Abstract
Introduction
We aimed to compare the efficacy of insulin degludec/insulin aspart (IDegAsp) and insulin degludec/liraglutide (IDegLira) in controlling glucose fluctuation and suppressing postprandial glucose levels using intermittently scanned continuous glucose monitoring.
Methods
Twenty-four patients with type 2 diabetes mellitus were randomly allocated to receive either IDegLira or IDegAsp followed by IDegAsp or IDegLira, respectively. A crossover study was conducted with intermittently scanned continuous glucose monitoring. We compared the postprandial blood glucose level, time in range, and time below range from a 3-day intermittently scanned continuous glucose monitoring period for each treatment group.
Results
The time in range was significantly higher in IDegLira than in IDegAsp. Postprandial glucose levels 90 and 120 min after breakfast and 60, 90, and 120 min after lunch were significantly lower for IDegLira than for IDegAsp. However, postprandial glucose levels 90 and 120 min after supper were significantly lower for IDegAsp than for IDegLira. There was no significant difference in the time below range between IDegLira and IDegAsp.
Conclusion
IDegLira was more effective in treating type 2 diabetes mellitus than IDegAsp, as indicated by a higher time in range and lower postprandial glucose level at breakfast and lunch. This study was registered with the University Hospital Medical Information Network Clinical Trial Registry (UMIN 000039221).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Basal-supported oral therapy has three unmet medical needs: hypoglycemia, weight gain, and glycemic control. |
Intensive insulin therapy is sometimes needed but patients are burdened by the high number of injections. |
This study aimed to compare the efficacy of IDegAsp and IDegLira in controlling glycemic variability and suppressing hypoglycemia. |
What was learned from the study? |
IDegLira had a higher time in range (70–180 mg/dL) than IDegAsp. |
IDegLira had a lower glucose variability curve after breakfast and lunch than IDegAsp. |
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive disease, and many patients require intensive insulin therapy with rapid-acting and long-acting insulin. Compared with conventional therapies, early implementation of strict glycemic control reduces the 10-year relative risk of total mortality, myocardial infarction, and microangiopathy [1]. Intensive insulin therapy requires several daily injections; however, 23.1% of patients with T2DM are burdened by the multiple injections [2]. Therefore, basal-supported oral therapy (BOT) with basal insulin is often performed. However, BOT has three major adverse effects: hypoglycemia [3], weight gain [4], and fluctuating glycemic control [5]. Hypoglycemia increases the cost of emergency transportation and hospitalization, triggers adverse socio-economic effects such as accidents, incapacity to work, and reduced work efficiency [6], and increases the risk of developing dementia [7, 8]. Approximately 57.9% of patients with T2DM reduce their insulin unit after the onset of severe hypoglycemia [9], and 79.0% of physicians indicate that the risk of hypoglycemia prevents them from initiating a more aggressive treatment [2]. Failure to intensify treatment of T2DM because of hypoglycemia leads to clinical inertia, thus failing to achieve the glycated hemoglobin (HbA1c) target [2]. Approximately half of patients with T2DM treated with basal insulin do not reach their HbA1c level target [5, 10, 11]. Weight gain due to insulin treatment also impedes HbA1c goal attainment [4, 5, 10, 11]. Additionally, weight gain increases the risk of cardiovascular disease [12]. A previous study showed that the mean HbA1c levels were 9.6% when insulin treatment was recommended, and these concerns regarding adverse effects could justify physicians’ hesitation to initiate insulin treatment [13].
Insulin degludec/insulin aspart (IDegAsp) (Ryzodeg®; Novo Nordisk A/S, Bagsværd, Denmark) is a fixed-ratio combination (FRC) containing insulin degludec (IDeg), long-acting basal insulin, and insulin aspart (IAsp), a rapid-acting insulin, at a ratio of 7:3. If HbA1c levels in patients with T2DM are above the target for treatment with basal insulin, bolus insulin or premixed insulin is recommended before the largest meal [14]. Once-daily IDegAsp therapy showed a significant reduction in HbA1c level compared with once-daily insulin glargine U-100 (IGla100); however, weight gain was comparable [15], and nocturnal hypoglycemia associated with strict glycemic control was suppressed [16]. Therefore, the insufficient therapeutic effect of BOT can be addressed by once-daily IDegAsp therapy that enhances the treatment and suppresses the risk of hypoglycemia without increasing the number of injections. Another formulation is insulin degludec/liraglutide (IDegLira) (Xultophy®; Novo Nordisk A/S, Bagsværd, Denmark), a once-daily FRC containing IDeg and liraglutide (Lira), a glucagon-like peptide 1 receptor agonist (GLP-1RA). Since GLP-1RA promotes insulin secretion in a glucose-dependent manner and suppresses glucagon secretion [17], the risk of hypoglycemia is lower than that for a hypoglycemic effect due to insulin action. IDegLira administered once daily resulted in significantly lower HbA1c levels and weight loss compared with once-daily IGla100; however, the rate of hypoglycemia was comparable [18]. Moreover, once-daily IDegLira showed a significant decrease in HbA1c level and weight loss compared with once-daily IDeg and a significant decrease in HbA1c level but weight gain compared with GLP-1RA injection therapy [19, 20]. This weight gain may occur at the time of insulin initiation. Therefore, in patients with T2DM where BOT and GLP-1RA treatment have an insufficient therapeutic effect, once-daily IDegLira can enhance treatment, reduce the risk of hypoglycemia, and improve weight loss or suppress weight gain without increasing the number of injections.
Thus, the use of IDegAsp and IDegLira to treat T2DM is expected to address the three unmet medical needs and maintain a life span and quality of life similar to those of a healthy person. However, no reports have directly compared the two FRCs. We aimed to determine the difference in efficacy for hypoglycemia and glycemic variability (GV) between IDegAsp and IDegLira using intermittently scanned continuous glucose monitoring (isCGM) in hospitalized patients with T2DM admitted for glycemic control.
Methods
Study Design and Participants
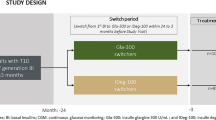
This randomized, open-label, crossover-controlled trial of patients with T2DM was conducted from February 2020 to October 2020. We explained the significance, purpose, and method of this study to the participants and obtained written informed consent from all participants before enrollment. The study was performed in accordance with the Declaration of Helsinki (1975, as revised in 2013). The protocol was reviewed and approved by the Ethics Committee of the Minami Osaka Hospital (No. 2019-16). We enrolled 24 participants with T2DM hospitalized in Minami Osaka Hospital for the purpose of improving glycemic control, diet and exercise therapy, diabetes education, and scrutiny of diabetic complications.
The inclusion criteria were as follows: (1) patients aged between 20 and 80 years; (2) patients diagnosed with T2DM at least 1 year before the initiation of the study and treated with oral hypoglycemic agents or long-acting insulin for at least 6 months before screening (however, patients using drugs containing IDeg, including IDegLira and IDegAsp, were excluded); and (3) patients with HbA1c level from 7.0% to 11.0%.
The exclusion criteria were as follows: (1) patients with a history of severe ketosis, diabetic coma, or precoma within 6 weeks of study initiation; (2) patients with severe hypoglycemia (diabetic coma or precoma, convulsions, and others, requiring the assistance of a third party) within 6 weeks of study initiation; (3) patients with severe renal dysfunction (estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2 or serum creatinine level ≥ 2.0 mg/dL) or patients with end-stage renal disease on dialysis; (4) patients with proliferative retinopathy (however, patients who underwent photocoagulation and had stable symptoms were eligible); (5) patients with a history of surgery for severe gastrointestinal disorders; (6) pregnant or breastfeeding women or those who became pregnant during the study; (7) patients with severe infections, before and after surgery, and those with severe trauma; (8) patients receiving systemic corticosteroids; (9) patients with severe liver dysfunction (aspartate aminotransferase or alanine aminotransferase level of ≥ 100 IU/L); (10) patients with a history of allergies to IDegLira or IDegAsp; (11) patients with malignant tumors or a history of malignant tumors; and (12) patients considered inappropriate participants by the physician. After applying the inclusion and exclusion criteria, 24 participants were obtained and assigned using blocked randomization with randomly selected block sizes at a ratio of 1:1 to receive either IDegLira or IDegAsp followed by IDegLira or IDegAsp, respectively, using Mujinwari by YK (Supplementary Fig. S1).
The study protocol is illustrated in Supplementary Fig. S2. At baseline, the day after obtaining consent for participation, blood samples were collected early in the morning after a fasting period of at least 10 h. If oral hypoglycemic agents were taken as pretreatment, the dosage was not changed during the study period. However, participants on dipeptidyl peptidase 4 inhibitor (DPP4i) therapy at the time of consenting to this study had to stop taking DPP4i. In the IDegLira-preceding group, participants were injected just before supper with a starting dose of 10 doses of IDegLira (1 dose of IDegLira contains IDeg 1U and Lira 0.036 mg). However, a lower dose could also be injected depending on the participants’ condition, including age, renal function, and the status of glycemic control. When long-acting insulin was used for the pretreatment, the starting dose could be increased to a maximum of 16 doses depending on the pretreatment insulin unit and state of glycemic control of the participants. In the IDegAsp-preceding group, participants were injected once daily just before supper with a starting unit of 4–20 units of IDegAsp. If long-acting insulin was used before this study, the starting units of IDegAsp were set so that the units of IDeg would be similar to the long-acting insulin unit in the pretreatment. During this study period, self-monitoring of blood glucose (SMBG) was performed four times a day before each meal and before bedtime. The doses of IDegLira and the units of IDegAsp were titrated to ensure that the preprandial glucose level at breakfast by SMBG was 100–119 mg/dL. The dose and unit titration algorithm for IDegLira and IDegAsp was based on the median preprandial glucose level at breakfast by SMBG for the last three consecutive days, as follows:
-
≥ 140 mg/dL: IDegLira, IDegAsp + 3 doses and unit increase.
-
120–139 mg/dL: IDegLira, IDegAsp + 2 doses and unit increase.
-
100–119 mg/dL: No change.
-
70–99 mg/dL: IDegLira, IDegAsp − 2 doses and unit reduction.
-
< 70 mg/dL or in symptomatic hypoglycemia and the appearance of gastrointestinal symptoms, the IDegLira doses and IDegAsp units were reduced by ≥ 3 at the discretion of the physician.
After participants achieved the target preprandial glucose level at breakfast, isCGM (Freestyle Libre Pro™; Abbott Diabetes Care, Alameda, CA) was attached to them for 15 days. To avoid glucose toxicity effects, hypoglycemia, and gastrointestinal symptoms due to FRC, at least 10 days were needed to adjust the doses of IDegLira and the units of IDegAsp until the start of isCGM attachment. On day 5 of isCGM attachment, the IDegLira-preceding group was switched to IDegAsp, and the IDegAsp-preceding group was switched to IDegLira. As a rule, the switching unit was set such that the amount of IDeg in each FRC at the time of the final injection was the same. When glucose levels were below 70 mg/dL or symptomatic hypoglycemia occurred, the dose was appropriately reduced. The IDegLira switching dose when changing from IDegAsp to IDegLira was set as IDegAsp unit × 0.7 doses when the number of IDegAsp units was 14 or less. When the number of IDegAsp units was 15 or more, IDegLira was started by switching 10 doses (up to 16 doses were possible). In the absence of hypoglycemia, hypoglycemic symptoms, or digestive symptoms, the dose of IDegLira was increased every 3 days. Finally, the dose of IDegLira reached the unit of IDegAsp × 0.7 doses. After switching, the doses of IDegLira and the units of IDegAsp were titrated between days 5 and 10 of isCGM attachment. The data for treatment period 1 (3 days from day 2 to 4 of isCGM) and data for treatment period 2 (from day 11 to 13 of isCGM) were used. Blood tests were performed the day after obtaining consent from the participants for this study, crossover day of each FRC (day 5 of isCGM attachment), and end date of the study (day 15 of isCGM attachment).
During hospitalization, a hospital diet of approximately 28 kcal/ideal body weight/day was provided to all participants at the same time. The calorie distribution was breakfast 30%, lunch 32%, and supper 38%.
Outcome Measures
The primary and secondary endpoints of this study were evaluated on the basis of three consecutive days of the isCGM for each treatment period. The primary endpoints were the percentage of reading and time of glucose levels of 70–180 mg/dL—time in range (TIR) and glucose levels 60, 90, and 120 min after each meal and percentage of reading and time of glucose levels below 70 mg/dL—time below range (TBR level 1) [21, 22]. The secondary endpoints were the percentage of reading and time of glucose levels of 180 mg/dL or higher—time above range (TAR); percentage of reading and time of glucose levels below 54 mg/dL—TBR level 2; percentage of reading and time of nocturnal (00:00–06:00) TBR level 1 [21]; 24-h mean glucose levels; 00:00–06:00 mean glucose levels; 24-h standard deviation (SD) of GV [23]; 24-h coefficient of variation (CV) of GV [24]; rate of 24-h CV of at most 36% [21]; and 24-h M value (the standard glucose level was set to 100 mg/dL) [25]. Additional secondary endpoints included the mean amplitude of glycemic excursion (MAGE) [25], mean postprandial glucose excursion (MPPGE) [26], mean of daily difference (MODD) [25], preprandial glucose level at each meal, and IDeg unit included in each FRC. Furthermore, the primary and secondary endpoints of the pretrial DPP4i therapy (DPP4i group or non-DPP4i group) were evaluated.
Statistical Analyses
Data are shown as means ± SDs unless otherwise noted. A two-tailed paired Student’s t test was used to compare GV indices [27], and a χ2 test was performed to determine the difference in frequency between the two groups. The carryover and period effects in this crossover study were verified using the repeated measured analysis of variance according to the Grieve method [28]. A Pearson product-moment correlation test was used to determine the correlation coefficient between the two variables. A p value of less than 0.05 was considered statistically significant. The sample size was calculated by the fasting plasma glucose difference between IDegLira and IDegAsp using a priori power analysis with a meaningful fasting plasma glucose difference of 23.0 mg/dL [29, 30], a common SD of 26.0 mg/dL, and a power of 0.8. The result was 24 [31]. All statistical analyses used EZR software version 1.37 [32].
Results
Participant Characteristics
Table 1 shows the characteristics of the study participants. A total of 24 participants were randomly assigned to the IDegLira and IDegAsp group or the IDegAsp and IDegLira group in a 1:1 ratio. All participants completed the study without dropout (Supplementary Fig. S1). The only adverse events were loose stools in one case and abdominal discomfort in another when using IDegLira; however, both events were mild, and the participants had no problem with continuing the study. In addition, severe hypoglycemia requiring the assistance of a physician or nurse did not occur during the study period. The mean number of days for injection dosage titration was 10.7 days for the IDegLira and IDegAsp group and 10.5 days for the IDegAsp and IDegLira group. The mean age of the participants was 65.4 years, the mean body mass index was 29.0 kg/m2, the mean HbA1c level was 8.8%, the mean C-peptide index (fasting C-peptide immunoreactivity × 100/fasting plasma glucose) was 1.4, and the mean eGFR was 65.3 mL/min/1.73 m2. There were no significant differences in all parameters between the groups.
Comparison of the Efficacy Between IDegLira and IDegAsp
Table 2 shows the isCGM parameters of glucose variability. Regarding the primary endpoint of this study, TIR was significantly higher in IDegLira than in IDegAsp (q = 0.022). There was no significant difference between IDegLira and IDegAsp in glucose levels 60 min after breakfast and 60 min after supper (q > 0.05). Postprandial glucose levels 90 (q = 0.016) and 120 (q = 0.002) min after breakfast, and 60 (q = 0.007), 90 (q = 0.002), and 120 (q = 0.001) min after lunch were significantly lower for IDegLira than for IDegAsp. However, postprandial glucose levels 90 (q = 0.030) and 120 (q = 0.016) min after supper were significantly lower for IDegAsp than for IDegLira. TBR level 1 was not significantly different between IDegLira and IDegAsp (q > 0.05). Carryover (p = 0.536) and period effect (p = 0.096) were not observed in this study.
Regarding the secondary endpoints of this study, TAR, TBR level 2, nocturnal (00:00–06:00) TBR level 1, 24-h mean glucose levels, 00:00–06:00 mean glucose levels, the rate of 24-h CV, the rate of 24-h CV of at most 36%, 24-h M value (target glucose level = 100 mg/dL), MAGE, MPPGE, MODD, and preprandial glucose level at breakfast were not significantly different between IDegLira and IDegAsp (q > 0.05). In contrast, IDegLira had a significantly lower effect than IDegAsp for 24-h SD of GV, preprandial glucose level at lunch, and preprandial glucose level at supper (q = 0.008, 0.008, and 0.007, respectively). IDeg units included in the FRC injection were significantly less in IDegAsp than in IDegLira (q = 0.016) (Table 2). There was almost no difference in the primary and secondary endpoints for pretrial DPP4i therapy (Supplementary Table S1).
Figure 1 shows the mean glucose variability curve measured by isCGM for three consecutive days. A lower glucose variability curve was demonstrated under IDegLira treatment than under IDegAsp treatment after breakfast and lunch. Conversely, a lower glucose variability curve was demonstrated under IDegAsp treatment than under treatment IDegLira after supper. The nocturnal glucose variability curve (00:00–06:00) was similar in both groups.
Three-day mean glycemic variability curve for all 24 participants obtained from isCGM data. The solid and dotted lines show the glycemic variability curves of participants injected with IDegLira and IDegAsp, respectively. isCGM intermittently scanned continuous glucose monitoring, IDegLira insulin degludec/liraglutide, IDegAsp insulin degludec/insulin aspart
Correlation Between CPI and CV in Patients with T2DM Treated with IDegLira and IDegAsp
This study analyzed the correlation between baseline CPI values and 24-h CV of GV during the treatment period with IDegLira and IDegAsp. A significant negative correlation was found between the CPI and 24-h CV of GV during the treatment period with IDegLira (r = − 0.430, p = 0.036) (Fig. 2a). In contrast, no correlation was found between the CPI and 24-h CV of GV during the treatment period by IDegAsp (Fig. 2b).
Relationship between the baseline CPI values and 24-h CV of glycemic variability. A Pearson product-moment correlation test was used to determine the correlation coefficient between the two variables. CPI C-peptide index, CV coefficient of variation; a IDegLira, insulin degludec/liraglutide; b IDegAsp, insulin degludec/insulin aspart
CPI Cutoff Value Increasing the Risk of Hypoglycemia in IDegLira
The cutoff value for the risk of hypoglycemia in patients with T2DM has been reported as 27% of 24-h CV of GV [33]. Using this cutoff value, we applied receiver operating characteristic (ROC) analysis to derive the CPI at risk of hypoglycemia in IDegLira. Assuming that the risk of hypoglycemia is avoided to satisfy the 24-h CV value of at most 27% blood glucose variability, the predictive ability was the highest when the CPI cutoff value was 1.350; the sensitivity was 66.7%, the specificity was 80.0%, and the area under the curve for the 24-h CV value of GV of at most 27% was 0.87 (95% confidence interval, 0.72–0.99) (Fig. 3).
Discussion
In this study, treatment using IDegLira significantly increased TIR and significantly reduced postprandial glucose levels, except for postprandial glucose levels at supper, compared with treatment using IDegAsp. A previous report indicated that the HbA1c levels were approximately 7.0% when the TIR in isCGM was 70% [34]; therefore, the target value for diabetes treatment using isCGM should be at least 70% in TIR [21]. Accordingly, we included patients with HbA1c levels of at least 7.0% in this study, and both IDegLira and IDegAsp achieved a TIR of at least 70%. However, TIR was higher in IDegLira than in IDegAsp, which decreased TBR, thereby improving glycemic control, minimizing hypoglycemia, and reducing the risk of diabetic complication. Lira reaches its maximum concentration after 8–12 h, and its half-life is 13 h in long-acting GLP-1RA [35], promoting insulin secretion in a glucose-dependent manner. In this study, the mean dose of IDegLira was approximately 15 doses; the content of Lira was approximately 0.6 mg in 15 doses. Lira had a dose-dependent effect of lowering postprandial blood glucose levels [36]. IDegLira lowered postprandial glucose levels at breakfast and lunch compared with that by IDegAsp. The peak postprandial glucose level after breakfast and lunch of IDegLira was 60 min; IDegLira may more greatly suppress the postprandial glucose levels because in Japanese people with T2DM, there is delay in postprandial insulin secretion [37]. Lira 0.6 mg in 15 doses slightly weakens the suppression of glucose increment after supper, which is the largest meal of the day. We postulate that the postprandial glucose level of IDegAsp at supper was significantly lower than that of IDegLira because the glucose-suppressing effect of IAsp in IDegAsp was stronger than that of Lira. Furthermore, as shown in Fig. 1, because of the effect of IAsp, the GV curve from the end of supper to approximately 00:00 in IDegAsp was lower than that in IDegLira. The glucose variability curve was similar in both groups from 00:00 to 06:00; there was no significant difference in the mean glucose levels and hypoglycemia rate between the two groups, assuming that the risk of nocturnal hypoglycemia did not differ between the two groups. There are no reports comparing the efficacy and safety of once-daily and twice-daily injection therapy of IDegAsp. Once-daily IDegAsp may be appropriate for patients with T2DM who follow an irregular diet and a high-calorie intake at supper, such as a carbohydrate-rich Japanese diet [38]. However, if a single injection of IDegAsp before the largest meal is inadequate, a twice-daily injection regimen may be recommended to improve TIR and postprandial glucose level at breakfast [14]. Comparison of the IDeg units in FRC between the two treatment groups showed that IDegAsp had a significantly lower IDeg unit than had IDegLira. This is because IAsp lowered the postprandial glucose level at supper, followed by the glucose levels at night and before breakfast (07:30), which may have reduced the unit of IDegAsp. IDegLira had a significantly smaller effect on 24-h SD of GV and 24-h CV of GV than had IDegAsp. This is because Lira stimulates insulin secretion in a glucose-dependent manner and corrects hyperglycemia. The HbA1c level is frequently used as an index for glycemic control, although it has been clarified that lowering HbA1c level has less effect in suppressing macroangiopathy than microangiopathy [39]. In contrast, it has been suggested that postprandial hyperglycemia is associated with cardiovascular events and all-cause mortality and is an independent risk factor for cardiovascular events [40, 41]. Treatment with basal insulin + GLP-1RA significantly suppresses 24-h SD of GV compared with premixed insulin treatment [42]; therefore, suppression of postprandial hyperglycemia by IDegLira is important for preventing macroangiopathy in patients with diabetes and improving their prognosis.
We found a significant negative correlation between the baseline CPI and 24-h CV of GV during the IDegLira treatment period and not during the IDegAsp treatment period. CPI is one of the endogenous insulin secretory capacity indicators since Lira promotes insulin secretion in a glucose-dependent manner. Conversely, IAsp has no such effect, and the difference in the functions of the remaining pancreatic β cells may reflect the difference between these two FRCs and GV. In patients with T2DM, more endogenous insulin secretory capacity suppresses postprandial glucose and reduces the 24-h CV of GV. In a study that used Lira as an index of fasting glucose at breakfast, the CPI of the Lira effective group was significantly higher than that of the Lira ineffective group [43]. The efficacy of Lira is associated with the remaining pancreatic β-cell function at initiation [44]. Moreover, because Lira suppresses GV in drug-naive patients with T2DM [36], treatment intervention with IDegLira in the early stages of diabetes may maintain glycemic homeostasis and prevent cardiovascular diseases. A GV (%CV) target of at most 36% is recommended as a clinical target when interpreting the CGM data [21]. This target is derived from the %CV of patients with T2DM when the GV and risk of hypoglycemia are considered [24]. In this study, the 24-h CV of GV target (at most 36%) was achieved at a high rate with no significant difference between IDegLira (83.3%) and IDegAsp (75.0%). In addition, when using insulin or sulfonylurea, the 24-h CV of GV should be reduced to below 27% to avoid hypoglycemia [33]. Since we used IDeg, the threshold of 24-h CV of GV was 27%. ROC analysis of the CPI when using IDegLira showed that the baseline CPI cutoff value was 1.350. Besides, the baseline CPI cutoff value for patients with T2DM who received Lira for 54 weeks and achieved an HbA1c level of below 7.0% was 1.86 [44]. Therefore, the CPI for preventing hypoglycemia and achieving an HbA1c level of below 7.0% will be approximately 1.6.
This study has some limitations. IDegLira and IDegAsp are not available in all healthcare economies. Additionally, our crossover study comprised a small sample size of 24 participants in a single facility. Furthermore, we analyzed the 2-week results of isCGM since we conducted this study during the hospital stay of the participants. Although a short-term study with Lira, which is similar to our study, showed that continuous glucose monitoring can be used to reduce mean glucose levels and also to improve GV [36], IDeg and Lira have long half-lives for insulin and GLP-1RA. Further, pancreatic β-cell function may improve after glucose toxicity is eliminated. Therefore, a slightly longer study period may be required for maximum effect. Although it was possible to analyze the index related to glucose variability obtained from isCGM data, a study of at least 1 year is required to comprehensively investigate the changes in body weight, HbA1c level, hypoglycemia, and adverse events, such as gastrointestinal symptoms, and calorie intake. In addition, long-term studies are needed to determine whether the incidence of cardiovascular disease differs between patients receiving IDegLira or IDegAsp.
Conclusions
Patients with T2DM treated with IDegLira had higher TIR and significantly suppressed glucose variability by lowering postprandial glucose level at breakfast and lunch. In contrast, IDegAsp significantly reduced postprandial glucose levels at supper; however, nocturnal hypoglycemia was not significantly different between the two groups. Both IDegLira and IDegAsp are useful because they lower the risk of hypoglycemia.
References
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in insulin therapy study. Diabet Med. 2012;29:682–9.
Little S, Shaw J, Home P. Hypoglycemia rates with basal insulin analogs. Diabetes Technol Ther. 2011;13:S53-64.
George D, Karim A, Florence M. Relationship of insulin dose, A1c lowering, and weight in type 2 diabetes: comparing insulin glargine and insulin detemir. Diabetes Technol Ther. 2010;12:1019–27.
Dale J, Martin S, Gadsby R. Insulin initiation in primary care for patients with type 2 diabetes: 3-year follow-up study. Prim Care Diabetes. 2010;4:85–9.
Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med. 2012;29:855–62.
Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–72.
Mattishent K, Loke YK. Bi-directional interaction between hypoglycaemia and cognitive impairment in elderly patients treated with glucose-lowering agents: a systematic review and meta-analysis. Diabetes Obes Metab. 2016;18:135–41.
Leiter LA, Yale JF, Chiasson JL, Harris S, Kleinstiver P, Sauriol L. Assessment of the impact of fear of hypoglycemic episodes on glycemic and hypoglycemia management. Can J Diabetes. 2005;29:186–92.
Giugliano D, Maiorino MI, Bellastella G, Chiodini P, Ceriello A, Esposito K. Efficacy of insulin analogs in achieving the hemoglobin A1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Care. 2011;34:510–7.
Blak BT, Smith HT, Hards M, Maguire A, Gimeno V. A retrospective database study of insulin initiation in patients with type 2 diabetes in UK primary care. Diabet Med. 2012;29:e191–8.
Wilding JPH. The importance of weight management in type 2 diabetes mellitus. Int J Clin Pract. 2014;68:682–91.
Ishii H, Iwamoto Y, Tajima N. An exploration of barriers to insulin initiation for physicians in Japan: findings from the Diabetes Attitudes, Wishes and Needs (DAWN) JAPAN study. PLoS One. 2012;7:e36361.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S111–24.
Onishi Y, Ono Y, Rabøl R, Endahl L, Nakamura S. Superior glycaemic control with once-daily insulin degludec/insulin aspart versus insulin glargine in Japanese adults with type 2 diabetes inadequately controlled with oral drugs: a randomized, controlled phase 3 trial. Diabetes Obes Metab. 2013;15:826–32.
Philis-Tsimikas A, Astamirova K, Gupta Y, et al. Similar glycaemic control with less nocturnal hypoglycaemia in a 38-week trial comparing the IDegAsp co-formulation with insulin glargine U100 and insulin aspart in basal insulin-treated subjects with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2019;147:157–65.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705.
Lingvay I, Pérez Manghi F, García-Hernández P, et al. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA. 2016;315:898–907.
Watada H, Kaneko S, Komatsu M, et al. Superior HbA1c control with the fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with a maximum dose of 50 units of insulin degludec in Japanese individuals with type 2 diabetes in a phase 3, double-blind, randomized trial. Diabetes Obes Metab. 2019;21:2694–703.
Linjawi S, Bode BW, Chaykin LB, et al. The efficacy of IDegLira (insulin degludec/liraglutide combination) in adults with type 2 diabetes inadequately controlled with a GLP-1 receptor agonist and oral therapy: DUAL III randomized clinical trial. Diabetes Ther. 2017;8:101–14.
Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593–603.
American Diabetes Association. Postprandial blood glucose. Diabetes Care. 2001;24:775–8. https://doi.org/10.2337/diacare.24.4.775.
Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631–40.
Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2017;40:832–8.
Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability; does it matter? Endocr Rev. 2010;31:171–82.
Torimoto K, Okada Y, Sugino S, Tanaka Y. Determinants of hemoglobin A1c level in patients with type 2 diabetes after in-hospital diabetes education: a study based on continuous glucose monitoring. J Diabetes Investig. 2017;8:314–20.
Jones B, Kenward MG. Design and analysis of cross-over trials. 3rd ed. Boca Raton: CRC; 2014.
Grieve AP. The two-period changeover design in clinical trials. Biometrics. 1982;38:517.
Kaku K, Araki E, Tanizawa Y, et al. Superior efficacy with a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with insulin degludec and liraglutide in insulin-naïve Japanese patients with type 2 diabetes in a phase 3, open-label, randomized trial. Diabetes Obes Metab. 2019;21:2674–83.
Kaneko S, Chow F, Choi DS, et al. Insulin degludec/insulin aspart versus biphasic insulin aspart 30 in Asian patients with type 2 diabetes inadequately controlled on basal or pre-/self-mixed insulin: a 26-week, randomised, treat-to-target trial. Diabetes Res Clin Pract. 2015;107:139–47.
Cohen J. A power primer. Psychol Bull. 1992;112:155–9.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Chandran SR, Tay WL, Lye WK, et al. Beyond HbA1c: comparing glycemic variability and glycemic indices in predicting hypoglycemia in type 1 and type 2 diabetes. Diabetes Technol Ther. 2018;20:353–62.
Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019;13:614–26.
Jackson SH, Martin TS, Jones JD, Seal D, Emanuel F. Liraglutide (Victoza): the first once-daily incretin mimetic injection for type-2 diabetes. Pharm Ther. 2010;35:498–529.
Mori Y, Taniguchi Y, Sezaki K, Yokoyama J, Utsunomiya K. Liraglutide narrows the range of circadian glycemic variations in Japanese type 2 diabetes patients and nearly flattens these variations in drug-naive type 2 diabetes patients: a continuous glucose monitoring-based study. Diabetes Technol Ther. 2011;13:1139–44.
Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract. 2004;66:S37-43.
Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–40.
Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12.
Cavalot F, Pagliarino A, Valle M, et al. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care. 2011;34:2237–43.
Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in type II diabetes: the epidemiological evidence. Diabetologia. 2001;44:2107–14.
Bajaj HS, Venn K, Ye C, et al. Lowest glucose variability and hypoglycemia are observed with the combination of a GLP-1 receptor agonist and basal insulin (VARIATION Study). Diabetes Care. 2017;40:194–200.
Kozawa J, Inoue K, Iwamoto R, et al. Liraglutide is effective in type 2 diabetic patients with sustained endogenous insulin-secreting capacity. J Diabetes Investig. 2012;3:294–7.
Usui R, Yabe D, Kuwata H, Murotani K, Kurose T, Seino Y. Retrospective analysis of safety and efficacy of liraglutide monotherapy and sulfonylurea-combination therapy in Japanese type 2 diabetes: association of remaining β-cell function and achievement of HbA1c target one year after initiation. J Diabetes Complications. 2015;29:1203–10.
Acknowledgements
We would like to thank all the study participants and Minami Osaka Hospital staff for their cooperation.
Funding
Social Medical Corporation Keigakukai Minami Osaka Hospital will be funding the journal's Rapid Service Fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Yuji Kawaguchi and Yasuro Kumeda; Methodology: Yuji Kawaguchi and Yasuro Kumeda; Data curation: Tomoe Hirota and Koji Masumoto; Investigation: Yuji Kawaguchi, Shoko Miyamoto, Yuriko Hajika, Narumi Ashida, Tomoe Hirota, Koji Masumoto, and Kenji Hamazaki; Formal Analysis: Yuji Kawaguchi, Jun Sawa, Kenji Hamazaki, and Yasuro Kumeda; Project administration: Yuji Kawaguchi; Writing-Original Draft: Yuji Kawaguchi and Yuriko Hajika; Validation: Shoko Miyamoto and Narumi Ashida; Supervision: Yasuro Kumeda.
Disclosures
Shoko Miyamoto, Yuriko Hajika, Narumi Ashida, Tomoe Hirota, Koji Masumoto, Jun Sawa, Kenji Hamazaki, and Yasuro Kumeda declare that they have no conflict of interest. Yuji Kawaguchi has received lecture honoraria or speaker fees from Sanofi K.K., Novo Nordisk Pharma, Takeda Pharmaceutical Co., Ono Pharmaceutical Co., Boehringer Ingelheim, Sumitomo Dainippon Pharma Co., Ltd., Astellas Pharma Inc., and Kowa Company Ltd.
Compliance with Ethics Guidelines
The study was performed in accordance with the Declaration of Helsinki (1975, as revised in 2013). The protocol was reviewed and approved by the Ethics Committee of the Minami Osaka Hospital (No. 2019-16). We explained the significance, purpose, and method of this study to the participants and obtained written informed consent from all participants before enrollment.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kawaguchi, Y., Miyamoto, S., Hajika, Y. et al. Efficacy of IDegLira Versus IDegAsp Therapy in Patients with Type 2 Diabetes: A Randomized Crossover Study by isCGM. Adv Ther 39, 2688–2700 (2022). https://doi.org/10.1007/s12325-022-02138-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02138-w