Abstract
Introduction
Antimicrobial resistance is an urgent medical challenge. In this two-part study, we investigated the epidemiology and management of carbapenem non-susceptible (Carb-NS) Gram-negative bacteria (GNB) in the UK.
Methods
We conducted a retrospective review of data from UK hospitals (ten in part 1, nine in part 2). In part 1, epidemiological data were collected from patients hospitalised between April 2017 and March 2018 with any laboratory detection of Carb-NS GNB, encompassing both colonisation and infection. In part 2, diagnosis and management pathways in a randomly selected population of adults from part 1 with confirmed Carb-NS GNB infection were assessed. Data were obtained from a detailed medical chart review for ≥ 3 months from index (collection date of first positive Carb-NS GNB sample).
Results
Of 42,340 GNB isolates from 36,098 patients colonised/infected with GNB in part 1, 7% were Carb-NS. In 157 patients included in part 2, 234 GNB index samples were collected, of which 197 (82%) were Carb-NS (median number of Carb-NS pathogens per patient, 1; range 1–3). The most frequent Carb-NS isolates were Pseudomonas aeruginosa (36%), Stenotrophomonas maltophilia (29%) and Klebsiella pneumoniae (10%). Median length of hospitalisation was 34 days. Median time from index to appropriate therapy was 3 days, with empirical therapy initiated a median of 1 day before index. Carb-NS infection was believed to contribute to 21 (28%) of 76 deaths during the study.
Conclusions
This study highlights the high incidence of Carb-NS GNB colonisation and infection in the UK and the need for improved management of patients with Carb-NS GNB infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Despite an increasing burden of resistance to broad-spectrum antimicrobials, including carbapenems, high-quality data on the management of multidrug-resistant Gram-negative bacterial and carbapenem-resistant Gram-negative bacterial infections are lacking. |
What did the study ask?/What was the hypothesis of the study? |
This observational study aimed to investigate the epidemiology, diagnosis and management pathway for carbapenem-resistant Gram-negative bacterial infections in the UK, to help improve understanding of patient profiles, infection-related treatments, outcomes and resource use. |
What were the study outcomes/conclusions? |
Carbapenem resistance was present in 7% of Gram-negative isolates in this study, resulting in a substantial requirement for hospitalisation and a significant rate of mortality. |
What has been learned from the study? |
This study suggests a need to improve the management of carbapenem-resistant Gram-negative bacterial infections in the UK. |
Specific improvements include reducing the time to availability of susceptibility tests to reduce the time to prescribing appropriate antibiotic therapy and improving the availability of antibiotics that are effective against these infections. |
Introduction
Globally, rates of antimicrobial resistance continue to rise [1, 2]. The significant and increasing burden of multidrug-resistant (MDR) and extensively drug-resistant (XDR) clones of Gram-negative bacteria (GNB) that are resistant to broad-spectrum antimicrobials, such as carbapenems, represents a particular challenge [3]. All pathogens classified as critical priority by the World Health Organization, due to the urgency of need for new antibiotics, are carbapenem-resistant (CR) GNB species, specifically CR Pseudomonas aeruginosa, CR Acinetobacter baumannii and CR/extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales [4, 5]. The last two also feature on the US Centers for Disease Control and Prevention Urgent Threats list [6].
Colonisation with CR GNB is a significant risk factor for subsequent infection [7, 8]. Furthermore, patients colonised with CR GNB or ESBL-producing GNB can transmit these organisms to other patients [9]. Other risk factors for CR GNB infection include prior exposure to broad-spectrum antimicrobials (including carbapenems) [10, 11]. Infections with CR GNB are associated with considerable morbidity [4] as well as substantial excess use of hospital resources, which have high attributable costs [12, 13]. Furthermore, there is uncertainty regarding the optimal therapeutic strategy for treatment of CR GNB infections [14].
The government body, UK Health Security Agency (formerly Public Health England), implemented the English Surveillance Programme for Antimicrobial Utilisation and Resistance in 2013 to monitor and report trends in antimicrobial resistance [15]. Later, in 2018, the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party published guidance for the treatment of infections due to MDR GNB [16]. Despite these efforts, high-quality data on the management of MDR GNB and CR GNB infections are lacking, particularly with regard to the few effective treatment options available at the time of study, which have variable outcomes [16,17,18].
Fortunately, several new antibacterial agents have recently been approved or are in late-stage clinical development, including several β-lactam–β-lactamase inhibitors (ceftazidime–avibactam, ceftolozane–tazobactam, meropenem–vaborbactam, imipenem–cilastatin–relebactam and aztreonam–avibactam) and agents of other classes, including eravacycline and cefiderocol [17]. However, resistance has already emerged against many of these agents [15], and additional novel agents with activity against CR GNB are likely to be required [18].
The CARBAR study aims to investigate the epidemiology of CR GNB infections and to describe the diagnosis and management pathway for patients with such infections across Europe, to help improve understanding of patient profiles, infection-related treatments, outcomes and resource use. The study was conducted in four European countries: France, Italy, Spain and the UK. Here, we report data from the UK arm of the study (CARBAR UK), the first country for which data are available.
Methods
The CARBAR UK study was a retrospective observational study undertaken in 10 hospitals in the UK (Table 1). The study comprised two parts. Part 1 aimed to describe the epidemiology of GNB colonisation or infection in the UK, including carbapenem-non-susceptible (Carb-NS) strains. The aim of part 2 was to describe the diagnosis and management pathway for a randomly selected subset of patients with Carb-NS GNB infections identified in part 1. Pathogens of interest included A. baumannii (plus other Acinetobacter species [spp.]), P. aeruginosa (plus other Pseudomonas spp.), Stenotrophomonas maltophilia, and species within the Enterobacterales order [19]. GNB isolates were classified as Carb-NS if they were Stenotrophomonas spp. or if their susceptibility to meropenem, ertapenem, imipenem or doripenem was classified as ‘intermediate’ or ‘resistant’ by local laboratory methodology.
Part 1 of the study included data from patients admitted to hospital (defined as an overnight stay) between 1 April 2017 and 31 March 2018 who had a GNB infection or who underwent screening and were positive for colonisation with Carb-NS GNB, irrespective of age. Anonymised data (microbiology results, patient demographics and clinical data, where available) collected from patient health records were analysed. Part 2 of the study included data from adults (≥ 18 years of age) with confirmed infection due to Carb-NS GNB regardless of the organism and site of infection. Infection was defined as the presence of relevant symptoms by detailed medical chart review, combined with laboratory evidence of infection in a clinical sample other than a screening swab. Patients included in part 2 were randomly selected by a data analyst using random number generation applied to a list of patients (identified by unique study code only) who had already been included in part 1 and met the part 2 eligibility criteria. If any of the randomly selected patients for inclusion in part 2 only had colonisation (rather than infection) then they were not included and the next randomly selected patient was chosen. The causal relationship between the organism identified at index and the infection was determined by the treating physician on the basis of chart review. In patients in which two or more carbapenem-resistant organisms were identified, it was possible that the index organism was not the one causing infection (as the index date was the date of the first positive sample). Patients were followed from the date of their first positive Carb-NS GNB sample (index date) for up to 3 months or until death or discharge, whichever occurred first. Collected data included patient demographics and baseline characteristics, microbiology results, antibiotic treatment, outcomes and resource use. Appropriate antibiotic therapy was defined as treatment with an antibiotic to which the organism was susceptible. Empirical antibiotic therapy was defined as the earliest relevant antibiotic that the patient took as part of their treatment during the study period (i.e., before microbiological analysis was completed and susceptibility results were available to guide therapy). XDR was defined as resistance to all non-colistin antibiotics tested.
For part 1 of the study, there was no fixed sample size (the intention was to include all patients meeting the inclusion criteria). For part 2, a sample size of approximately 200 was anticipated. It was calculated that, for 200 patients, the width of the 95% confidence interval (CI) for the proportion of patients remaining on a treatment would be between 8% and 14%, and that the width of the 95% CI for the length of time until changing to the most effective treatment would be 0.52 for a mean of 4 days (with a sample size of 150, the latter CI width remained acceptable at 0.6 days). Data were analysed using descriptive statistics. Where data were missing for a certain variable, the number of patients or samples for whom data were missing are reported, and these patients or samples are not included in the denominator for any descriptive statistics for that variable. Details of the numbers of patients or samples for which data were missing are summarised per variable in Supplementary Table 1. Post hoc analyses were performed to assess the effects of (1) prescription of inappropriate antibiotics and (2) delayed antibiogram availability (3-day delay) on the length of hospital stay and mortality. Patients were classified as ‘prescribed resistant’ if they were prescribed an inappropriate antibiotic to which their infection was resistant (based on the results of the antibiogram) or ‘prescribed susceptible’ if prescribed appropriate antibiotics. Outcomes for each group were then compared using Kaplan–Meier curves with hazard ratios (HRs) calculated via Cox proportional hazards models. Length of hospital stay was calculated from date of hospital admission rather than index date, as patients may have received empirical therapy prior to the index date (defined as the date on which the first positive sample was collected).
Results
Part 1: Epidemiology of GNB Infection/Colonisation, Including Carb-NS GNB
In total, 36,098 patients colonised or infected with GNB were included from the 10 participating UK centres (Table 1). Patients were a mean of 59.9 years of age (standard deviation [SD] 24.0) and 60% were female (Supplementary Table 2). A total of 42,340 GNB isolates were cultured from these patients, of which 81% were Enterobacterales and 19% were non-fermenters. The most prevalent GNB species was Escherichia coli, representing 52% of all isolates, followed by P. aeruginosa (12%) and Klebsiella pneumoniae (9%).
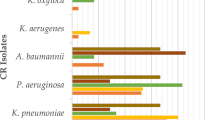
Of the 42,340 GNB isolates, 3094 (7%) were Carb-NS GNB (Table 1). The proportions of GNB isolates that were Carb-NS differed widely across hospitals, from 2% in Dundee to 37% in Birmingham. Amongst Carb-NS isolates, non-fermenters represented just over 50%. The most frequent species were P. aeruginosa (26%), E. coli (21%) and S. maltophilia (18%). The distribution of Enterobacterales and non-fermenters among the Carb-NS isolates differed among hospitals, with Enterobacterales predominating in Birmingham and Manchester. Combined, the five species of interest (E. coli, K. pneumoniae, P. aeruginosa, Acinetobacter spp. and S. maltophilia) represented 75% of all pathogens identified and 78% of the Carb-NS isolates. In the Carb-NS subset, isolates tested were predominantly from patients located in general medicine wards (12%) and in the intensive care unit (ICU; 11%).
Overall, there were 32,859 antibiotic sensitivity tests performed on Carb-NS isolates (n = 3094) across the 10 centres. Among the antibiotics tested most frequently (i.e., those for which > 1000 sensitivity tests were performed), susceptibility rates ranged from 30% for cefuroxime to 76% for amikacin and gentamicin (Supplementary Table 3). Among the carbapenems, meropenem had the highest susceptibility rate (55%).
Part 2: Management of Carb-NS GNB Infections
Patient Population
In part 2 of the study, 157 patients with Carb-NS GNB infection were selected from nine of the 10 hospitals that participated in part 1. Most of the participating UK centres contributed between 15 and 25 patients with Carb-NS infections. However, only four patients were included from Glasgow and 11 from Cardiff. Heartlands Hospital, Birmingham, was unable to provide data for part 2 of the study.
The mean age of patients included in part 2 of the study was 63.3 (SD 15) years (similar to the age of patients in part 1) and 38% were female (a lower percentage than in part 1). The most frequent primary diagnoses at hospital admission were respiratory disease (15%), cancer (10%), cardiovascular disease (10%), gastrointestinal disease (10%) and infectious diseases (10%). Impaired renal function was recorded for 47% of patients (68/144). For those with data available (n = 57), the mean estimated glomerular filtration rate was 57.1 mL/min/1.73 m2 (SD 90.0; median 38.0; range 7.0–697.0). At least one comorbidity was reported for 80% of patients (median 2; range 0–7) and the mean Charlson Comorbidity Index score was 3.1 (SD 2.7) (Table 2).
Very few patients (8%; n = 12) were known to have travelled internationally in the previous 12 months, nine of whom were known to have been hospitalised abroad (information was missing for the other three patients). A substantial number of patients had been admitted to another hospital (18%; n = 28) or a residential care/nursing home (2%; n = 3) prior to entering the study.
Treatment History
The 157 patients participating in part 2 of the study underwent 176 medical/surgical procedures in the 6 months prior to the index date, the most frequent of which were venous catheter placement (performed in 35 patients [22%]), abdominal surgery or grafts (20 patients [13%]) and urinary catheter placement (19 patients [12%]). The most frequently administered antibiotics in the 6 months prior to the index date were co-amoxiclav (administered to 52 patients [33%]), piperacillin/tazobactam (51 patients [32%]) and meropenem (47 patients [30%]).
In the 6 months prior to index, 85 patients (57%) had a history of a culture positive for GNB, with the most frequently identified pathogens being E. coli (identified in 31 patients [36%]) and P. aeruginosa (30 patients [35%]) followed by S. maltophilia (16 patients [19%]), K. pneumoniae (14 patients [16%]) and Citrobacter spp. (8 patients [9%]).
Microbiological Characteristics
The most frequent sample types obtained for microbiological analysis in patients included in part 2 were urine samples (52%; n = 81/157), followed by sputum (45%; n = 70/157), blood (43%; n = 67/157), rectal swabs (39%; n = 39/157) and wound swabs (36%; n = 57/157) (the total number of samples exceeded the total number of patients as multiple samples were taken in some patients). During the 3-month observation period, microbiological samples for the 157 patients were requested by different hospital departments and wards, with requests from the ICU (34%) and haematology department (13%) being the most frequent. Pathogen identification methods are provided in Supplementary Table 4.
A total of 234 isolates were identified from the 293 samples collected on the index date, of which 197 (84%) were Gram-negative and 162 were Carb-NS (Table 3). The number of Gram-negative pathogens per patient ranged from 1 to 3 (mean 1.3 [SD 0.5]; median 1), and the number of Carb-NS GNB per patient ranged from 1 to 2 (mean 1.1 [SD 0.2]; median 1). The most frequently isolated Gram-negative pathogens at index were P. aeruginosa (62 isolates, of which 94% [n = 58/62] were Carb-NS), S. maltophilia (47 isolates, all Carb-NS), E. coli (22 isolates, of which 41% [n = 9/22] were Carb-NS) and K. pneumoniae (22 isolates, of which 73% [n = 16/22] were Carb-NS). The mean number of GNB pathogens per patient was 1.3 (SD 0.5; median 1; range 1–3) and the mean number of Carb-NS GNB per patient was 1.1 (SD 0.2; median 1; range 1–2).
Of the GNB isolates recovered, 128/197 (65%) were regarded as the causal pathogen at index (of which 112/128 [88%] were Carb-NS isolates), 27/197 (14%) were regarded as not causal and 42/197 (21%) were regarded as of indeterminate causality. Of the Carb-NS isolates regarded as causal pathogens, the most frequent were P. aeruginosa (42%) and S. maltophilia (27%).
For the 157 patients in part 2, 3527 susceptibility tests were performed on Carb-NS GNB isolates during the study period. The most frequently tested antibiotics (> 5% of all sensitivity tests) were gentamicin (8%; n = 297/3527), meropenem (8%; n = 295/3527), ciprofloxacin (8%; n = 287/3527) and ceftazidime (8%; n = 270/3527). Susceptibility was highest with colistin (88% susceptible), followed by tobramycin (73%), amikacin (68%), gentamicin (67%) and trimethoprim/sulfamethoxazole (62%). Six isolates were considered to be XDR (Supplementary Table 5) including one A. baumannii isolate, three K. pneumoniae isolates and two S. maltophilia isolates (assumed to be Carb-NS based on intrinsic resistance). Overall, the XDR rate (per isolate) was 3%. Four isolates (all A. baumannii) were susceptible only to colistin.
Antibiotic Therapy
The median time from the index date until the availability of the first antimicrobial susceptibility results (antibiogram) was 4 days (interquartile range [IQR] 3–6; mean 4.8; n = 157). Similarly, the median time from the index date to the first antibiogram confirming susceptibility to the prescribed antibiotic was 4 days (IQR 3–6; mean 5.4; n = 152).
Empirical therapy (the first relevant antibiotic administered during the study period) was initiated a median of 1 day prior to the index date (IQR was from 5 days pre-index to 0 days pre-index [i.e., on the index date]; mean 5.4 days before the index date; n = 149). The antibiotics prescribed empirically are shown in Supplementary Table 6.
The median time from the index date to the initiation of appropriate antibiotic therapy was 3 days (IQR 0–7; mean 7.2; n = 105; Fig. 1). The median time from initiation of empirical therapy to initiation of appropriate antibiotic therapy was 3 days (IQR 0–11; mean 13.7; n = 105; Fig. 1).

aEmpirical therapy is the earliest relevant antibiotic that the patient was prescribed as part of their treatment during the study period. Some patients were administered an antibiotic before the index date (interquartile range 0–5 days prior to index date)
Median time to treatment milestones between the initiation of empirical therapy and the initiation of appropriate antibiotic therapy.
The median total duration of antibiotic therapy, from first empirical therapy to last recorded therapy, was 20.5 days (IQR 10–60.2; mean 113.8; n = 150). In total, 30% of patients received a combination of antibiotics as empirical treatment. A notable proportion of patients received a carbapenem (13%).
Patient Outcomes
The median overall length of hospital stay was 34 days (range 2–237; mean 47). Of the 118 patients (75%) who were discharged from hospital during the study period, the majority (78%) returned to their own home, while 16% were transferred to another hospital, 3% were discharged to residential care/nursing home and 3% to hospice care. Median length of ICU stay (59 patients) was 23 days (range 1–137; mean 29). For species recovered in ≥ 5 patients, the longest ICU stays were observed with Enterobacter spp. (median 29 days), followed by P. aeruginosa (median 28 days).
Of 113 patients with data available, 56 (50%; 36% of total population) were barrier-nursed in an isolation facility (i.e., side room or cohort bay) for infection-control purposes during the study period. Some of these patients required more than one episode of care in isolation. The median overall number of days in isolation per patient was 29.0 (range 0–293; mean 43.9) and the median number of days of isolation per episode (76 episodes) was 22.5 (range 1–293; mean 32.3). Isolation care was most frequently delivered in ICU facilities (27%).
Respiratory support (invasive or non-invasive) was required by 55 patients (35%) for a total of 64 episodes. Of these episodes, 38 (59%) required invasive ventilation and 24 (38%) required non-invasive ventilation. The median duration of ventilation per patient was 15 days (range 0–489).
Microbiological cure, as indicated by a test showing resolution of infection, was achieved in 33/37 patients (89%) with data available and occurred a median of 9 days (range 1–89) from the index date. Recurrent infection with the same causative pathogen within 30 days of discharge was recorded for 4% of patients (5/112), all of whom were infected with Carb-NS P. aeruginosa. At the end of the observation period, 76 patients had died (51%; n = 148; status unknown for the remaining nine patients). Of these, 54% (n = 41/76) occurred while the patient was in hospital and 46% (n = 35/76) occurred after discharge from hospital. Carb-NS GNB infection was considered to be at least a contributing factor in 21/76 (28%) deaths.
Post hoc analyses showed that administration of inappropriate antibiotics significantly increased the duration of hospital stay (HR 1.87; CI 1.35, 2.58), but had a non-significant effect on the time from hospital admission to death (HR 0.95; CI 0.62, 1.47), compared with administration of appropriate antibiotics (Fig. 2).
Discussion
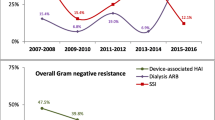
These real-world data provide valuable insights into the prevalence of Carb-NS GNB and current standards of care in the UK for the diagnosis and management of Carb-NS GNB infections. GNB infections appear to be frequent across the UK, with significant variations between hospitals. Overall, 7% of isolates were Carb-NS, representing a considerable proportion of infections and colonisations. Previous studies have demonstrated that CR rates are > 60% for non-fermenters and < 10% for Enterobacterales globally [20].
Of all Carb-NS isolates recovered, 35% were from one centre (Heartlands Hospital, Birmingham). Carb-NS isolates represented 39% of all isolates at this hospital, a rate approaching those seen in CR hotspots in southern and south-eastern Europe [21]. Without the Birmingham data, the proportion of Carb-NS isolates in our study was 5%. However, the overall impact of Heartlands Hospital data was limited by its lack of participation in part 2 of the study.
Approximately half of the pathogens that were Carb-NS were within the order Enterobacterales. The most frequent individual species overall were P. aeruginosa followed by E. coli; a significant proportion of isolates were S. maltophilia. In comparison, a US study found that the most prominent species associated with CR GNB infection (292,742 infections in total) are P. aeruginosa (60%) and A. baumannii (22%) [22].
The median time from index to confirmatory antibiotic susceptibility in this study was 4 days, which is similar to that reported previously (2–5 days) [23]. Culture-based techniques generally require 2–3 days to isolate and identify pathogens and perform phenotypic antimicrobial susceptibility tests. However, various factors may result in a delay in the availability of susceptibility test results, including delays in transportation of samples and laboratory inefficiencies. In addition, given the significant implications of a Carb-NS test result, additional confirmatory testing is often performed prior to result authorisation and communication to clinical staff. Novel, more rapid diagnostics have the potential to reduce delays, but such methods are expensive [24] and have not been widely adopted.
The time to availability of a susceptibility test (4 days) was longer than time to appropriate antibiotic therapy. In some institutions, a preliminary indication of a resistant organism may trigger a switch to a more appropriate agent before official availability of a susceptibility test. Poor clinical response may also lead to a switch of antibiotic therapy. The present results indicate that inappropriate antibiotic treatment had no significant effect on the time from admission to death, but that it increased the length of hospital stay versus appropriate therapy.
The most frequently used empirical antibiotics to treat the index infection were meropenem (11%), piperacillin/tazobactam (9%) and co-amoxiclav (9%), with 30% of patients receiving multiple agents. This pattern of prescribing reflects the desire for routine widespread coverage of potential MDR GNB, e.g., ESBL, while also taking account of other factors such as the severity of illness and the presence of risk factors for resistant infection. Notably, the susceptibility rate for individual carbapenems was moderately high (meropenem, 55%; imipenem, 40%), highlighting that cross-resistance amongst carbapenems is not complete.
Six isolates (3%) were considered to be XDR, including one A. baumannii isolate. This finding is encouraging, given the strong association between XDR and excess mortality [25]. However, the overall resistance rates documented here and elsewhere, coupled with the challenges posed when XDR is encountered, highlight the need for new antibiotics, ideally with new mechanisms of action.
CR GNB infections are associated with excess morbidity and mortality, as well as significantly increased resource use. Half of the patients died over the course of the study, and in 28% of deaths, Carb-NS GNB infection was considered a potential contributing factor. The healthcare costs associated with asymptomatic colonisation with CR GNB during a hospital outbreak have been shown to be up to six times higher than in patients without colonisation and are even higher in infected patients [26].
We consider the real-world setting as a strength of the current study, as the results are likely to be applicable to real-world clinical practice at hospitals beyond those included in the study. However, the study was not well controlled (there were many disparate variables), there was no comparator group and a larger number of patients would have been required to demonstrate a survival advantage. The retrospective observational design of the study is associated with risk of bias (e.g., due to lack of blinding in the collection, analysis or interpretation of the data). Part 2 of the study was based on a chart review, meaning that some data were incomplete and some were recorded inconsistently. In particular, the index organism was not always the causal organism, and as such, the data from part 2 represent overall trends and should be interpreted with appropriate caution. The study was conducted prior to the January 2020 European Committee on Antimicrobial Susceptibility Testing update replacing the ‘intermediate’ susceptibility category with ‘susceptible with increased exposure’ [27]. Under the revised guidance, a proportion of ‘carbapenem-intermediate’ isolates would have been classified as ‘carbapenem-susceptible’. A further limitation was that methods relevant to the study (e.g., criteria for choosing empirical antibiotic therapy, laboratory analyses for susceptibility testing) may have varied between the study centres.
Conclusions
Antimicrobial resistance, and particularly resistance to carbapenems, is an urgent challenge in the healthcare setting. This study provides an up-to-date view of Carb-NS GNB infections across the UK and suggests a need to improve the management of these infections. Specific improvements include reducing the time to availability of susceptibility tests to reduce the time to prescribing appropriate antibiotic therapy and improving the availability of antibiotics that are effective against these infections. These improvements may reduce durations of hospitalisation and improve other clinical outcomes.
References
Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist. 2019;12:3903–10. https://doi.org/10.2147/IDR.S234610.
Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115(15):E3463–70. https://doi.org/10.1073/pnas.1717295115.
Bassetti M, Peghin M, Vena A, Giacobbe DR. Treatment of infections due to MDR Gram-negative bacteria. Front Med (Lausanne). 2019;6:74. https://doi.org/10.3389/fmed.2019.00074.
World Health Organization (WHO).WHO publishes list of bacteria for which new antibiotics are urgently needed. 2017. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. Accessed 1 July 2020.
Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–27. https://doi.org/10.1016/S1473-3099(17)30753-3.
Centers for Disease Control and Prevention (CDC). Antibiotic resistance threats in the United States, 2019. 2019. https://stacks.cdc.gov/view/cdc/82532. Accessed 1 July 2020.
Khawcharoenporn T, Laichuthai W. Subsequent carbapenem-resistant Enterobacteriaceae (CRE)-associated infections among hospitalized patients with CRE colonization: impact of antibiotic use and other factors. Infect Control Hosp Epidemiol. 2020;41(9):1084–89. https://doi.org/10.1017/ice.2020.220.
Chen X, Liu Q, Liu WE, Yan Q. Risk factors for subsequential carbapenem-resistant Klebsiella pneumoniae clinical infection among rectal carriers with carbapenem-resistant Klebsiella pneumoniae. Infect Drug Resist. 2020;13:1299–305. https://doi.org/10.2147/IDR.S247101.
Alhmidi H, Cadnum JL, Koganti S, et al. Shedding of multidrug-resistant gram-negative bacilli by colonized patients during procedures and patient care activities. Am J Infect Control. 2020;48(11):1336–40. https://doi.org/10.1016/j.ajic.2020.06.004.
Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother. 2006;50(1):43–8. https://doi.org/10.1128/AAC.50.1.43-48.2006.
Richter SE, Miller L, Needleman J, et al. Risk factors for development of carbapenem resistance among Gram-negative rods. Open Forum Infect Dis. 2019;6(3):ofz027. https://doi.org/10.1093/ofid/ofz027.
Vargas-Alzate CA, Higuita-Gutiérrez LF, López-López L, Cienfuegos-Gallet AV, Jiménez Quiceno JN. High excess costs of infections caused by carbapenem-resistant Gram-negative bacilli in an endemic region. Int J Antimicrob Agents. 2018;51(4):601–7. https://doi.org/10.1016/j.ijantimicag.2017.12.012.
Merrick B, Tan MKI, Bisnauthsing K, Goldenberg SD. Healthcare resource use in hospitalized patients with carbapenem-resistant Gram-negative infections. J Hosp Infect. 2021;110:7–14. https://doi.org/10.1016/j.jhin.2020.12.021.
Lee CS, Doi Y. Therapy of infections due to carbapenem-resistant Gram-negative pathogens. Infect Chemother. 2014;46(3):149–64. https://doi.org/10.3947/ic.2014.46.3.149.
UK Health Security Agency. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR). Report 2019 to 2020. 2019. https://webarchive.nationalarchives.gov.uk/ukgwa/20211022024510/https://www.gov.uk/government/publications/english-surveillance-programme-antimicrobial-utilisation-and-resistance-espaur-report. Accessed 1 July 2020.
Hawkey PM, Warren RE, Livermore DM, et al. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother. 2018;73(suppl_3):iii2–78. https://doi.org/10.1093/jac/dky027.
Doi Y. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis. 2019;69(Suppl 7):S565–75. https://doi.org/10.1093/cid/ciz830.
Morris S, Cerceo E. Trends, epidemiology, and management of multi-drug resistant Gram-negative bacterial infections in the hospitalized setting. Antibiotics (Basel). 2020;9(4):196. https://doi.org/10.3390/antibiotics9040196.
Adeolu M, Alnajar S, Naushad S, Gupta RS. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int J Syst Evol Microbiol. 2016;66(12):5575–99. https://doi.org/10.1099/ijsem.0.001485.
Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in Gram-negative bacteria. Clin Infect Dis. 2019;69(Suppl 7):S521–8. https://doi.org/10.1093/cid/ciz824.
Brolund A, Lagerqvist N, Byfors S, et al. Worsening epidemiological situation of carbapenemase-producing Enterobacteriaceae in Europe, assessment by national experts from 37 countries, July 2018. Euro Surveill. 2019;24(9):1900123. https://doi.org/10.2807/1560-7917.ES.2019.24.9.1900123.
Cai B, Echols R, Magee G, et al. Prevalence of carbapenem-resistant Gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis. 2017;4(3):ofx176. https://doi.org/10.1093/ofid/ofx176.
Banerjee R, Humphries R. Clinical and laboratory considerations for the rapid detection of carbapenem-resistant Enterobacteriaceae. Virulence. 2017;8(4):427–39. https://doi.org/10.1080/21505594.2016.1185577.
Reyes S, Nicolau DP. Precision medicine for the diagnosis and treatment of carbapenem-resistant Enterobacterales: time to think from a different perspective. Expert Rev Anti Infect Ther. 2020;18(8):721–40. https://doi.org/10.1080/14787210.2020.1760844.
Kofteridis DP, Andrianaki AM, Maraki S, et al. Treatment pattern, prognostic factors, and outcome in patients with infection due to pan-drug-resistant gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 2020;39(5):965–70. https://doi.org/10.1007/s10096-019-03784-9.
Rodriguez-Acevedo AJ, Lee XJ, Elliott TM, Gordon LG. Hospitalization costs for patients colonized with carbapenemase-producing Enterobacterales during an Australian outbreak. J Hosp Infect. 2020;105(2):146–53. https://doi.org/10.1016/j.jhin.2020.03.009.
Kahlmeter G, Giske CG, Kirn TJ, Sharp SE. Point-counterpoint: differences between the European Committee on Antimicrobial Susceptibility Testing and Clinical and Laboratory Standards Institute recommendations for reporting antimicrobial susceptibility results. J Clin Microbiol. 2019;57(9):e01129–19. https://doi.org/10.1128/jcm.01129-19.
Acknowledgements
We thank the participants of this study. We would also like to thank Dr Abid Hussain from Heartlands Hospital, Birmingham, for his contribution to part 1 of this study, as well as Karen Bisnauthsing and Antonio Querol-Rubiera for assistance with data collection.
Funding
This study and the journal fees for Rapid Service and Open Access were funded by Shionogi Europe. Shionogi Europe were responsible for the design of the study, with data collected from the investigators on their behalf by OPEN VIE; Shionogi Europe funded the medical writing support used in manuscript development. Shionogi Europe contributed to discussions around where and when to publish the article, while the ultimate responsibility for these aspects lay with the manuscript authors.
Medical Writing/Editorial Assistance
Medical writing assistance was provided by Ben Caldwell of Axis, a division of Spirit Medical Communications Group Limited, an OPEN Health company, Manchester, UK, with funding from Shionogi Europe.
Author Contributions
SDG, DM, CL and SL conceived and designed the study; AM contributed to design of the data collection sheet; MA, GB, ARD, AM, DM, CM, SDG, DAE, CL, SL, BJP, APRW, KG and LJ collected, analysed and interpreted data and critically reviewed each draft of the manuscript.
Disclosures
Mahableshwar Albur received lecturing fees from Pfizer and Shionogi; Andrew R. Dodgson received fees for lectures and/or advisory boards from Eumedica, Menarini, MSD, Pfizer and Shionogi; Benjamin J. Parcell received payment from Shionogi to take part in this study; David A. Enoch has served on advisory boards for MSD, Pfizer and Shionogi; A. Peter R. Wilson was supported in part by the National Institute for Health Research University College London Hospitals Biomedical Research Centre; Lim Jones received grants from Shionogi, via pH Associates (trading as OPEN VIE), during the conduct of the study; Aleks Marek has nothing to disclose; Gavin Barlow received grants from Shionogi during the course of the study; Christianne Micallef received personal fees from Mundipharma and Pfizer, outside the submitted work; Simon D. Goldenberg received personal fees from Astellas, Enterobiotix, MSD, Pfizer, Shionogi and Tillotts outside the submitted work; Christopher Longshaw, Sara Lopes and Karan Gill are employees of Shionogi; Davide Manissero is a former employee of Shionogi.
Compliance with Ethics Guidelines
Ethical approval for the study was obtained via the Heath Research Authority system from an independent National Health Service Research Ethics Committee (reference 18/ES/0066) and from the Research and Development Department at each participating hospital.
Informed Consent
Part 1: as the data were collected by a member of the direct care team and anonymised prior to release to pH Associates (trading as OPEN VIE), no patient consent was required for this part of the study as patient confidentiality was maintained within the direct care team throughout, and no direct patient participation was required. Part 2: for living patients eligible for participation in part 2, patient informed consent was required where data were to be collected by an external researcher rather than the patient’s direct care team. Living patients were asked to provide their consent for access to their clinical records at one centre (Guy’s & St Thomas’ NHS Foundation Trust). In all cases, data for deceased patients were collected by the direct care team, to preserve patient confidentiality. No consent was sought from relatives of the deceased, to avoid causing a burden or distress to the recently bereaved.
Data Availability
The datasets generated during and/or analysed during the current study are not publicly available due to the need to protect patient anonymity. However, data analyses may be available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Manissero: Formerly of Shionogi Europe, London, UK
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Goldenberg, S.D., Dodgson, A.R., Barlow, G. et al. Epidemiology, Outcomes and Resource Utilisation in Patients with Carbapenem Non-susceptible Gram-Negative Bacteria in the UK: A Retrospective, Observational Study (CARBAR UK). Adv Ther 39, 3602–3615 (2022). https://doi.org/10.1007/s12325-022-02177-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02177-3