–concentration ranges was calculated and the occurrence of bleeding events at each range was determined by random sampling.
The treatment courses for joint bleeding that were considered in the model were as per the 2020 Chinese guidelines for the treatment of hemophilia, which recommend increasing the plasma concentration of FVIII to 40–60 IU/dL for a treatment duration of 1–2 days, provided that access to coagulation factors is not limited [24]. Data from the expert survey also revealed that the rFVIII dose after an episode of bleeding varied from 15 to 40 IU/kg; and according to the ADVATE and myPKFiT® instructions, the study also set the maximum (40 IU/kg) and minimum injection dose (10 IU/kg) of rFVIII (Table 1). The administration frequency during bleeding was usually 1–2 times a day and the treatment duration varied from 1 to 3 days according to the severity of bleeding (Supplementary Table2 ). The model assumed that the dose of each rFVIII injection and the dosing frequency were constant for all the patients, whereas the average treatment duration may vary from person to person and a lognormal distribution was assumed. The risk of surgery, death, and production of FVIII inhibitors were not considered because of the low probability of these events occurring within a study duration of 1 year.
Utility Inputs
There is no related study to evaluate the health state utilities for Chinese patients with hemophilia A. The model used the average EQ-5D data of all patients with hemophilia in the study conducted by Neufeld et al. [29] to measure the utility value of patients in different periods; this showed that the health utility value of patients with hemophilia in the bleeding and non-bleeding periods was 0.64 and 0.84, respectively (Supplementary Table 3). In the model, it was assumed that patients’ health utility values were constant.
Cost Inputs
The study considered direct medical costs, including prophylaxis drug and bleeding treatment costs. The price of ADVATE® was 471.33 USD/1000 IU in yaozhi.com in 2020 and the price per unit (IU) was 0.47 USD [30]. According to the expert experience, in the treatment of patients with hemophilia A, the injection cost was very low because most of the patients preferred self-injection. Hence, the intravenous injection cost was not considered in this model. The model assumed that the price per unit was constant (Supplementary Table 4).
Data Analysis
In the base case analysis, total costs, AJBR, and QALYs were calculated for patients receiving standard prophylaxis and PK-guided prophylaxis. DES provides a random simulation of individual patients based on the distribution of the basic characteristics of patients and the probability of the occurrence of events. The occurrence and sequence of events were determined by random sampling.
Scenario analysis was also conducted to assess the stability of the model and reliability of the obtained results in different rFVIII unit price, single bleeding injection dose of rFVIII, utility values, and different fixed-dose prophylaxis treatment scenarios.
Results
Base Case Analysis Results
The basic analysis results showed that in the standard prophylaxis (30 IU/kg), about 8.2% of patients’ trough concentration was less than 1 IU/dL, and 29.1% of patients’ trough concentration was greater than 5 IU/dL. However, in PK-guided prophylaxis, trough concentrations of 94.3% of patients were 1–5 IU/dL; as a result of the individual dose adjustment. Not all the patients (100%) could reach the trough level of 1–5 IU/dL in the PK-guided individualized prophylaxis, because according to instructions of ADVATE®, the maximum single injection dose was set to 40 IU/kg. Under this circumstance, 4.6% of the patients had a high clearance, resulting in a dose of greater than 40 IU/kg when the trough concentration was equal to 1 IU/dL (Supplementary Fig. 2).
The results showed that the total dose (315,352 IU) and single dose (28 IU/kg) of PK-guided individualized prophylaxis were both lower than those of standard prophylaxis (338,645 IU and 30 IU/kg) (Table 2). The results also suggested that 34.5% of patients using PK-guided individualized prophylaxis had single injection dose of less than 30 IU/kg (Supplementary Table 5 ). However, the reduction in the dose of rFVIII prophylaxis injection did not result in an increase in bleeding events, and conversely, AJBR was slightly lower in PK-guided individualized prophylaxis (1.527) than standard prophylaxis (1.601) in the 1-year time horizon. Additionally, the proportion of AJBR greater than 12 times was also lower (Supplementary Fig. 3, 0.1% vs 0.4%) and consequently the total injection dose of rFVIII for treatment of bleeding was lower (9646 IU vs 10,085 IU) (Table 2).
Cost-Effectiveness Analysis Results
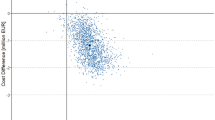
The rFVIII injection for prophylaxis cost of PK-guided individualized prophylaxis (148,641.47 USD) was lower than that of standard prophylaxis (159,620.93 USD). Additionally, the cost of rFVIII in the treatment of bleeding was also lower (4546.43 USD vs 4753.39 USD). Therefore, the total cost of PK-guided individualized prophylaxis was lower than that of standard prophylaxis (Table 3). In addition, the PK-guided prophylaxis had a slightly higher QALY than the standard prophylaxis (0.8384 vs 0.8383). The scatter diagram (Fig. 2) which was obtained by the simulation results of 10,000 patients also showed that in PK-guided prophylaxis, the total treatment cost of more than half the number of patients was lower than that in the standard prophylaxis.
Scenario Analysis
Scenario analysis (Table 4) showed that the changes in rFVIII price, bleeding treatment dose, utility values, and the limitation of prophylaxis injection dose had no obvious effect on the results. In some scenarios, PK-guided prophylaxis was preferable to standard prophylaxis with higher QALYs but lower costs. However, when setting the prophylaxis treatment scenario in a different regimen with respect to the base case (30 IU/kg/72 h and 20 IU/kg/48 h), PK-driven prophylaxis led to a reduction of AJBR with ICERs of 23,966,013.52 USD and 8,849,710.81 USD, respectively, which was higher than three times the per capita GDP in 2020 in China (per capita GDP was 10,779.04 USD in 2020).
Discussion
Patients with hemophilia have a higher mortality risk correlated with age and severity, when compared to the general population [31]. Multiple guidelines have recommended standard prophylaxis as the mainstay of treatment for severe hemophilia A [2, 24, 25], since regular FVIII injection can significantly reduce the number of bleeding events and prevent joint damage [9, 32], and improve the quality of life. However, complete eradication of bleeding events by fixed-dose prophylaxis could not be achieved [15, 16]. Furthermore, the high costs of hemophilia treatment have been one of the main factors hampering the introduction of prophylaxis in developing countries [33]. Increasing the prophylaxis injection dose for a successful management of bleeding events is not feasible because of the higher cost associated with it. In addition, as a result of the different pharmacokinetic curves of patients, fixed-dose prophylaxis is not suitable for all patients, which may lead to over- or underinjection, resulting in waste of resources or insufficient prevention [34]. Hence, PK-guided prophylaxis has gained importance as it customizes individual dosing regimen based on the individual PK parameters and is known to be better in effectiveness and cost [20, 21, 23].
The aim of the study was to simulate adult patients with severe hemophilia A and compare the prophylaxis cost, bleeding treatment cost, AJBR, and QALYs between standard prophylaxis and PK-guided prophylaxis in 1 year using the DES model. The DES model was chosen in our study owing to the advantages it offers, such as interpretation of individual entities (PK-guided individualized prophylaxis emphasizes injection dose adjustment at the individual level) [35], it deals with heterogeneity (the injection dose of FVIII in patients with hemophilia is affected by individual parameters, such as age, weight, PK parameters, etc.) [36], it is flexible in nature with no fixed period [37], and unlike other models, the occurrence of events is determined by random sampling, hence the uncertainty is fully considered [38].
Compared with standard prophylaxis, PK-guided individualized prophylaxis using myPKFiT® for patients showed a lower prophylaxis injection dose, consequently reducing the total cost (prophylaxis cost and bleeding treatment cost). The AJBR associated with the PK-guided prophylaxis was lower than standard prophylaxis and was associated with higher QALYs.
The results of cost-effectiveness analysis of this model were similar to the previously published economic evaluation of hemophilia treatment studies. Iannazzo et al. compared the cost-effectiveness analysis of PK-guided individualized prophylaxis with that of standard prophylaxis [20]. From the perspective of the Italian healthcare system, this study analyzed AJBR and direct medical cost under different treatment regimens. The study showed that when the prophylaxis injection dose of 30 IU/kg was injected once every 2 days, the PK-guided individualized prophylaxis had lower AJBR (0.845 vs 1.012) and total cost (€ 260,662 vs € 265,859) than standard prophylaxis. The AJBR observed in this study is slightly lower in PK-guided prophylaxis than the result of our study. The reason for the difference may be that the maximum single injection dose set in this model was 40 IU/kg even if the FVIII injection dose calculated by PK parameter was higher than 40 IU/kg. The standard was far lower than the 100 IU/kg set in the previous study by Iannazzo et al., hence some patients in this study may have had a higher AJBR due to lower injection dose. PK-guided prophylaxis has also shown benefits in children with hemophilia A. When comparing the direct and indirect costs of treatment with standard and PK-driven prophylaxis, the latter showed effectiveness with a total saving of € 54,797.40 (− 10.67%) proving PK-driven prophylaxis an effective option for children with hemophilia as well [39]. Besides, in a study by Li et al., eight patients aged 5–16 years with severe hemophilia A, who after a 6-month low-dose prophylaxis regimens (phase I) were given PK-tailored prophylaxis for the next 6 months (phase II), showed a reduction in median AJBR from 7.8 in phase I to 1.4 in phase II, an increase in median infusion frequency, and mean annual total factor consumption in phase II, but the FVIII consumption remained at approximately half the standard prophylaxis [40].
This study also has some limitations. Firstly, this model only simulates the efficacy and cost of patients with severe hemophilia A within 1 year. Therefore, only short-term results of different treatment methods are obtained and cannot be applied for long-term cost-effectiveness. Secondly, this model does not consider the formation of target joints. Since the number of annual bleedings after preventive treatment is small, the probability of target joint formation is low, and the model can only calculate the number of bleedings but cannot determine the specific location of bleeding. Hence, the occurrence of bleeding in the specific target joints cannot be ascertained. Thirdly, the model did not consider the wastage of drugs. Nevertheless, our study provides initial support for PK-guided dosing regimen using myPKFiT® in Chinese adult patients with severe hemophilia A.
Conclusions
PK-guided individualized prophylaxis appeared to be a dominant treatment compared with standard prophylaxis for adult patients with severe hemophilia A in China with slightly higher QALYs but lower total costs.
References
Samuelson Bannow B, Recht M, et al. Factor VIII: long-established role in haemophilia A and emerging evidence beyond haemostasis. Blood Rev. 2019;35:43–50.
Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(Suppl 6):1–158.
World Federation of Hemophilia. The WFH Report on the Annual Global Survey 2018-Hemophilia World News. https://news.wfh.org/now-available-the-wfh-report-on-the-annual-global-survey-2018/. Accessed 30 Mar 2020.
Dou X, Poon M-C, Yang R. Haemophilia care in China: achievements in the past decade. Haemophilia. 2020;26:759–67.
Ozelo MC, Matta MAP, Yang R. Meeting the challenges of haemophilia care and patient support in China and Brazil. Haemophilia. 2012;18(Suppl 5):33–8.
Miners A. Revisiting the cost-effectiveness of primary prophylaxis with clotting factor for the treatment of severe haemophilia A. Haemophilia. 2009;15:881–7.
Colombo GL, Di Matteo S, Mancuso ME, Santagostino E. Cost-utility analysis of prophylaxis versus treatment on demand in severe hemophilia A. Clin Outcomes Res. 2011;3:55–61.
Roosendaal G, Lafeber FP. Pathogenesis of haemophilic arthropathy. Haemophilia. 2006;12(Suppl 3):117–21.
Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44.
Nilsson IM, Berntorp E, Löfqvist T, Pettersson H. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25–32.
Gringeri A, Lundin B, von Mackensen S, Mantovani L, Mannucci PM, ESPRIT Study Group. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT Study). J Thromb Haemost. 2011;9:700–10.
Nilsson IM, Hedner U, Ahlberg A. Haemophilia prophylaxis in Sweden. Acta Paediatr Scand. 1976;65:129–35.
Pettersson H, Nilsson IM, Hedner U, Noréhn K, Ahlberg A. Radiologic evaluation of prophylaxis in severe haemophilia. Acta Paediatr Scand. 1981;70:565–70.
Ahnström J, Berntorp E, Lindvall K, Björkman S. A 6-year follow-up of dosing, coagulation factor levels and bleedings in relation to joint status in the prophylactic treatment of haemophilia. Haemophilia. 2004;10:689–97.
Kasper CK, Dietrich SL, Rapaport SI. Hemophilia prophylaxis with factor VIII concentrate. Arch Intern Med. 1970;125:1004–9.
Schimpf K, Fischer B, Rothmann P. Hemophilia A prophylaxis with factor VIII concentrate in a home-treatment program: a controlled study. Scand J Haematol Suppl. 1977;30:79–80.
Collins PW, Björkman S, Fischer K, et al. Factor VIII requirement to maintain a target plasma level in the prophylactic treatment of severe hemophilia A: influences of variance in pharmacokinetics and treatment regimens. J Thromb Haemost. 2010;8:269–75.
Li Z, Wu J, Zhao Y, Liu R, et al. Influence of medical insurance schemes and charity assistance projects on regular prophylaxis treatment of the boys with severe haemophilia A in China. Haemophilia. 2018;24:126–33.
Sun J, Zhao Y, Yang R, Guan T, Iorio A, Chinese HERO study group. The demographics, treatment characteristics and quality of life of adult people with haemophilia in China—results from the HERO study. Haemophilia. 2017;23:89–97.
Iannazzo S, Cortesi PA, Crea R, Steinitz K, Mantovani LG, Gringeri A. Cost-effectiveness analysis of pharmacokinetic-driven prophylaxis vs. standard prophylaxis in patients with severe haemophilia A. Blood Coagul Fibrinolysis. 2017;28:425–30.
Mingot-Castellano ME, Parra R, Núñez R, Martorell M. Improvement in clinical outcomes and replacement factor VIII use in patients with haemophilia A after factor VIII pharmacokinetic-guided prophylaxis based on Bayesian models with myPKFiT®. Haemophilia. 2018;24:e338–43.
Nagao A, Yeung CHT, Germini F, Suzuki T. Clinical outcomes in hemophilia A patients undergoing tailoring of prophylaxis based on population-based pharmacokinetic dosing. Thromb Res. 2019;173:79–84.
Pasca S, Zanon E. Savings without changing: how to use the MyPKfit® device to improve treatment strategies in a cohort of patients with haemophilia A. Thromb Res. 2019;183:1–3.
Thrombosis and Hemostasis Group, Chinese Society of Hematology, Chinese Medical Association/Hemophilia Treatment Center Collaborative Network of China. Chinese guidelines on the treatment of hemophilia (version 2020). Chin J Hematol. 2020;41:265–71. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7364913/. Accessed 11 Mar 2021.
Rayment R, Chalmers E, Forsyth K, et al. Guidelines on the use of prophylactic factor replacement for children and adults with haemophilia A and B. Br J Haematol. 2020;190:684–95.
Björkman S, Oh M, Spotts G, et al. Population pharmacokinetics of recombinant factor VIII: the relationships of pharmacokinetics to age and body weight. Blood. 2012;119:612–8.
den Uijl IEM, Fischer K, Van Der Bom JG, Grobbee DE, Rosendaal FR, Plug I. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17:41–4.
Chen B, Xia A, Yu K. Determination of pharmacokinetic parameters in adult patients with severe hemophilia A and the efficacy of individualized preventive treatment. J Math Med. 2019;32:1590–2.
Neufeld EJ, Recht M, Sabio H, et al. Effect of acute bleeding on daily quality of life assessments in patients with congenital hemophilia with inhibitors and their families: observations from the dosing observational study in hemophilia. Value Health. 2012;15:916–25.
Yaozhi.com drug bidding information query-Baiyinzhi. https://db.yaozh.com/yaopinzhongbiao?comprehensivesearchcontent=%E7%99%BE%E5%9B%A0%E6%AD%A2&. Accessed 23 Mar 2021.
Hay CRM, Nissen F, Pipe SW. Mortality in congenital hemophilia A—a systematic literature review. J Thromb Haemost. 2021;19(Suppl 1):6–20.
Iorio A, Marcucci M, Cheng J, et al. Patient data meta-analysis of Post-Authorization Safety Surveillance (PASS) studies of haemophilia A patients treated with rAHF-PFM. Haemophilia. 2014;20:777–83.
Schrijvers LH, Uitslager N, Schuurmans MJ, Fischer K. Barriers and motivators of adherence to prophylactic treatment in haemophilia: a systematic review. Haemophilia. 2013;19:355–61.
Megías-Vericat JE, Bonanad S, Haya S, et al. Bayesian pharmacokinetic-guided prophylaxis with recombinant factor VIII in severe or moderate haemophilia A. Thromb Res. 2019;174:151–62.
Caro JJ. Pharmacoeconomic analyses using discrete event simulation. Pharmacoeconomics. 2005;23:323–32.
Karnon J, Haji Ali Afzali H. When to use discrete event simulation (DES) for the economic evaluation of health technologies? A review and critique of the costs and benefits of DES. Pharmacoeconomics. 2014;32:547–58.
Caro JJ, Möller J, Getsios D. Discrete event simulation: the preferred technique for health economic evaluations? Value Health. 2010;13:1056–60.
Simpson KN, Strassburger A, Jones WJ, Dietz B, Rajagopalan R. Comparison of Markov model and discrete-event simulation techniques for HIV. Pharmacoeconomics. 2009;27:159–65.
Pasca S, Milan M, Sarolo L, Zanon E. PK-driven prophylaxis versus standard prophylaxis: when a tailored treatment may be a real and achievable cost-saving approach in children with severe hemophilia A. Thromb Res. 2017;157:58–63.
Li P, Chen Z, Cheng X, et al. PK-tailored tertiary prophylaxis in patients with severe hemophilia A at Beijing Children’s Hospital. Pediatr Investig. 2019;3:45–9.
Acknowledgements
Funding
Funding for this study and the Journal’s Rapid Service Fee were provided by Takeda Pharmaceutical Company Ltd.
Medical Writing/Editorial Assistance
We acknowledge medical writing assistance provided by Dr Sunita Rana and Dr Amit Bhat of Indegene Pvt Ltd. Support for this assistance was funded by Takeda Pharmaceutical Company Ltd.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
(I) Conception and design: Han Y, Gu C; (II) Administrative support: Huang H; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: Han Y, Gu C; (V) Data analysis and interpretation: Gu C, Han Y; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Prior Presentation
The study was presented at Virtual ISPOR EU 2021.
Disclosures
Congling Gu and Hui Huang are employees of Takeda International Trading Co. Ltd, Beijing, China. Yi Han has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gu, C., Huang, H. & Han, Y. Cost-Effectiveness Analysis of Pharmacokinetic-Guided Prophylaxis Versus Standard Prophylaxis in Adults with Severe Hemophilia A in China. Adv Ther 39, 3777–3788 (2022). https://doi.org/10.1007/s12325-022-02220-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02220-3