Abstract
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare blood disorder characterized by anemia and debilitating fatigue. Limited evidence characterizes the association between hemoglobin, an indicator of anemia and disease activity, and patient-reported fatigue scales. This review identifies benchmarks for clinically meaningful improvements in patients with and without PNH.
Methods
MEDLINE, Embase, Cochrane, and PsycINFO databases were searched along with Google Scholar to identify publications for patients with and without PNH. Full-text articles and conference abstracts of clinical trials or observational studies that examined patient-reported fatigue or associations between fatigue and hemoglobin were included.
Results
Fourteen publications were included in this study. Four clinical trials conducted in patients with PNH reported that patients achieved and sustained clinically meaningful improvements in fatigue. However, these studies did not examine the association between fatigue and hemoglobin. Ten studies conducted in patients with cancer and anemia (with or without chemotherapy) demonstrated an association between increased hemoglobin and improvements in fatigue (P < 0.05). The greatest incremental gain in fatigue improvement was observed when hemoglobin increased from 11 to 12 g/dL.
Conclusion
Evidence among patients with cancer without PNH demonstrates that increased hemoglobin levels are associated with clinically significant improvements in fatigue. Future studies should validate this relationship among patients with PNH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Fatigue is among the most common unrelieved symptoms reported by patients with paroxysmal nocturnal hemoglobinuria (PNH). |
This targeted literature review (TLR) identified benchmarks related to change in fatigue following treatment with C5 complement inhibitors among patients with PNH. |
Evidence from this TLR demonstrates that a substantial proportion of patients with PNH experience limited clinical benefit related to fatigue. |
Among patients with cancer and anemia, increased hemoglobin levels were associated with clinically meaningful improvements in patient-reported fatigue. |
Future studies are needed to validate the relationship between hemoglobin and patient-reported fatigue among patients with PNH. |
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, chronic, and life-threatening blood disease caused by a somatic mutation of the PIG-A gene in hematopoietic stem cells [1]. PNH stem cells and their progeny lack glycosylphosphatidylinositol (GPI)-anchored surface proteins, including key regulators of the complement pathway, and complement dysregulation leads to chronic intravascular and extravascular hemolysis [1]. While evidence is limited, the prevalence of PNH is estimated to be 12–13 cases per million persons [2], and an estimated 5000–6000 patients live with PNH in the USA [3, 4]. Retrospective studies suggest that 10-year survival of patients with untreated PNH ranges from 50% to 70% [5]. Two C5 complement inhibitors, eculizumab (Soliris®) and ravulizumab (Ultomiris®), are currently approved in the USA and the European Union.
Clinical manifestations of PNH are heterogeneous and may include core complications such as hemolytic anemia, thrombosis, and bone marrow failure, as well as other symptoms such as fatigue, abdominal pain, and renal impairment [6,7,8,9,10]. Evidence suggests that among non-fatal patient-reported symptoms, fatigue is experienced by as many as 80% of patients with PNH [11]. Fatigue is a common effect of hemolytic anemia that may restrict patients’ ability to perform daily activities and have a profound impact on their quality of life (QoL). A cross-sectional analysis using data from the International PNH Registry found that patients who had experienced fatigue were significantly more likely to be hospitalized than those who had not ( ~25% vs. ~15%; P < 0.01) [9]. Given the burden and severity of fatigue among patients with PNH, routine assessment of this debilitating symptom may represent a critical component of treatment and management among these patients.
Numerous instruments exist that measure patient-reported symptoms such as fatigue and QoL. For example, originally designed to capture anemia-associated fatigue in patients with cancer, the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale (previously referred to as the Functional Assessment of Cancer Therapy [FACT]-Fatigue scale) is a 13-item questionnaire that assesses patient-reported tiredness, weakness, and difficulty conducting usual activities as a result of fatigue over the past 7 days [12, 13]. In addition to being validated for use among patients with cancer [14], the content validity of the FACIT-Fatigue scale was also confirmed in larger cohorts of patients with PNH with diverse clinical and demographic characteristics [15]. Other instruments include the FACT-Anemia scale, a 20-question scale that includes the 13 questions of the FACIT-Fatigue scale in addition to seven anemia-related questions [16]; the German Exhaustion Scale, an 8-item instrument based on the FACT-Anemia and FACT-Fatigue scales [17]; the Fatigue Symptom Inventory (FSI), a 14-item measure that assesses the frequency and severity of fatigue as well as its perceived disruptiveness with patient QoL [18]; and the simple, single-item linear analog scale assessments (LASAs) that assess patient perceptions related to fatigue and physical well-being [19].
Benchmarks for the FACIT-Fatigue scale have been identified for the US general population (mean = 43.6 points; standard deviation [SD] = 9.4 points) and patients with cancer and anemia (mean = 23.9 points; SD = 12.6 points) [20]. Anchor-based analyses of fatigue and clinical indicators such as hemoglobin, performance status, and response to treatment have established that an increase or decrease of 3 points or more represents a minimum clinically meaningful difference in patients with cancer and anemia [21] and has been used among patients with PNH [22]; the European Medicines Agency has adopted a slightly more conservative level of 4 points [23]. Investigators of this study conducted a targeted literature review (TLR) to identify benchmarks for patient-reported fatigue among patients with PNH. In addition, this study aimed to identify clinical evidence that reported the association between hemoglobin level, a key measure of hemolysis, and patient-reported fatigue or QoL among patients with and without PNH.
Methods
This TLR utilized relevant guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Cochrane Handbook for Systematic Reviews of Interventions. While the PRISMA guidelines were developed for systematic literature reviews, they are broadly applicable to the methodology implemented in this study.
Eligibility Criteria
Participant Intervention Comparison Outcome Study (PICOS) criteria were used to identify studies for inclusion in this TLR (Supplementary Table 1). Studies must have enrolled patients aged 18 years or older with PNH. However, as a result of limited availability of literature among patients with PNH, the TLR was expanded to include patients with cancer and anemia who might or might not be receiving chemotherapy. Similar to patients with PNH, patients with cancer and anemia report low hemoglobin values and fatigue as one of the most frequently reported symptoms accompanying cancer and cancer treatment [24].
Several outcomes of interest were examined in this TLR, including benchmarks related to mean patient-reported fatigue scores at baseline, change in patient-reported fatigue score over follow-up, and the proportion of patients who experienced clinically meaningful improvements for patient-reported fatigue scores following treatment. In addition, this TLR examined the association between hemoglobin and patient-reported fatigue or QoL. This included the change in patient-reported fatigue by level of hemoglobin response, the proportion of patients who achieved a clinically meaningful improvement in patient-reported fatigue or QoL by level or hemoglobin response, and the association between incremental improvements in hemoglobin level and incremental improvements in patient-reported fatigue or QoL.
Clinical trials and observational studies were eligible for inclusion in this TLR. Studies must have been conducted in the USA, where both eculizumab and ravulizumab were approved for use at the time of study conduct. However, multicountry studies that included US sites, and studies conducted in North American or Western European countries without US sites were also eligible for this TLR if investigators presented results stratified by geographic region. Published, English-language full-text articles (no date restriction) and conference abstracts (published in 2017 or later) were included in this TLR.
Search Strategy and Study Selection
Four electronic databases, MEDLINE, Embase, Evidence-Based Medicine Reviews–Cochrane Central Register of Controlled Trials, and PsycINFO (Supplementary Table 2), were searched using pre-defined search terms that reflect the PICOS elements described earlier (Supplementary Table 3). This search was limited to patients with PNH. A Google Scholar search was conducted for patients with cancer and anemia using pre-defined search terms consistent with the electronic database search; patents were not included. Google Scholar has been shown to efficiently identify relevant, in addition to highly cited, publications when compared with electronic databases [25]. Electronic searches were conducted on November 6, 2019.
Unique results identified by the four electronic databases were divided among three reviewers. Using the same PICOS criteria, reviewers screened the title and abstract of results. Publications that clearly did not meet the criteria were excluded. The full text of published articles and conference abstracts were then screened by two reviewers to determine whether they met all eligibility criteria. Reasons for exclusion were documented. Differences in the decision to exclude publications were reconciled between reviewers. Results from the Google Scholar search were sorted by relevance and titles of the first 100 search results were reviewed for inclusion in this TLR.
Data Extraction and Synthesis of Results
Data were extracted from selected studies using a standardized data extraction form. Specifically, publication details (i.e., author, year, title, journal, disease area, abstract) and study characteristics (i.e., study population, fatigue instrument, endpoint, and primary results) were collected. All data recorded in the data extraction form were audited; the primary abstractor and auditor resolved any discrepancies. All data extracted in this TLR were synthesized by patient population (i.e., patients with PNH; patients with cancer and anemia).
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Study Selection
A total of 144 unique full-text articles and conference abstracts were identified through the four electronic databases (Fig. 1). After reviewing the title, abstract, and full-text of all unique references identified through the four electronic databases, we excluded 140/144 publications (97%). Among these references, the most common reason for exclusion was date range (n = 75 [54%] unique references pertained to conference abstracts that had been published more than 2 years prior to the date of the electronic search). Among the first 100 results of the Google Scholar, 90 results were excluded on the basis of a review of the title and abstract. A total of 14 relevant publications met all eligibility criteria and were included in this TLR. Among these 14 publications, 4 (29%) were conducted among patients with PNH and were identified through the electronic database search [22, 26,27,28]. Each of these four publications reported benchmarks related to patient-reported fatigue; however, none of these studies reported results describing the association between hemoglobin level and patient-reported fatigue or QoL. Ten publications (71%) included in this TLR were identified through the Google Scholar search [17, 29,30,31,32,33,34,35,36,37] and were conducted among patients with cancer and anemia who might or might not be receiving chemotherapy. Each of these studies described the association between hemoglobin level and patient-reported fatigue, while two studies described the association between incremental improvement in hemoglobin level and incremental improvement in patient-reported fatigue or QoL.
Benchmarks Related to Patient-Reported Fatigue Among Patients with PNH
Change from Baseline in Patient-Reported Fatigue
Each of the four studies conducted among patients with PNH reported the mean change from baseline of FACIT-Fatigue score (Table 1). The earliest study conducted among patients with PNH included in this TLR, the TRIUMPH Study (NCT00122330), was a double-blind, randomized, placebo-controlled, multicenter, phase 3 trial comparing eculizumab to placebo [22]. Eighty-seven complement inhibitor-naïve adult patients with PNH were enrolled and randomized to receive eculizumab (n = 43) or placebo (n = 44) for 26 weeks. After 26 weeks of follow-up, Hillmen et al. [22] reported that FACIT-Fatigue scores improved on average by 6.4 points (standard error [SE] = 1.2 points) from baseline for patients who received eculizumab, and decreased by a mean of 4.0 points (SE = 1.7 points) from baseline for patients who received placebo.
The SHEPHERD Study (NCT00130000) was a single-arm, open-label, phase 3 study that examined the long-term safety and efficacy of the complement inhibitor eculizumab [26]. Among 97 adult patients who received eculizumab for 52 weeks, FACIT-Fatigue scores significantly improved within 1 week of treatment initiation, and the improvement was maintained over 52 weeks of follow-up [26]. Specifically, investigators reported a mean change from baseline of 12.2 points [SE = 1.1 points] in FACIT-Fatigue score at 52 weeks of follow-up [26].
The “301 Study” (NCT02946463) was an open-label, multicenter, phase 3 study evaluating the non-inferiority of ravulizumab to eculizumab in complement inhibitor-naïve patients with PNH [28]. A total of 246 adult patients were randomized to receive ravulizumab (n = 125) or eculizumab (n = 121) for 26 weeks [28]. Least squares mean increase from baseline (95% confidence interval [CI]) in FACIT-Fatigue score was 7.07 points (95% CI 5.55–8.60 points) and 6.40 points (95% CI 4.85–7.96 points) for patients with PNH who received ravulizumab and eculizumab, respectively.
Finally, the “302 Study” (NCT03056040) was an open-label, multicenter, phase 3 study evaluating the non-inferiority of ravulizumab to eculizumab in patients with PNH who showed stability during previous eculizumab treatment [27]. A total of 195 adult patients previously treated with eculizumab were randomized to receive ravulizumab (n = 97) and eculizumab (n = 98) for 26 weeks. Least squares mean change from baseline in FACIT-Fatigue score was 2.01 points (SE = 0.7 points) and 0.54 points (SE = 0.7 points) for patients who received ravulizumab and eculizumab, respectively [27].
Proportion of Patients Who Experienced a Clinically Meaningful Improvement in Patient-Reported Fatigue
Two publications reported the proportion of patients who experienced a clinically meaningful improvement in patient-reported fatigue [27, 28]. Among patients with PNH who had not previously been treated with a complement inhibitor enrolled in the 301 Study, more than half experienced an increase of at least 3 points in FACIT-Fatigue score during follow-up (61.6% of patients who received eculizumab; 58.7% of patients who received ravulizumab) [28]. Meanwhile, the percentage of patients with at least a 3-point improvement in FACIT-Fatigue score was reduced among patients with PNH and prior complement inhibitor treatment with ravulizumab (37.1%) and eculizumab (33.7%) in the 302 Study [27].
Association Between Hemoglobin Response and Patient-Reported Fatigue or QoL Among Patients with Cancer and Anemia
FACIT-Fatigue or FACT-Anemia Scales
Eight studies assessed fatigue using the FACIT-Fatigue or FACT-Anemia scales among patients with cancer and anemia [29,30,31,32,33,34,35, 37] (Table 2). In general, studies reported that increases in hemoglobin levels were associated with improvements in fatigue; two studies reported a correlation coefficient of 0.27 between hemoglobin levels and the FACT-Anemia scale [34, 37], and two additional studies reported statistically significant P values, but not coefficients [30, 31].
Several studies examined the association between patient-reported fatigue, as measured by the FACIT-Fatigue or FACT-Anemia scale, and hemoglobin response. A study in 607 patients with solid tumors and anemia [29] reported that 55.0% of patients whose hemoglobin level increased by 2 g/dL by the end of a 12-week treatment period also reported a clinically meaningful improvement of at least 3 points in FACIT-Fatigue score; compared to 39.8% among non-responders (P = 0.0004). In another study, 59.5% of patients whose hemoglobin level increased by 2 g/dL or more also reported a clinically meaningful improvement in FACT-Fatigue score [31]. In addition, patients with cancer who had greater increases in hemoglobin level also reported greater improvements from baseline in patient-reported fatigue [30,31,32,33,34,35]. A study comparing hemoglobin responders (i.e., improvement in hemoglobin level of at least 2 g/dL) vs. non-responders [34] reported both greater mean final FACT-Anemia score (59.7 points vs. 57.5 points) and a larger mean improvement in patient-reported fatigue from baseline (5.0 points vs. 0.9 points), respectively. Similarly, one study [35] reported a mean improvement from baseline on the FACIT-Fatigue scale of 5.6 points, a clinically meaningful improvement, for patients whose hemoglobin level increased by at least 2 g/dL compared to a 1.0-point improvement for non-responders. Two publications defined hemoglobin response using three categories: decrease or increase of less than 2 g/dL, and increase of at least 2 g/dL [30, 31]. In both studies, patients whose hemoglobin levels improved by at least 2 g/dL reported clinically meaningful improvements in FACIT-Fatigue score. Smaller increases in FACIT-Fatigue score, though clinically meaningful in one study [31], were observed for patients whose hemoglobin increased between 0 and less than 2 g/dL during treatment, and FACIT-Fatigue scores decreased from baseline for patients whose hemoglobin level decreased during treatment [30, 31]. Finally, one study reported clinically meaningful improvements in FACIT-Anemia scores of 4.8, 7.7, and 11.0 points for patients whose hemoglobin levels increased 0–2 g/dL, 2–4 g/dL, and more than 4 g/dL, respectively [32].
Additional Patient-Reported Fatigue Scales
Three studies assessed the association between hemoglobin level and patient-reported fatigue using alternative, validated instruments among patients with cancer and anemia (Table 2), including the German Exhaustion Scale [17], the LASA for overall QoL [37], and the FSI [36]. Changes in hemoglobin level were associated with changes in patient-reported fatigue or QoL in each of these studies. For example, Reinhardt et al. [17] observed that mean increase in hemoglobin level was correlated with improvement in mean fatigue score (r = 0.31, P < 0.0001). Similarly, Crawford et al. [37] reported a positive correlation between hemoglobin levels and the LASA overall QoL score and noted that the relationship between a patient’s change in hemoglobin level and change in LASA overall QoL scores was direct and significant (P < 0.01) when hemoglobin level was between 8 and 14 g/dL. Finally, Jacobsen et al. [36] reported that a greater decline in hemoglobin was related to a greater increase in fatigue disruptiveness (i.e., the degree to which fatigue interfered with the patient’s general level of activity, ability to bathe and dress, normal work activity, ability to concentrate, relations with others, enjoyment of life, and mood in the past week; P < 0.05).
In addition, two studies reported associations according to hemoglobin response [17, 36]. In one publication, the greatest improvement in patient-reported fatigue was observed for patients whose hemoglobin increased by more than 2 g/dL during follow-up [17]. In another publication, Jacobsen et al. [36] observed that greater declines in hemoglobin were significantly related to greater increases in fatigue disruptiveness (P < 0.01) and fatigue duration (i.e., the number of days the patient reported experiencing fatigue in the past week; P < 0.05) among patients with cancer whose hemoglobin level declined to 12 g/dL or less during follow-up.
Association Between Incremental Increases in Hemoglobin Level and Incremental Increases in Patient-Reported Fatigue or QoL Among Patients with Cancer and Anemia
Two studies [34, 37] assessed the association between incremental improvements in hemoglobin levels and incremental improvements in patient-reported fatigue or QoL among patients with cancer and anemia (Table 2). In addition to the positive association between hemoglobin level and overall QoL, as measured by LASA, across the clinically relevant range of 8–14 g/dL, Crawford et al. [37] reported that the greatest incremental gain in patient-reported overall QoL was observed when hemoglobin level increased from 11 to 12 g/dL. Another publication that used linear regression analysis reported that a 1 g/dL increase in hemoglobin level was associated with a mean increase of 2.1 points on the FACT-Anemia scale [34]. In addition, confirming the results reported by Crawford et al. [37], Cartenì et al. [34] reported that independent of baseline hemoglobin level, the greatest increase in patient-reported fatigue as measured by the FACT-Anemia scale was observed when patients reached a hemoglobin level of 11 g/dL and approached a hemoglobin level of 12 g/dL.
Discussion
This TLR was conducted to identify benchmarks for patient-reported fatigue, as well as clinical evidence that reported the association between hemoglobin level and patient-reported fatigue among patients with and without PNH. Fatigue is among the most common unrelieved symptoms reported by patients with PNH [11]. However, only a few studies investigating fatigue among patients with PNH were identified in this TLR, none of which published baseline measures of fatigue as assessed by patient-reported instruments. Studies from the International PNH Registry, which includes aggregate results for pediatric and adult patients from all global regions, show mean FACIT-Fatigue scores from 30.5–34 points [11, 38]. This evidence demonstrates that patients with PNH experience a greater, clinically meaningful level of fatigue compared to the US general population (mean FACIT-Fatigue score = 43.6 points [20]).
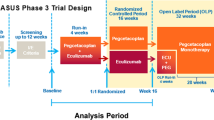
Evidence from the studies identified in this TLR demonstrates that currently available treatments, such as C5 complement inhibitors eculizumab and ravulizumab, result in improvements in patient-reported fatigue, assessed as a change from baseline. However, without absolute values at baseline and follow-up, it is not possible to contextualize these improvements relative to the levels of fatigue experienced by the US general population [22]. Importantly, clinically meaningful improvements in fatigue were reported only among studies that enrolled complement inhibitor-naïve patients, suggesting that improvement of fatigue in complement inhibitor-experienced patients remains an unmet need [22, 26, 28]. Among patients previously treated with eculizumab who were enrolled in the 302 Study, improvements in patient-reported fatigue were attenuated (i.e., 2.0 and 0.5 points among ravulizumab and eculizumab patients, respectively) [27]. Moreover, 63% of ravulizumab patients and 66% of eculizumab patients did not achieve a clinically meaningful improvement in FACIT-Fatigue score [27]. This TLR demonstrates that complement inhibitor-experienced patients with PNH experienced lesser benefit related to patient-reported fatigue despite treatment with current C5 complement inhibitors, warranting further investigation into other therapies. The C3 complement inhibitor pegcetacoplan has been investigated in two phase 3 trials among complement inhibitor-naïve (PRINCE; NCT04085601; results expected in 2021) [39] and complement inhibitor-experienced (PEGASUS; NCT03500549) [40] patients with PNH. Centrally targeted at the C3 level, pegcetacoplan broadly inhibits multiple complement activation pathways resulting in improved therapeutic benefit and symptom control. Among complement inhibitor-experienced patients with PNH enrolled in the PEGASUS trial who were previously treated with eculizumab and had a hemoglobin level below 10.5 g/dL, FACIT-Fatigue score increased by 9.2 points for patients treated with pegcetacoplan, compared with a 2.7-point decrease with eculizumab, by week 16 [40]. In addition, 73% of patients treated with pegcetacoplan achieved a clinically meaningful improvement of at least 3 points for FACIT-Fatigue score, as compared with 0% among patients treated with eculizumab [40].
It is of note that the FACIT-Fatigue scale was originally designed for patients with cancer, but it has long been a standard assessment for QoL among patients with PNH as a result of the lack of PNH-specific instruments. In this regard, an instrument for measuring aplastic anemia (AA)/PNH-specific QoL (QLQ-AA/PNH) is currently under the final phase of development [41]. The 54-item QLQ-AA/PNH has shown good internal consistency (e.g., Cronbach’s alpha of the fatigue scale was 0.88) and may already be used in some clinical studies. Upon finalization of the scoring algorithm, QLQ-AA/PNH may be used to provide more tailored QoL measurements for patients with PNH [41].
No studies conducted among patients with PNH examined the association between hemoglobin levels and patient-reported fatigue and QoL. Given this evidence gap, it was necessary to expand the scope of this TLR to include patients with cancer and anemia, a patient population that also exhibits a high burden of fatigue [20]. Among patients with cancer and anemia, substantial evidence demonstrates that increased hemoglobin levels are associated with clinically meaningful improvements in patient-reported fatigue, and the greatest incremental gain on the basis of fatigue was observed when hemoglobin level increased from 11 to 12 g/dL. Hemoglobin level represents a direct indicator of clinical severity among patients with PNH [42]. The results reported among patients with cancer and anemia must be confirmed among patients with PNH. For example, a recent case report on a Japanese woman with AA/PNH without severe hemolytic presentation found that complement inhibitor led to improvement in patient-reported fatigue and QoL, suggesting that patients with severe fatigue but mild disease activity and anemia may also benefit from complement inhibitors [43]. Nonetheless, findings from this TLR provide clinicians with hematological targets that may translate into clinically meaningful improvements in patient-reported fatigue and QoL outcomes.
The results of this TLR are subject to some limitations. It is possible that not all relevant studies were captured through our search of four electronic databases as a result of incorrect indexing. Because our search was limited to 2017 and later for published conference abstracts, relevant studies conducted prior to this period may not be included. However, it was expected that abstracts more than 2 years old would be published as full-text manuscripts and potentially identifiable by our search. Also, screening by reviewers might have missed relevant articles, although all reviewers were involved in the development, testing, and implementation of the PICOS criteria used in this TLR. In addition, our electronic database search identified several publications that utilized data collected by the International PNH Registry. Because stratified data were not reported for adult patients (i.e., results were reported for pediatric and adult patients combined) or by geographic location (i.e., USA/North America and Western Europe), these studies were excluded from this TLR. However, because burden of disease may differ by geographic region and race/ethnic group, excluding studies that do not present stratified results is warranted. Finally, publication bias may have also affected outcomes reported and the studies published.
Conclusions
This study identified benchmarks related to change in fatigue following treatment with C5 complement inhibitors among patients with PNH. A substantial proportion of patients experience limited clinical benefit related to fatigue, which reflects an ongoing unmet need in this patient population. Given the lack of studies identified in this TLR, it is clear that the association between fatigue and hemoglobin level, an indicator of disease severity, remains underevaluated among patients with PNH. Routine use of the FACIT-Fatigue scale, among other psychometric instruments, should be considered during the management of patients with PNH. Evidence from patients with cancer and anemia indicates that the greatest gains in improving patient-reported fatigue and QoL occur when hemoglobin increased from 11 and 12 g/dL. Future studies should validate this relationship among patients with PNH.
References
Hill A, DeZern AE, Kinoshita T, Brodsky RA. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers. 2017;3(1):17028.
Jalbert JJ, Chaudhari U, Zhang H, Weyne J, Shammo JM. Epidemiology of PNH and real-world treatment patterns following an incident PNH diagnosis in the US. Blood. 2019;134(Supplement_1):3407.
Hill A, Platts PJ, Smith A, et al. The incidence and prevalence of paroxysmal nocturnal hemoglobinuria (PNH) and survival of patients in Yorkshire. Blood. 2006;108(11):985.
Bektas M, Copley-Merriman C, Khan S, Sarda SP, Shammo JM. Paroxysmal nocturnal hemoglobinuria: role of the complement system, pathogenesis, and pathophysiology. J Manag Care Spec Pharm. 2020;26(12-b Suppl):S3–S8.
Socié G, Schrezenmeier H, Muus P, et al. Changing prognosis in paroxysmal nocturnal haemoglobinuria disease subcategories: an analysis of the International PNH Registry. Intern Med J. 2016;46(9):1044–53.
Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124(18):2804–11.
Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2009;113(26):6522–7.
Mohammed AA, El-Tanni H, Atiah TA, Atiah AA, Atiah MA, Rasmy AA. Paroxysmal nocturnal hemoglobinuria: from bench to bed. Indian J Hematol Blood Transfus. 2016;32(4):383–91.
Schrezenmeier H, Muus P, Socie G, et al. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica. 2014;99(5):922–9.
Muus P, Szer J, Schrezenmeier H, et al. Evaluation of paroxysmal nocturnal hemoglobinuria disease burden: the patient's perspective. A report from the international PNH registry. In: Blood conference: 52nd annual meeting of the American Society of Hematology, ASH, vol. 116, no. 21; 2010.
Schrezenmeier H, Röth A, Araten DJ, et al. Baseline clinical characteristics and disease burden in patients with paroxysmal nocturnal hemoglobinuria (PNH): updated analysis from the International PNH Registry. Ann Hematol. 2020;99(7):1505–14.
Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manag. 1997;13(2):63–74.
Webster K, Cella D, Yost K. The functional assessment of chronic illness therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1(1):79.
Revicki DA, Rentz AM, Luo MP, Wong RL. Psychometric characteristics of the short form 36 health survey and functional assessment of chronic illness therapy-fatigue subscale for patients with ankylosing spondylitis. Health Qual Life Outcomes. 2011;9(1):36.
Weitz I, Meyers G, Lamy T, et al. Cross-sectional validation study of patient-reported outcomes in patients with paroxysmal nocturnal haemoglobinuria. Intern Med J. 2013;43(3):298–307.
Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13–9.
Reinhardt U, Tulusan A, Angermund R, Lutz H. Increased hemoglobin levels and improved quality-of-life assessments during epoetin alfa treatment in anemic cancer patients: results of a prospective, multicenter German trial. Oncologist. 2005;10(3):225–37.
Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7(4):301–10.
Glaspy J, Bukowski R, Steinberg D, Taylor C, Tchekmedyian S, Vadhan-Raj S. Impact of therapy with epoetin alfa on clinical outcomes in patients with nonmyeloid malignancies during cancer chemotherapy in community oncology practice. Procrit Study Group. J Clin Oncol. 1997;15(3):1218–34.
Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528–38.
Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manag. 2002;24(6):547–61.
Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233–43.
European Medicines Agency. Soliris (eculizumab). 2020.
Holzner B, Kemmler G, Greil R, et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol. 2002;13(6):965–73.
Shariff SZ, Bejaimal SA, Sontrop JM, et al. Retrieving clinical evidence: a comparison of PubMed and Google Scholar for quick clinical searches. J Med Internet Res. 2013;15(8):e164.
Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111(4):1840–7.
Kulasekararaj AG, Hill A, Rottinghaus ST, et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study. Blood. 2019;133(6):540–9.
Lee JW, Sicre de Fontbrune F, Wong Lee Lee L, et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood. 2019;133(6):530–9.
Kallich JD, Tchekmedyian NS, Damiano AM, Shi J, Black JT, Erder M. Psychological outcomes associated with anemia-related fatigue in cancer patients. Oncology (Williston Park). 2002;16(9 Suppl 10):117–24.
Smith RE Jr, Glaspy JA, Tchekmedyian NS, Austin MD, Kallich JD. Hemoglobin increase is associated with improved health-related quality of life in patients with cancer not receiving chemotherapy. Support Cancer Ther. 2003;1(1):49–54.
Berndt E, Kallich J, McDermott A, Xu X, Lee H, Glaspy J. Reductions in anaemia and fatigue are associated with improvements in productivity in cancer patients receiving chemotherapy. Pharmacoeconomics. 2005;23(5):505–14.
Gabrilove JL, Cleeland CS, Livingston RB, Sarokhan B, Winer E, Einhorn LH. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol. 2001;19(11):2875–82.
Glaspy J, Jadeja J, Justice G, et al. Darbepoetin alfa given every 1 or 2 weeks alleviates anaemia associated with cancer chemotherapy. Br J Cancer. 2002;87(3):268–76.
Cartenì G, Giannetta L, Ucci G, et al. Correlation between variation in quality of life and change in hemoglobin level after treatment with epoetin alfa 40,000 IU administered once-weekly. Support Care Cancer. 2007;15(9):1057–66.
Cella D, Kallich J, McDermott A, Xu X. The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: results from five randomized clinical trials. Ann Oncol. 2004;15(6):979–86.
Jacobsen PB, Garland LL, Booth-Jones M, et al. Relationship of hemoglobin levels to fatigue and cognitive functioning among cancer patients receiving chemotherapy. J Pain Symptom Manag. 2004;28(1):7–18.
Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95(4):888–95.
Ueda Y, Obara N, Yonemura Y, et al. Effects of eculizumab treatment on quality of life in patients with paroxysmal nocturnal hemoglobinuria in Japan. Int J Hematol. 2018;107(6):656–65.
Apellis Pharmaceuticals, Inc. A study to evaluate the efficacy and safety of APL-2 in patients with PNH (PRINCE) 2020. https://ClinicalTrials.gov/show/NCT04085601. Accessed 9 Oct 2020.
Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021;384(11):1028–37.
Niedeggen C, Singer S, Groth M, et al. Design and development of a disease-specific quality of life tool for patients with aplastic anaemia and/or paroxysmal nocturnal haemoglobinuria (QLQ-AA/PNH)-a report on phase III. Ann Hematol. 2019;98(7):1547–59.
Barcellini W, Fattizzo B. Clinical applications of hemolytic markers in the differential diagnosis and management of hemolytic anemia. Dis Mark. 2015;2015:635670.
Sakai K. Even mild hemolysis in paroxysmal nocturnal hemoglobinuria could severely compromise the quality of life due to long-term sustained intolerant fatigue. Leuk Res Rep. 2020;14:100224.
Acknowledgements
Funding
This study was funded by Apellis Pharmaceuticals, Inc., MA, USA. The study sponsor reviewed and provided feedback on the manuscript. The journal’s Rapid Service and Open Access Fees were provided by Apellis Pharmaceuticals, Inc., MA, USA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Authors contributed to the conception and design of the study; drafting and critical revision of the article for important intellectual content; and approved the final version to be submitted. All authors agree to be accountable for all aspects of this study. Sangeeta Krishnan: all of the above; Sanjana Sundaresan: drafting and critical revision of the article; final approval; Colin Kunzweiler: all of the above; Melody Wu: all of the above; Sanjana Sundaresan: all of the above; Lynn Huynh: all of the above; Mei Sheng Duh: all of the above; Carmelita P Escalante: drafting and critical revision of the article; final approval.
Medical Writing, Editorial, and Other Assistance
Apellis Pharmaceuticals, Inc. and Swedish Orphan Biovitrum AB reviewed and provided feedback on the manuscript.
Prior Presentation
Part of the materials contained in this manuscript was accepted as an abstract to the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 2020 Virtual Conference, May 18–20, 2020.
Disclosures
Sangeeta Krishnan and Sujata Sarda are employees of Apellis Pharmaceuticals, Inc. and own stock/stock options. Sanjana Sundaresan, Lynn Huynh, and Mei Sheng Duh are employees of Analysis Group Inc., which has received consultancy fees from Apellis Pharmaceuticals, Inc. Carmelita P Escalante reports no personal fees from Apellis Pharmaceuticals, Inc. before or during the conduct of the study, and no conflicts outside the submitted work. Melody Wu and Colin Kunzweiler were employees of Analysis Group Inc. at the time of study conduct. Melody Wu is now an employee of Takeda. Colin Kunzweiler is now an employee of Vertex Pharmaceuticals.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Informed consent is not applicable because the article does not contain any patient-identifiable information.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Krishnan, S., Sarda, S., Kunzweiler, C. et al. Literature Review of Fatigue Scales and Association with Clinically Meaningful Improvements in Outcomes Among Patients With and Without Paroxysmal Nocturnal Hemoglobinuria. Adv Ther 39, 1959–1975 (2022). https://doi.org/10.1007/s12325-022-02111-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02111-7