Abstract
Heart failure (HF) continues to increase in prevalence, representing a significant burden to healthcare systems in the USA. Despite several established HF therapies, particularly for HF with reduced ejection fraction (HFrEF), rates of HF hospitalizations and cardiovascular (CV) mortality remain very high. Type 2 diabetes (T2D) is an important risk factor for HF, with the two conditions often occurring concurrently. Several CV outcomes trials have shown that the sodium–glucose cotransporter 2 inhibitor (SGLT2i) class of antihyperglycemic drugs reduces the risk of HF-related outcomes in patients with T2D and either established CV disease or multiple CV risk factors. Subsequently, there have been large clinical studies that have investigated the effects of SGLT2is in patients with HFrEF, with or without T2D, which have shown that both dapagliflozin and empagliflozin have significant reductions in hospitalization for HF and CV mortality. These data led to US Food and Drug Administration approval of dapagliflozin and empagliflozin as a novel treatment pathway for patients with HFrEF; empagliflozin has subsequently been approved for the treatment of HF regardless of ejection fraction. A clinical practice algorithm can assist cardiologists in identifying patients who may be eligible for SGLT2i treatment as well as the appropriate timeframe for initiating therapy and the parameters for patient monitoring. Given the evidence that SGLT2is are beneficial in the management of HF, specifically HFrEF, irrespective of underlying T2D, evidence-based recommendations and greater clinician familiarity can facilitate the integration of SGLT2is into general HF therapeutic management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cardiovascular mortality and heart failure (HF) hospitalizations remain high for those with HF with reduced ejection fraction (HFrEF). |
New therapeutic options for HF are needed to improve outcomes. |
Sodium–glucose cotransporter 2 inhibitors (SGLT2is) beneficially affect HFrEF. |
SGLT2is improve HFrEF outcomes irrespective of type 2 diabetes status. |
Dapagliflozin and empagliflozin are US Food and Drug Administration approved for the management of HFrEF; empagliflozin is also approved for HF with preserved ejection fraction. |
Introduction
Heart failure (HF) affects more than 37.7 million adults worldwide and carries a substantial risk of morbidity and mortality [1, 2]. In the USA, National Health and Nutrition Examination Survey data (2013 to 2016) suggest that 6.2 million adults (at least 20 years old) have HF [3]; this number is projected to increase to more than eight million by 2030 [3, 4]. HF is the primary cause of more than one million hospitalizations annually in the USA, with approximately 25% of patients being readmitted or dying from cardiovascular (CV) causes within 6 months of discharge [5]. In 2017, one in eight deaths in the USA was attributed to HF [3]. Consequently, poor HF-related outcomes pose a significant burden to the US healthcare system with an estimated total healthcare cost of approximately $30.7 billion in 2012 and a projected increase up to $69.8 billion in 2030 [3]. Despite the availability and use of guideline-recommended medications, HF carries a poor prognosis and, therefore, new treatment approaches are required. Several large CV outcome trials have shown significant reductions in hospitalization for HF (HHF) during treatment with the glucose-lowering therapy sodium–glucose cotransporter 2 inhibitors (SGLT2is) in patients with type 2 diabetes (T2D) and established CV disease (CVD) or multiple CV risk factors [6,7,8,9,10,11]. Recently, evidence from placebo-controlled randomized clinical trials demonstrated that SGLT2is improve CV outcomes in patients who have HF with reduced ejection fraction (HFrEF) with or without T2D [12, 13]. This narrative review considers current guidelines for the treatment of HF, the mechanism of the hemodynamic and cardioprotective effects of SGLT2 inhibition, and clinical considerations when initiating therapy with SGLT2is for HF in practice.
Methods
Studies relevant to the topic of this narrative review article were identified by non-systematic literature searches of the PubMed database. Search terms were those related to SGLT2i therapy (“SGLT-2 inhibitor”, “sodium–glucose cotransporter-2 inhibitor”, “canagliflozin”, “dapagliflozin”, “empagliflozin”, “ertugliflozin”, “sotagliflozin”) and “heart failure”. The search was limited to publications in English published between July 2015 and November 2021 that reported clinical trials and meta-analyses of SGLT2is. Publications that reported CV and/or renal outcomes in patients treated with SGLT2is were included in this review.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by the author.
A New Pathway for the Treatment of HFrEF
Various treatment guidelines exist for the management of HF, and some have been modified recently to incorporate SGLT2i therapy. In 2019, the consensus recommendations and guidelines of the American College of Cardiology (ACC)/American Heart Association (AHA), the European Society of Cardiology (ESC)/European Association for the Study of Diabetes, and the American Diabetes Association pertinent to HF were updated to include SGLT2i therapy in patients with T2D and established atherosclerotic CVD [14,15,16]. Additionally, the ACC/AHA recommended the use of SGLT2is as a primary treatment for prevention of the development of HF in patients with T2D who are at risk [15].
More recently, the 2021 guidelines of the ESC and Heart Failure Association of the ESC recommend dapagliflozin and empagliflozin for the reduction of HHF and death in all patients with HFrEF [17]. These guidelines also recommend the use of SGLT2is in individuals with T2D and HFrEF to reduce HHF and CV death (dapagliflozin, empagliflozin, and sotagliflozin) and in patients with T2D who are at risk of CV events to reduce HHF, major CV events, end-stage kidney dysfunction, and CV death (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, and sotagliflozin) [17]. Further, the 2021 update to the Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment [18] recommends that the use of SGLT2is (dapagliflozin and empagliflozin) be considered as part of therapy for patients with and without T2D who have HFrEF and are already receiving β-blockers, an angiotensin receptor–neprilysin inhibitor (ARNi)/angiotensin-converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB), and/or aldosterone antagonists, if not contraindicated. The aforementioned recommendations were based on demonstrated benefits of SGLT2is on HF outcomes and indicate that the drug class constitutes a new therapeutic option for HFrEF.
SGLT2is in Patients with T2D: Summary of the CV Outcome Trials
The CV safety of SGLT2is has been assessed in several large CV outcome trials in patients with T2D and established CVD (empagliflozin [EMPA-REG OUTCOME] and ertugliflozin [VERTIS-CV]) as well as T2D and atherosclerotic CVD or multiple CVD risk factors (canagliflozin [CANVAS] and dapagliflozin [DECLARE–TIMI 58]) [7, 10, 11, 19]. The study designs and patient populations of these CV outcome trials varied (Fig. 1a) [7, 10, 11, 19], and these differences most likely had an effect on the individual study results, but a consistent finding was a significant reduction in HHF compared with placebo [7, 10, 11, 19].
Summary of HF-related outcomes observed in trials with SGLT2is in a patients with T2D [7, 10, 11, 19] and b patients with HFrEF [12, 13, 22, 30]. CI confidence interval, CV cardiovascular, CVD cardiovascular disease, HF heart failure, HFpEF HF with preserved ejection fraction, HFrEF HF with reduced ejection fraction, HHF hospitalization for HF, HR hazard ratio, MACE major adverse CV events (CV death, non-fatal myocardial infarction, or non-fatal stroke), NNT number needed to treat, NR not reported, NYHA New York Heart Association, PBO placebo, SGLT2i sodium–glucose cotransporter 2 inhibitor, SOTA sotagliflozin, T2D type 2 diabetes. *Primary study end point; †Secondary or other end points; ‡Defined as an unplanned hospitalization for HF or an urgent visit resulting in intravenous therapy for heart failure; §Defined in SOLOIST-WHF as hospitalizations and urgent visits for HF
The EMPA-REG OUTCOME and CANVAS studies found a significant reduction (14%) in the relative risk of 3-point major adverse CV event (MACE) with empagliflozin and canagliflozin, respectively, compared with placebo in patients with T2D and established CVD (EMPA-REG OUTCOME) or high CV risk (CANVAS) (Fig. 1a) [7, 10]. Significant reductions in the risk of HHF of 35% with empagliflozin and of 33% with canagliflozin were also observed [6,7,8, 10]. The DECLARE–TIMI 58 study (patients with T2D and a majority at high CV risk; thus, this was a study of the “primary prevention” of HF) and VERTIS-CV study (patients with established CVD and T2D) found that dapagliflozin and ertugliflozin were noninferior to placebo for reducing the risk of the composite 3-point MACE end point, but both agents significantly reduced the risk of HHF (by 27% and 30%, respectively) as well as the risk of the composite of HHF or CV death (by 17% and 12%, respectively) (Fig. 1a) [11, 19].
A meta-analysis of data from the EMPA-REG OUTCOME, CANVAS, and DECLARE–TIMI 58 trials found that irrespective of atherosclerotic CVD or history of HF at baseline, treatment with SGLT2is was associated with significant reductions in the risk of HHF or CV death (by 23%) and the risk of HHF (by 31%) [9].
SGLT2is in Patients with HFrEF Regardless of T2D Status
The marked effects of SGLT2is on HF outcomes in CV outcome trials, in which all patients had T2D by definition, led researchers to speculate whether these agents have a benefit in patients with HFrEF without T2D. Therefore, the effects of SGLT2is on CV outcomes among patients with and without T2D are being actively investigated, with published data available from the DAPA-HF [12] and EMPEROR-Reduced [13] studies (Fig. 1b).
DAPA-HF and EMPEROR-Reduced were both randomized, placebo-controlled studies (median follow-up 16–18 months) that evaluated the efficacy and safety of SGLT2is (dapagliflozin and empagliflozin, respectively) plus standard HF therapy at baseline in patients with HFrEF (left ventricular [LV] ejection fraction [LVEF] ≤ 40%; New York Heart Association [NYHA] class II–IV) [12, 13, 20]. Both studies included a high proportion of patients without T2D (58% in DAPA-HF and 50% in EMPEROR-Reduced) [12, 13]. The results of both studies were remarkably consistent. In DAPA-HF, the relative risk for the primary outcome, a composite of worsening HF (defined as an unplanned hospitalization or an urgent visit resulting in intravenous therapy for HF) or CV death, was reduced by 26% with dapagliflozin versus placebo (P < 0.001), and the secondary outcome of HHF or CV death was reduced by 25% (P < 0.001) (Fig. 1b) [12]. Similarly, the risk of HHF or CV death was 25% lower with empagliflozin than with placebo in the EMPEROR-Reduced study (P < 0.001) [13]. Both dapagliflozin and empagliflozin reduced the risk of HHF by approximately 30% (both P < 0.001) [12, 13]. The risk of CV death significantly decreased by 18% with dapagliflozin in the DAPA-HF study (hazard ratio [HR] 0.82 [95% confidence interval (CI) 0.69–0.98]) [12]. In both studies, the effects of SGLT2is on these outcomes were observed early in the course of treatment (i.e., early separation from placebo) and were similar between patients with and without T2D, suggesting a nonglycemic mechanism leading to improved CV outcomes [12, 13].
Baseline characteristics in both DAPA-HF and EMPEROR-Reduced were very similar. The mean LVEF in DAPA-HF was 31% vs 27% in EMPEROR-Reduced [12, 13]. The mean estimated glomerular filtration rate (eGFR) was 66 and 62 mL/min/1.73 m2 in DAPA-HF and EMPEROR-Reduced, respectively [12, 13]. Though patients with LVEF < 40% and NYHA class II–IV HF were enrolled, the majority of patients in both trials had NYHA class II HF. A subsequent meta-analysis of data from these studies demonstrated that both dapagliflozin and empagliflozin have a significant effect on the composite end point of HHF or CV death in a range of patient subgroups: with or without T2D, body mass index < 30 or ≥ 30 kg/m2, eGFR < 60 or ≥ 60 mL/min/1.73 m2, with or without a history of HHF, aged ≤ 65 or > 65 years, men or women [21].
Further evidence of the benefit of SGLT2i therapy in patients with HF has come from the SOLOIST-WHF study (Fig. 1b) [22]. In this study, patients with T2D, recently hospitalized for worsening HF, were randomized to receive sotagliflozin or placebo. Patients had HFrEF (LVEF < 50%) or HF with preserved LVEF (LVEF ≥ 50%; HFpEF) and received prior treatment with an intravenous diuretic [22]. The trial was terminated early because of loss of funding from the sponsor, which resulted in the primary and secondary end points being amended. The revised primary end point of total number of deaths from CV causes and HHF and urgent visits for HF was reduced by 33% in patients receiving sotagliflozin (P < 0.001), with effects seen both in patients with HFrEF (HR 0.72 [95% CI 0.56–0.94]) and HFpEF (HR 0.48 [95% CI 0.27–0.86]). Sotagliflozin also significantly reduced the first secondary end point in the hierarchy (total number of HHF and urgent visits for HF) but had no effect on other secondary end points [22].
On the basis of the findings from the DAPA-HF study, dapagliflozin became the first SGLT2i to be approved in the USA as a treatment in adults with HFrEF (NYHA class II–IV HF) to reduce the risk of CV death and HHF [23]. More recently, on the basis of data from the EMPEROR-Reduced study, empagliflozin received US Food and Drug Administration (FDA) approval for use in adult patients with HFrEF (NYHA class II–IV HF) to reduce the risk of CV death and HHF [24]. Empagliflozin has since been approved for the treatment of HFpEF on the basis of the results of the EMPEROR-Preserved study [24].
Mitigation of potential risk factors for HF, such as chronic kidney disease (CKD), is an important consideration when choosing treatment interventions for HF; the renal protective effects of SGLT2is can serve as the basis for their use in patients with renal impairment [25]. These effects were investigated in several studies including the aforementioned EMPEROR-Reduced study in patients with HFrEF as well as the CREDENCE (canagliflozin; in patients with T2D), DAPA-CKD (dapagliflozin; in patients with or without T2D), and SCORED (sotagliflozin; in patients with T2D) studies in patients with baseline CKD [13, 26,27,28].
Of the three studies in patients with baseline CKD, DAPA-CKD was the only one to include patients without T2D. In this randomized, double-blind study of patients with CKD, approximately one-third of whom did not have T2D, the effect of dapagliflozin on renal and CV outcomes was compared with that of placebo [26]. The primary outcome was a composite of a sustained decline in eGFR by at least 50%, onset of end-stage kidney disease, or death from renal or CV causes, and the CV secondary outcome was a composite of HHF and CV death [26]. Dapagliflozin significantly reduced the risk of the primary outcome by 39% (P < 0.001) as well as the risk of HHF or CV death by 29% (P = 0.009). Interestingly, the effect of dapagliflozin on the primary end point was even more marked in patients without T2D (reduced risk by 50%) than in patients with T2D (reduced risk by 36%) [26]. Renal outcomes and CV mortality are the focus of the EMPA-KIDNEY study, which is looking at empagliflozin in patients with CKD. Data from these studies may contribute to the body of clinical evidence for SGLT2i therapy in patients with HF. Additionally, the National Kidney Foundation advocates the use of SGLT2is in appropriate patients to improve renal and CV outcomes [29].
Ongoing Clinical Trials
Several clinical trials currently investigating SGLT2i treatment effects on HF and CV and renal outcomes have application to clinical practice in specific patient populations. For example, the DELIVER study with dapagliflozin is investigating effects on CV and HF outcomes in patients with HFpEF and will add to the data from SOLOIST-WHF [22] and EMPEROR-Preserved [30] that indicate that SGLT2is may be useful approaches to managing this subtype of HF, which presently has few therapeutic options available. Data from these studies may help confirm the beneficial effects of SGLT2is on clinical outcomes in patients with HF and/or CKD and contribute to the growing body of clinical evidence for SGLT2i therapy in patients with HF. Ongoing dapagliflozin (DAPA ACT HF-TIMI 68, DICTATE-AHF) studies are also assessing use in the acute HF inpatient population, adding to the data from SOLOIST-WHF and EMPA-RESPONSE-AHF that suggest that SGLT2i initiation is safe and beneficial during acute HF hospitalization (HFrEF and HFpEF) once the patient has been stabilized.
Mechanisms Underlying the Cardioprotective Effects of SGLT2is on HF
SGLT2is decrease plasma glucose levels by reducing the renal glucose reabsorptive capacity in the proximal tubule thereby resulting in glucosuria [31]. This mechanism of action is insulin independent and does not increase the risk of hypoglycemia [31]. In addition to reductions in glycated hemoglobin, fasting plasma glucose, and postprandial plasma glucose [32,33,34,35], SGLT2is’ effects on glucosuria have also been associated with weight loss and reduced body fat [36]. In addition to these metabolic improvements, reductions in blood pressure (BP) and beneficial effects on CV and renal complications were observed with SGLT2is in patients with T2D [32,33,34,35].
Mounting evidence from CV outcome trials and real-world studies indicates that SGLT2is have cardioprotective effects that improve HF outcomes, and this can be attributed to multiple mechanisms (Fig. 2) [25, 37]. Inhibition of the SGLT2 receptor in the proximal tubule, in addition to blocking glucose reabsorption, also blocks reabsorption of sodium and therefore leads to an osmotic diuresis and natriuresis, resulting in reductions in both preload and afterload [38]. The BP-lowering effects of SGLT2is are out of proportion with their diuretic effect, and animal studies have shown that this drug class also leads to a downregulation of the sympathetic nervous system that is thought to play an important role in the cardio- and renoprotective benefits [39, 40].
A reduction in cardiac remodeling has been attributed to inhibition of the myocardial sodium–hydrogen exchanger by SGLT2is [41]. SGLT2i-related effects on mitochondrial calcium influx are associated with reductions in LV hypertrophy and cardiac remodeling, which have the potential to mitigate systolic dysfunction [42, 43]. These improvements in ventricular loading are thought to be mediated by the changes in preload and afterload that occur with osmotic diuresis and natriuresis, which can lead to reductions in BP, intravascular volume, and arterial stiffness (a predictor of HF outcome) [44,45,46,47]. Reductions in BP have been observed without any parallel changes in heart rate; thus, other factors are thought to impact BP changes, such as non-fluid body weight reductions and direct vascular effects [25]. However, in patients with symptomatic HF and T2D, no reversal in LV remodeling was observed following treatment with the SGLT2i dapagliflozin, suggesting that other mechanisms are responsible for improved HF outcomes [48].
SGLT2is are also associated with conversion of the primary myocardial energy substrate from glucose to fatty acids, resulting in increased ketone body formation, which may have anti-arrhythmogenic effects [45]. The SGLT2i-related increase in β-hydroxybutyrate levels may shift cardiomyocyte fuel utilization from fatty acids and glucose toward the more fuel-efficient ketones, thereby lowering oxygen consumption and improving myocardial function [25, 38]. In addition, SGLT2is may affect adenosine triphosphate utilization, resulting in improved myocardial energetics, which is thought to affect LV remodeling [49].
Considerations for the Use of SGLT2is in Clinical Practice for HFrEF
Factors to Consider for the Integration of SGLT2is into the HF Treatment Plan
Although treatment algorithms and standards of care for the treatment of HF are well established, the prognosis of HF is still poor, and there is an unmet need for effective preventative therapy [50]. On the basis of data from CV outcome trials and, more recently, studies in patients with HF regardless of the presence of T2D, the guidelines and consensus recommendations of the ACC, AHA, and ESC now endorse the use of specific SGLT2is in patients with T2D and with established CVD or at risk of developing CVD, as well as in almost all patients with HFrEF [14,15,16,17,18, 51].
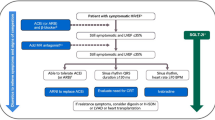
Four out of five HFrEF patients would be eligible for SGLT2i initiation [52]. Currently, dapagliflozin and empagliflozin are the only SGLT2is approved in the USA for use in this patient population [23, 24]. With increasing data supporting the use of this drug class as a key pathway in the treatment of HFrEF, more cardiologists need to become comfortable using SGLT2is in clinical practice. Figure 3 provides a potential treatment algorithm, which incorporates patient assessment, initiation of treatment with SGLT2is, and patient monitoring, for clinicians to use to integrate SGLT2is into their clinical practice for HF [23, 24, 37, 53,54,55]. In both the DAPA-HF and EMPEROR-Reduced studies, all enrolled patients had HFrEF with LVEF < 40%, NYHA class II–IV symptoms, elevated N-terminal pro-brain natriuretic peptide, and very good background medical therapy including beta-blockers, ACEis/ARBs/ARNis, and mineralocorticoid receptor antagonists (MRAs) [12, 13]. The eGFR cutoffs in the studies were < 30 mL/min/1.73 m2 for dapagliflozin and < 20 mL/min/1.73 m2 for empagliflozin, though the mean eGFRs in the two trials were 66 mL/min/1.73 m2 and 62 mL/min/1.73 m2, respectively [12, 13].
Treatment algorithm: prescribing SGLT2is for HFrEF in clinical practice by cardiologists: patient assessment, treatment initiation, monitoring, and patient counseling [23, 24, 37, 53,54,55]. Modified from Vardeny O, Vaduganathan M. Practical guide to prescribing sodium-glucose cotransporter 2 inhibitors for cardiologists. JACC Heart Fail 2019;7:169–172, ©2019, with permission from Elsevier Inc. DKA diabetic ketoacidosis, eGFR estimated glomerular filtration rate, HCP healthcare provider, HFrEF heart failure with reduced ejection fraction, LVEF left ventricular ejection fraction, NYHA New York Heart Association, QD once daily, SGLT2i sodium–glucose cotransporter 2 inhibitor, T2D type 2 diabetes
Specific Safety Considerations
SGLT2is are generally well tolerated and considered to have a favorable risk–benefit profile [56]; however, with the introduction of any new therapeutic agent, such as SGLT2is, into a patient’s treatment plan, the physician should take into consideration adverse events (AEs) sometimes seen in SGLT2i users (Table 1) [17, 18, 51, 53]. Patients with underlying comorbidities and concomitant medications should be monitored for the following serious (although rare) AEs: volume depletion, renal injury, and diabetic ketoacidosis (DKA) [17, 53]. Baseline medications are also an important consideration, particularly agents that have a diuretic effect [57, 58], or, in the patients with comorbid T2D, those agents that can precipitate hypoglycemia if used in conjunction with SGLT2is (i.e., insulin and sulfonylureas) [58].
The concomitant effects on diuresis can be a concern, especially when SGLT2is are prescribed in combination with ACEis/ARBs/ARNis, MRAs, and/or loop diuretics [44, 56], which may necessitate dose adjustments of loop diuretics depending on the patient’s volume status at the time of initiation [57]. However, a sub-analysis of the CANVAS study of canagliflozin found that a greater benefit on MACE outcomes was seen in patients receiving diuretics versus those not receiving these drugs [59]. If volume depletion or hypotension is considered a risk in a patient receiving concomitant SGLT2i and diuretic therapies, consideration should be given to dose reduction or cessation of diuretic therapy, similar to how one would initiate an ARNi in clinical practice [58].
Genitourinary infections, including urinary tract infections and genital mycotic infections, are attributed to glucosuria and are one of the most common AEs of SGLT2is. Several clinical trials have shown that urinary tract infections were not significantly increased with SGLT2is compared with placebo, whereas the incidence of genital mycotic infections was three- to fourfold higher with SGLT2is [58]. These genitourinary infections are usually mild and easy to treat. Physicians can assist patients in mitigating the risk of these infections by stressing the importance of personal hygiene [58].
SGLT2is have been associated with a reduction in the eGFR over the initial 1–4 weeks of treatment as a result of hemodynamic changes in the glomerulus; however, this is usually a transient effect, with the eGFR normalizing to a stable level within 1–3 months [60]. Sufficient data now support the renoprotective effects of SGLT2is, including data from the CREDENCE, DAPA-CKD, SCORED, and EMPEROR-Reduced trials [13, 26,27,28].
SGLT2is have a very low risk of hypoglycemia, which is limited almost exclusively to patients with T2D on background glucose-lowering therapy, such as a sulfonylurea or insulin [58]. Consequently, patients with T2D may require adjustments in insulin or sulfonylurea dosage when initiating SGLT2i therapy [58]. The risk of major hypoglycemia (i.e., hypoglycemia requiring assistance) with dapagliflozin in patients with HF appears to be limited to those with T2D, because no major hypoglycemic events were observed in patients without T2D in the DAPA-HF study and a subsequent exploratory analysis thereof [12, 26, 61]. The incidences of hypoglycemic events (defined as a plasma glucose level ≤ 70 mg/dL or events requiring assistance) in the EMPEROR-Reduced study in patients with HFrEF was 2.2% in empagliflozin-treated patients with T2D and 0.7% in those without T2D [13]. Similarly, the incidences of severe hypoglycemic events (defined as those requiring assistance) were 0.6% and 0.0%, respectively [62].
To mitigate the risk of DKA when initiating SGLT2i treatment, minor reductions to the insulin dosage can be made; in cases of acute illness, patients should be monitored and if acidosis worsens, consideration should be given to temporarily interrupting SGLT2i therapy [17, 18, 58, 63]. Prior to initiating treatment with an SGLT2i, clinical conditions known to predispose to an increased risk for DKA should be resolved [17, 18, 58, 63]. The phenomenon known as euglycemic DKA has been reported in SGLT2i-treated patients, but it may go undiagnosed as it presents with normal or slightly elevated blood glucose; consequently, serum ketones or urine ketones need to be assessed in SGLT2i-treated patients who exhibit DKA symptoms [64].
Patient Case Study
The following patient case has been developed to illustrate the use of SGLT2i therapy for a patient with HFrEF. Patient A.K. (Table 2) has HFrEF and is clinically stable, receiving optimal guideline-recommended HF treatments (ARNi, β-blocker, aldosterone antagonist, and furosemide). Following a review of A.K.’s medical history, including an assessment of kidney function and volume status (Fig. 3), A.K.’s cardiologist decided to initiate treatment with the SGLT2i dapagliflozin 10 mg once daily to further reduce the risk of HHF and CV death in this patient with HFrEF. Patient A.K.’s furosemide 20 mg dose was held until the 2–4-week follow-up appointment with his cardiologist, at which time there was a repeat assessment of volume status and basic metabolic panel. Postural hypotension/volume status, as well as kidney function and acidosis will be monitored by A.K.’s cardiologist on a regular basis throughout treatment. Patient A.K. was counseled about potential AEs, including genital infections and potential signs of hypovolemia such as rapid weight loss, dizziness, and/or postural hypotension. In line with a multidisciplinary approach to the management of patients with HFrEF, patient A.K.’s primary care physician was advised about the new medication regimen and given guidance about recommended assessments during follow-up (body weight, renal function, BP, and serial basic metabolic panel).
Conclusions
A growing body of clinical evidence demonstrates that SGLT2is are beneficial when added to current standard of care for the treatment of HFrEF in patients with or without T2D. However, physicians may require further practical guidance regarding the integration of SGLT2is into their clinical management of patients with HFrEF, specifically a clinical practice algorithm considering a patient’s unique profile of HF-related risk factors and concomitant medications to guide the selection of SGLT2i therapy. The clinical evidence supporting SGLT2i therapy for HFrEF is being reflected in cardiology guidelines to include SGLT2is into a standard-of-care-treatment regimen for patients with HFrEF. Ongoing clinical trials may provide further evidence to broaden the range of patients who may benefit from the cardio- and renoprotective benefits of SGLT2is and further define the physiological mechanisms responsible for SGLT2i-associated clinical outcomes in HF.
References
Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30–41.
Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96.
Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–596.
Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–19.
Dei Cas A, Khan SS, Butler J, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015;3(2):136–45.
Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016;37(19):1526–34.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Rådholm K, Figtree G, Perkovic V, et al. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation. 2018;138(5):458–68.
Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–35.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24.
Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323.
Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–646.
Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487–93.
McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726.
Maddox TM, Januzzi JL Jr, Allen LA, et al. 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772–810.
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
McMurray JJV, DeMets DL, Inzucchi SE, et al. The dapagliflozin and prevention of adverse-outcomes in heart failure (DAPA-HF) trial: baseline characteristics. Eur J Heart Fail. 2019;21(11):1402–11.
Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396(10254):819–29.
Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–28.
US Food and Drug Administration. Farxiga® (dapagliflozin) tablets, for oral use [prescribing information]. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202293s018lbl.pdf. Accessed October 6, 2021.
US Food and Drug Administration. Jardiance® (empagliflozin) tablets, for oral use [prescribing information]. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/204629s033lbl.pdf. Accessed March 22, 2022.
Butler J, Hamo CE, Filippatos G, et al. The potential role and rationale for treatment of heart failure with sodium-glucose co-transporter 2 inhibitors. Eur J Heart Fail. 2017;19(11):1390–400.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46.
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306.
Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–39.
Tuttle KR, Brosius FC 3rd, Cavender MA, et al. SGLT2 inhibition for CKD and cardiovascular disease in type 2 diabetes: report of a scientific workshop sponsored by the National Kidney Foundation. Am J Kidney Dis. 2020;77(1):94–109.
Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61.
Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes. 2013;62(10):3324–8.
Markham A. Ertugliflozin: first global approval. Drugs. 2018;78(4):513–9.
Plosker GL. Dapagliflozin: a review of its use in patients with type 2 diabetes. Drugs. 2014;74(18):2191–209.
Plosker GL. Canagliflozin: a review of its use in patients with type 2 diabetes mellitus. Drugs. 2014;74(7):807–24.
Scott LJ. Empagliflozin: a review of its use in patients with type 2 diabetes mellitus. Drugs. 2014;74(15):1769–84.
Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60(2):215–25.
Seferović PM, Fragasso G, Petrie M, et al. Sodium-glucose co-transporter 2 inhibitors in heart failure: beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22(9):1495–503.
Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136(17):1643–58.
Herat LY, Magno AL, Rudnicka C, et al. SGLT2 inhibitor-induced sympathoinhibition: a novel mechanism for cardiorenal protection. JACC Basic Transl Sci. 2020;5(2):169–79.
Matthews VB, Elliot RH, Rudnicka C, Hricova J, Herat L, Schlaich MP. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J Hypertens. 2017;35(10):2059–68.
Baartscheer A, Schumacher CA, Wüst RCI, et al. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia. 2017;60(3):568–73.
Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2017;2(9):1025–9.
Verma S, Mazer CD, Yan AT, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. 2019;140(21):1693–702.
O’Meara E, McDonald M, Chan M, et al. CCS/CHFS heart failure guidelines: clinical trial update on functional mitral regurgitation, SGLT2 inhibitors, ARNI in HFpEF, and tafamidis in amyloidosis. Can J Cardiol. 2020;36(2):159–69.
Custodio JS Jr, Duraes AR, Abreu M, Albuquerque Rocha N, Roever L. SGLT2 inhibition and heart failure-current concepts. Heart Fail Rev. 2018;23(3):409–18.
Staels B. Cardiovascular protection by sodium glucose cotransporter 2 inhibitors: potential mechanisms. Am J Med. 2017;130(6 Suppl):S30–9.
Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180–93.
Singh JSS, Mordi IR, Vickneson K, et al. Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: the REFORM trial. Diabetes Care. 2020;43(6):1356–9.
Verma S, McMurray JJV, Cherney DZI. The metabolodiuretic promise of sodium-dependent glucose cotransporter 2 inhibition: the search for the sweet spot in heart failure. JAMA Cardiol. 2017;2(9):939–40.
Butler J, Handelsman Y, Bakris G, Verma S. Use of sodium-glucose co-transporter-2 inhibitors in patients with and without type 2 diabetes: implications for incident and prevalent heart failure. Eur J Heart Fail. 2020;22(4):604–17.
Das SR, Everett BM, Birtcher KK, et al. 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117–45.
Monzo L, Ferrari I, Cicogna F, Tota C, Calo L. Sodium-glucose co-transporter-2 inhibitors eligibility in patients with heart failure with reduced ejection fraction. Int J Cardiol. 2021;341:56–9.
Vardeny O, Vaduganathan M. Practical guide to prescribing sodium-glucose cotransporter 2 inhibitors for cardiologists. JACC Heart Fail. 2019;7(2):169–72.
US Food and Drug Administration. Invokana® (canagliflozin) tablets, for oral use [prescribing information]. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204042s032lbl.pdf. Accessed October 6, 2021.
US Food and Drug Administration. Steglatro® (ertugliflozin) tablets, for oral use [prescribing information]. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209803s000lbl.pdf. Accessed October 6, 2021.
Opingari E, Partridge ACR, Verma S, Bajaj HS. SGLT2 inhibitors: practical considerations and recommendations for cardiologists. Curr Opin Cardiol. 2018;33(6):676–82.
Seferovic PM, Ponikowski P, Anker SD, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(10):1169–86.
Fitchett D. A safety update on sodium glucose co-transporter 2 inhibitors. Diabetes Obes Metab. 2019;21(Suppl 2):34–42.
Yu J, Arnott C, Neuen BL, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline diuretic use: a post hoc analysis from the CANVAS Program. ESC Heart Fail. 2021;8(2):1482–93.
Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. New Engl J Med. 2016;375(4):323–34.
Petrie MC, Verma S, Docherty KF, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323(14):1353–68.
Anker SD, Butler J, Filippatos G, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status—results from the EMPEROR-Reduced trial. Circulation. 2021;143(4):337–49.
Cherney DZI, Udell JA. Use of sodium glucose cotransporter 2 inhibitors in the hands of cardiologists: with great power comes great responsibility. Circulation. 2016;134(24):1915–7.
Mistry S, Eschler DC. Euglycemic diabetic ketoacidosis caused by SGLT2 inhibitors and a ketogenic diet: a case series and review of literature. AACE Clin Case Rep. 2021;7(1):17–9.
Acknowledgements
Funding
The development of this manuscript and the journal’s Rapid Service and Open Access Fees were funded by AstraZeneca (Wilmington, DE, USA).
Authorship
The author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, takes responsibility for the integrity of the work as a whole, and has given their approval for this version to be published.
Author Contributions
Nisha B. Jhalani has contributed to the development and review of the manuscript and approved the final version of the manuscript for submission.
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by Catherine Rees and Kate Palmer, inScience Communications, Springer Healthcare (Philadelphia, PA, USA) in accordance with Good Publication Practice (GPP-3), and funded by AstraZeneca.
Disclosures
Nisha B. Jhalani has served on speakers bureaus for AstraZeneca and Boehringer Ingelheim and has participated on advisory boards for AstraZeneca.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by the author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jhalani, N.B. Clinical Considerations for Use of SGLT2 Inhibitor Therapy in Patients with Heart Failure and Reduced Ejection Fraction: A Review. Adv Ther 39, 3472–3487 (2022). https://doi.org/10.1007/s12325-022-02169-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02169-3