Abstract
Introduction
Atopic dermatitis (AD) is associated with significant quality-of-life and economic burdens. Real-world evidence is needed to identify optimal treatment pathways for AD. Here we evaluate real-world effectiveness of systemic therapies for moderate-to-severe AD in the USA.
Methods
Data (September 2016 to December 2019) were from the IQVIA Health Plan Claims data set (IQVIA, Danbury, CT) from patients aged 12 years or older with AD (ICD-9/10-CM, 691.8/L20.x) initiating a systemic immunosuppressive (SIS) agent (methotrexate, cyclosporine, mycophenolate, or azathioprine) or dupilumab and continuously enrolled for at least 6 months before and after the index date. Indicators of non-response (i.e., adding on/switching systemic therapy, AD-related inpatient/emergency room visits, or incident staphylococcal/streptococcal skin infection) and predictors of non-response were evaluated. Descriptive statistics and Kaplan–Meier rates and times were obtained; Cox regression models were used.
Results
In 3249 patients, 45.4% exhibited at least one indicator of non-response, with median time to non-response being longer for dupilumab than for any SIS therapy (27.0 vs 4.0–7.7 months, respectively). Key non-response predictors were age, geographic region, and baseline number of annual AD-related medical visits.
Conclusion
Non-response was common in patients with AD who required systemic treatment, and non-response indicators occurred significantly more frequently with SIS treatment than with dupilumab treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Moderate-to-severe atopic dermatitis, a chronic inflammatory skin disease, affects 6.6 million adults in the USA. |
Systemic therapies such as conventional immunosuppressive agents and the biologic agent dupilumab are recommended treatment options in adult patients with moderate-to-severe atopic dermatitis who do not respond to topical treatments or phototherapy. |
This study evaluated the real-world treatment effectiveness of current systemic therapies for atopic dermatitis based on specific indicators of non-response in US patients with moderate-to-severe disease who initiated these therapies. |
What was learned from the study? |
Indicators of non-response occurred significantly more frequently with conventional systemic immunosuppressive therapy than with dupilumab treatment; key predictors of non-response were age, geographic region, and baseline number of annual atopic dermatitis-related medical visits. |
Factors that predict non-response should be considered before initiation of systemic therapy in patients with moderate-to-severe atopic dermatitis. |
Introduction
Atopic dermatitis (AD), a common chronic inflammatory skin disease characterized by intense pruritus and eczematous lesions [1], is associated with significant impairment in health-related quality of life in patients and caregivers [2,3,4]. Worldwide, AD affects up to 23% of the pediatric population and 17% of the adult population each year [5], and its burden on patients, payers, and society is significant [2, 6, 7]. In 2017, it was estimated that moderate-to-severe AD would affect up to 6.6 million adults in the USA alone [8].
For adults with moderate-to-severe AD who do not respond adequately to topical treatments or phototherapy or following treatment with short courses of systemic corticosteroids (SCSs) as bridging therapy [9], the alternative treatment options are conventional systemic immunosuppressive (SIS) agents (methotrexate, cyclosporine, mycophenolate, azathioprine [10]) and the biologic agent dupilumab [11]. Although conventional SIS agents are not approved by the US Food and Drug Administration (FDA) [10], they are clinically recommended for a subset of patients with refractory or severe AD [9]. However, guidelines do not recommend long-term use of these agents because of the possibility of cumulative toxicity [10].
Because systemic therapies for AD are rapidly evolving, it is necessary to understand treatment effectiveness based on evidence that reflects real-world practice. This study was conducted to evaluate the real-world effectiveness of current systemic AD therapies, based on indicators of non-response among patients in the USA with moderate-to-severe AD who were newly initiating these therapies.
Methods
Study Design
We conducted a retrospective cohort analysis based on data from the IQVIA Health Plan Claims data set (IQVIA, Danbury, CT), which is composed of data from over 100 different health plans in the USA. Data were used from patients newly initiating systemic AD therapies from September 28, 2016 (6 months before FDA approval of dupilumab [12]) to December 31, 2019. The systemic AD treatments considered were based on American Academy of Dermatology clinical guidelines and reviews of treatments for moderate-to-severe AD [10, 13].
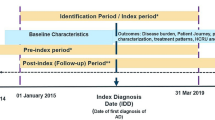
Three patient cohorts were examined: a SIS cohort, which included patients newly initiating (i.e., no claims in the baseline period) a SIS therapy (methotrexate, cyclosporine, mycophenolate, or azathioprine); a dupilumab cohort, which included patients newly initiating dupilumab therapy; and an overall cohort, which included all patients newly initiating either a SIS or dupilumab therapy. The index date was defined as the initiation date (i.e., date of the first claim) of systemic treatment (Fig. 1). The baseline period was defined as the 6-month period before the index date. The follow-up period was at least 6 months and spanned from the index date to the end of insurance eligibility or data availability, whichever occurred first.
Participants: Patient Inclusion and Exclusion Criteria
Patients were required to be aged at least 12 years and to have at least two claims for the same index systemic AD treatment (methotrexate, cyclosporine, mycophenolate, azathioprine, or dupilumab) by March 28, 2017, or later to coincide with the availability of dupilumab [12]. Patients were also required to have been continuously enrolled for at least 6 months before and after the index date and have been given two or more AD diagnoses (ICD-9/10-CM, 691.8/L20.x) during their enrollment, including one during the baseline period. Patients were excluded if they had any claims for any SIS or dupilumab during the baseline period. Data are de-identified and compliant with the Health Insurance Portability and Accountability Act (HIPAA). This study was an analysis of secondary data and was exempt from institutional review board approval.
Outcomes
Demographics and clinical characteristics were evaluated in the baseline period and/or on the index date. Indicators of non-response of patients to their index treatment in the follow-up period (excluding the index date) were also evaluated. In lieu of data on clinical response (e.g., at least 50% improvement in Eczema Area and Severity Index score), which are not available in a claims database, we considered non-response to be indicated by adding on or switching to a different moderate-to-severe AD treatment (i.e., dupilumab, SIS, SCS, and/or phototherapy), having an AD-related inpatient or emergency room (ER) visit, or having staphylococcal or group A streptococcal skin infection.
We also evaluated the annual frequency of medical visits and healthcare costs. Medical visits included inpatient admission; ER visits; outpatient visits and other visits that occurred during the follow-up period (excluding the index date) and that were calculated for all causes; and visits for AD-related, infection-related, and staphylococcal-related or group A streptococcal-related causes.
Analyses
Patient demographics and clinical characteristics were described using mean, standard deviation (SD), and median for continuous variables; frequencies and proportions were reported for categorical variables (overall and by index treatment) and compared between SIS index treatment and dupilumab cohorts using χ2 tests [14]. For each cohort, mean annual frequency of medical visits and mean annual costs were estimated by dividing the total number of visits as well as total costs in the follow-up period by the total number of follow-up years per patient, and then calculating the mean value across the cohort.
The proportion of patients with an indicator of non-response was reported for the overall group and by index treatment. Kaplan–Meier rates [with 95% confidence intervals (CIs)] provided estimates of the time to the earliest indicator of non-response across index treatments and were compared using log-rank tests [14, 15].
To identify predictors of non-response in patients new to systemic therapy, we explored the univariate associations between baseline factors and the indicators. Univariate Cox regression models were fitted, with the time to a non-response indicator defined as the dependent variable and baseline characteristics as independent variables. We then fitted three different multivariate Cox regression models for the overall sample and for the subgroup of patients initiating dupilumab [14, 15].
The multivariate models were model 1, which included clinically important variables; model 2, which included variables having p < 0.25 in univariate analyses; and model 3, which included variables having p < 0.05 in univariate analyses. Patient demographics and other baseline characteristics were included as covariates in all multivariate models (models 1–3). All variables in each model are listed in detail in Supplementary Table S1 (overall sample) and Supplementary Table S2 (dupilumab subgroup). Hazard ratios, 95% CIs, and p values are reported for each baseline covariate. We confirmed that the proportionality assumption was held through a visual assessment using Schoenfeld residuals and using an interaction term between time and the variables of interest [14, 15].
Results
Patient Demographics and Clinical Characteristics
In the overall cohort (n = 3249 patients), the mean age (SD) was 40.6 (± 16.1) years, and 54.2% of patients were female. The most prevalent comorbidities (Table 1 and Supplementary Table S3) were atopic conditions [35.5%; including allergic rhinitis (26.6%) and asthma (20.7%)] followed by psychological conditions (27.8%), uncomplicated hypertension (18.5%), infections (17.9%), and AD-related conditions (16.1%). Most patients initiated systemic AD treatment with dupilumab (n = 2455; 75.6%), and patients in this cohort were on average younger than those in the SIS cohort (39.8 years ± 15.5 vs 43.4 years ± 17.4; p < 0.001). Chronic inflammatory conditions were more prevalent in the SIS cohort than in the dupilumab cohort (24.7% vs 6.5%; p < 0.001) and were highest in the azathioprine (36.5%) and methotrexate (32.1%) cohorts (data not shown).
Baseline Treatments
Compared with patients in the dupilumab cohort, patients in the SIS cohort were more likely to have used SCSs (57.9% vs 48.3%; p < 0.001) or high-potency topical corticosteroids at baseline (49.2% vs 44.5%; p = 0.019) (Table 1).
Follow-Up Medical Visits and Healthcare Costs
During the follow-up period [median, 433 days; interquartile range (IQR) 287–632 for the overall population), outpatient visits were the most common type of medical visit (mean per year ± SD, 18.3 ± 20.8); most patients (99.5%) had at least one outpatient visit during the period (Supplementary Table S1). Mean per year all-cause healthcare costs were $34,483 ± 32,484 in the follow-up period (Supplementary Table S2).
Patients who initiated systemic therapy with any SIS (median follow-up, 477 days; IQR, 316–691) had a higher mean number of annual all-cause outpatient visits (21.6 ± 21.0 vs 17.3 ± 20.7) and ER visits (0.6 ± 2.9 vs 0.5 ± 1.1) (Supplementary Table S1) than patients in the dupilumab cohort (median follow-up, 412 days; IQR, 279–611), which translated into higher mean annual all-cause medical costs ($11,640 ± 32,293 vs $6038 ± 18,233, respectively). However, the dupilumab cohort had substantially higher mean annual AD-related total healthcare costs ($29,946 ± 12,048 vs $8633 ± 16,848), mostly composed of higher mean pharmacy costs ($29,392 ± 11,629 vs $6689 ± 10,539), than the SIS cohort (Supplementary Table S2).
Indicators of Treatment Non-Response
During follow-up, 45.4% of the overall cohort exhibited at least one indicator of non-response. Adding on/switching to another moderate-to-severe AD therapy (44.7%) was the most common indicator across all index treatments; the rate was highest for patients treated with mycophenolate mofetil (72.3%) and lowest for those treated with dupilumab (37.5%) (Fig. 2). Dupilumab had the lowest Kaplan–Meier rate of non-response at month 12 (35.4%) and the longest median time to non-response (27.0 months) compared with methotrexate (59.6%; median time to non-response, 7.7 months), cyclosporine (68.7%; median time to non-response, 5.6 months), mycophenolate (70.9%; median time to non-response, 4.0 months), and azathioprine (67.4%; median time to non-response, 5.8 months). Comparisons of non-response indicator rates for all other index treatments versus dupilumab were statistically significant at p < 0.001 (Fig. 3).
Predictors of Non-Response to Index Treatment
Results of the multivariate analysis that included only clinically important baseline characteristics (model 1) in the overall cohort are shown in Fig. 4 and Supplementary Table S4. The variables significantly (p < 0.05) associated with increased hazard ratios (95% CI) of non-response were age at index date [1.06 (1.02–1.09)]; residing in the South [1.44 (1.19–1.73)], the Northeast [1.31 (1.06–1.62)], or the Midwest [1.43 (1.17–1.75)] of the USA relative to the West; having respiratory conditions [1.52 (1.00–2.30)]; having an upper respiratory tract infection [1.32 (1.15–1.51)]; having received SCSs in the baseline period [1.67 (1.48–1.88)]; having received phototherapy in the baseline period [1.52 (1.20–1.93)]; number of all-cause ER visits per year [1.04 (1.02–1.06)]; and number of AD-related outpatient visits per year [1.01 (1.00–1.02)]. The only statistically significant factor associated with a lower hazard ratio of non-response was dupilumab versus a SIS agent as index treatment [0.46 (0.41–0.52)].
Forest plot of hazard ratios (95% CI) for predictors of indicators of non-response to index treatment (overall population and dupilumab subgroup). AD atopic dermatitis, CI confidence interval, ER emergency room, HMO health maintenance organization, I/T/CDHC/HAS/U Indemnity/Traditional/Consumer Directed Healthcare/Health Savings Account/Unknown, POS point of service, PPO preferred provider organization, SCS systemic corticosteroid, SIS systemic immunosuppressant. *p < 0.05, **p < 0.001
Results of the multivariate analysis for the dupilumab cohort that included only clinically important baseline characteristics are shown in Fig. 4 and Supplementary Table S5. As in the overall population, the variables significantly (p < 0.05) associated with an increased hazard ratio (95% CI) of non-response were age at index date [1.08 (1.04–1.13)], residing in the South [1.40 (1.08–1.81)] or the Northeast [1.40 (1.05–1.87)] of the USA relative to the West, having an upper respiratory tract infection [1.38 (1.16–1.63)], having received SCS agents in the baseline period [1.74 (1.50–2.01)], having received phototherapy in the baseline period [1.37 (1.03–1.82)], number of all-cause ER visits per year [1.06 (1.02–1.11)], and number of AD-related outpatient visits per year [1.01 (1.01–1.02)]. Results of the multivariate models 2 and 3 for the full cohort and the dupilumab cohort, based on variables that showed statistical significance in the univariate regressions, are listed in Supplementary Tables S4 and S5.
Discussion
This retrospective cohort study highlights the challenges of treating patients with AD who require systemic therapy. Some of these challenges include the high cost associated with treatment and the frequency of concomitant corticosteroid use. Additionally, almost half the patients in the overall cohort who newly initiated systemic AD therapies had an indicator of treatment non-response during follow-up; this proportion was larger among patients initiated on any SIS treatment than among patients initiated on dupilumab therapy. The median time to non-response for each of the SIS index treatment cohorts was also substantially shorter than that for the dupilumab cohort (4.0–7.7 months vs 27.0 months), with significantly higher rates of non-response indicators at all time points across the 24-month period.
As for baseline patient characteristics, the relatively larger proportions of patients with chronic inflammatory conditions in the SIS cohort must be interpreted with caution because the reason for prescribing a SIS therapy may not have been for the treatment of AD but for the treatment of other inflammatory conditions. However, it is likely that including patients with chronic inflammatory conditions did not impact the results of the current study because similar results were observed after a sensitivity analysis that excluded patients with baseline evidence of chronic inflammatory conditions (i.e., excluding patients for whom the index prescription may have been for chronic inflammatory conditions instead of AD).
We defined non-response as adding/switching AD treatment, having an AD-related medical visit, or having a skin infection. Lack of adherence or discontinuation was not considered a lack of response because of the intermittent nature of the use of these AD treatments in actual clinical practice.
We also identified key predictors of non-response. We observed that, across the overall and dupilumab cohorts, patients with respiratory conditions, with infections, or receiving SCSs or phototherapy at baseline had 32–74% higher hazard of an indicator of non-response. To the extent that the use of additional treatments at baseline represents more severe disease, these outcomes highlight the significance of AD severity as an indicator of non-response to systemic AD treatment. In addition, we found that regional differences were notable indicators of non-response. These differences may be explained through different population characteristics and climate conditions across regions. Analogously, in the overall cohort, dupilumab as index treatment was associated with a greater than 50% reduced hazard of non-response. Separately, univariate analysis indicated that dupilumab was associated with a lower mean number of annual medical visits than were SISs.
The factors of non-response identified herein may provide information to enhance the clinical treatment of patients with AD before initiating systemic therapy. AD treatment response rates have improved since the availability of dupilumab; the results of our study show that the rate of non-response was reduced by nearly 50% with dupilumab therapy compared with SIS agents, and patients remained on dupilumab treatment for a median of 27 months. Nevertheless, approximately 35% of patients who initiated dupilumab showed a non-response after 12 months. Variables associated with a significantly higher hazard of non-response and related to baseline treatment appear to be driven by a lack of efficacy, with add-ons or switches largely driving the difference in treatment non-response between index treatment cohorts. Time-on-therapy differences between index treatment cohorts, in addition to the lack of efficacy, could partly be explained by differences in safety.
Limitations
Our findings must be considered in light of the study limitations. We applied claims-based indicators as proxy variables to determine indicators of non-response; however, given the nature of the data, the reliability of these indicators could not be medically confirmed. The reasons for switching treatment are not always known and may be unrelated to response in many cases. For example, a patient may have had a flare that was resolved by brief treatment with cyclosporine, and then subsequently for other reasons used a different treatment for another flare; this would not be an indication of treatment failure with cyclosporine. Poor adherence to SIS therapies (or even to dupilumab or concomitant topical therapies) could have resulted in suboptimal disease control and thus impacted measures of non-response; however, we did not assess adherence in this study. Because SIS therapies are associated with a substantial risk of side effects, considerable patient monitoring is required [10]. A previous retrospective real-world study showed that fewer than one-third of patients with AD receiving SIS therapy persisted with treatment [16]. Additionally, the duration of follow-up achieved in this study (median, approximately 430 days) was constrained by time intervals imposed on the database query to obtain comparable follow-up between the SIS and dupilumab cohorts. Therefore, it is possible that rates and predictors of non-response could have been different with longer follow-up.
Another limitation is that AD is not always correctly identified using ICD codes. We have mitigated this limitation by incorporating both ICD codes and use of treatment (SIS therapy or dupilumab) [17], although the possibility of misclassification remains.
Some medications are not recorded in the claims database, such as over-the-counter medication or medication received during an inpatient stay. Hence, AD-related costs may have been underestimated. The current study was conducted to additionally assess only direct healthcare costs. Indirect costs, such as work productivity and health-related quality of life, were not included in the evaluation.
Finally, the generalizability of the current findings is limited to the populations represented within the database (i.e., insured US patients, with Medicaid and Medicare patients and those residing in the West representing only a small proportion) and to patients meeting the inclusion and exclusion criteria.
Conclusions
By evaluating baseline characteristics and applying indicators of non-response to a retrospective cohort of patients with moderate-to-severe AD, the current study showed the challenges of treating AD with available systemic treatments. Indicators of treatment non-response are frequent outcomes among patients new to systemic AD therapy. To enhance the treatment of patients with AD, the factors used to predict non-response identified in the current study may be considered before initiation of systemic therapy.
References
Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1.
Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM. The burden of atopic dermatitis in US adults: health care resource utilization data from the 2013 National Health and Wellness Survey. J Am Acad Dermatol. 2018;78(1):54–61.e1.
Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–7.
Kwatra SG, Gruben D, Fung S, DiBonaventura M. Psychosocial comorbidities and health status among adults with moderate-to-severe atopic dermatitis: a 2017 US national health and wellness survey analysis. Adv Ther. 2021;38(3):1627–37.
Bylund S, von Kobyletzki LB, Svalstedt M, Svensson A. Prevalence and incidence of atopic dermatitis: a systematic review. Acta Derm Venereol. 2020;100(12):adv00160.
Shrestha S, Miao R, Wang L, Chao J, Yuce H, Wei W. Burden of atopic dermatitis in the United States: analysis of healthcare claims data in the Commercial, Medicare, and Medi-Cal databases. Adv Ther. 2017;34(8):1989–2006.
Eichenfield LF, DiBonaventura M, Xenakis J, et al. Costs and treatment patterns among patients with atopic dermatitis using advanced therapies in the United States: analysis of a retrospective claims database. Dermatol Ther (Heidelb). 2020;10(4):791–806.
Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–90.
Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49–57.
Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327–49.
Fishbein AB, Silverberg JI, Wilson EJ, Ong PY. Update on atopic dermatitis: diagnosis, severity assessment, and treatment selection. J Allergy Clin Immunol Pract. 2020;8(1):91–101.
Dupixent (dupilumab) injection, for subcutaneous use in adults. Prescribing information. Regeneron. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf. Accessed 05 July 2022.
Gooderham MJ, Hong HC-H, Eshtiaghi P, Papp KA. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3):S28–36.
van Belle G, Fisher LD, Heagerty PJ, Lumley T. Biostatistics: a methodology for the health sciences. 2nd ed. Hoboken: Wiley; 2004.
Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Ann Rev Public Health. 1999;20(1):145–57.
Armstrong AW, Huang A, Wang L, et al. Real-world utilization patterns of systemic immunosuppressants among US adult patients with atopic dermatitis. PLoS ONE. 2019;14(1):e0210517.
Hsu DY, Dalal P, Sable KA, et al. Validation of International Classification of Disease Ninth Revision codes for atopic dermatitis. Allergy. 2017;72(7):1091–5.
Acknowledgements
Funding
This study was funded by Pfizer Inc., New York, NY, USA. The funding for the journal’s Rapid Service and Open Access Fees were sponsored by Pfizer Inc., New York, NY, USA.
Medical Writing and Editorial Assistance
Editorial/medical writing support under the guidance of the authors was provided by Gauri Saal, MA, and Jared Mackenzie, PhD, at ApotheCom, San Francisco, CA, USA, and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–4).
Author Contributions
Concept and design: MHL, BE, IF, MSD, JCC, DEM, MD. Acquisition, analysis, or interpretation of data: JJW, MHL, BE, IF, MSD, JCC, NY, CF, DEM, MD. Drafting of the article: JCC, CF, MD. All authors critically revised the article for important intellectual content. All authors had full access to all data in the study and agreed to be accountable for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Disclosures
Jashin J Wu is or has been an investigator, consultant, or speaker for AbbVie, Amgen, Eli Lilly, Janssen, Novartis; a consultant for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dr. Reddy's Laboratories, Eli Lilly, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Regeneron, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, and Zerigo Health; and a speaker for AbbVie, Amgen, Bausch Health, Novartis, Regeneron, Sanofi Genzyme, Sun Pharmaceutical, and UCB. Jashin J Wu was affiliated with the Dermatology Research and Education Foundation, Irvine, CA, USA, at the time the study was conducted (current affiliation: Department of Dermatology, University of Miami, Miller School of Medicine, Miami, FL, USA). Marie-Hélène Lafeuille, Bruno Edmond, Iman Fakih, and Mei Sheng Duh are employees of Analysis Group, which received research funding from Pfizer Inc. Joseph C. Cappelleri, Claire Feeney, Daniela E. Myers, and Marco DiBonaventura are employees and shareholders of Pfizer Inc. Natalie Yin was an employee of Pfizer Inc. at the time of the study (current affiliation: US Dermatology Partners Practice, Denver, CO, USA).
Compliance with Ethics Guidelines
Data are de-identified and compliant with the Health Insurance Portability and Accountability Act (HIPAA). This study was an analysis of secondary data and was exempt from institutional review board approval. Permission to publish analysis of the data was provided by IQVIA, the company owning the database.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wu, J.J., Lafeuille, MH., Emond, B. et al. Real-World Effectiveness of Newly Initiated Systemic Therapy for Atopic Dermatitis in the United States: A Claims Database Analysis. Adv Ther 39, 4157–4168 (2022). https://doi.org/10.1007/s12325-022-02197-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02197-z