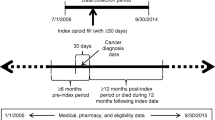
Abstract
Introduction
Opioid-induced constipation (OIC) prescription medications (OIC-Rx) like methylnaltrexone subcutaneous (SC) have shown efficacy in treating OIC in the emergency department (ED). This study aimed to describe and compare healthcare resource utilization (HRU) and healthcare costs in ED patients with OIC receiving OIC-Rx versus those not receiving OIC-Rx.
Methods
Adult patients with OIC during an ED encounter were identified from a hospital-based ED encounters database (2016–2019) and classified on the basis of receipt of OIC-Rx (OIC-Rx versus No OIC-Rx cohorts). Entropy balancing was used to reweight characteristics of the two cohorts. HRU and healthcare costs were measured and compared during the ED encounter and 30-day post-discharge period.
Results
Among 11,135 patients in the OIC-Rx cohort (21,474 in the No OIC-Rx cohort), 93% received methylnaltrexone SC. Patients in the OIC-Rx cohort had 0.7 fewer inpatient days per OIC ED encounter and 64% decreased odds of being hospitalized versus the No OIC-Rx cohort (both p < 0.001). During the post-discharge period, the OIC-Rx cohort had 35% decreased odds of any re-encounter (p < 0.001). The OIC-Rx cohort had a $732 reduction in costs per OIC ED encounter versus the No OIC-Rx cohort (p < 0.001), driven by larger hospitals and patients with Medicare or Commercial insurance. During the post-discharge period, the OIC-Rx cohort had a $421 reduction in costs associated with any re-encounter versus the No OIC-Rx cohort (p = 0.004).
Conclusion
Patients receiving OIC-Rx in the ED had decreased odds of being hospitalized and fewer re-encounters in the 30-day post-discharge period versus patients who did not receive OIC-Rx, resulting in cost savings for insurance agencies and healthcare providers.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Why carry out this study? |
Prescription medications for opioid-induced constipation (OIC) like methylnaltrexone subcutaneous have shown efficacy in treating OIC in the emergency department (ED). |
It is possible that this efficacy will translate into reduced healthcare resource utilization and healthcare cost savings, but there is no evidence available to date among patients with OIC in US clinical practice. |
What was learned from the study? |
Patients receiving prescription medications for OIC in the ED had decreased odds of being hospitalized and fewer re-encounters in the 30-day post-discharge period versus patients who did not receive prescription medications for OIC. |
These findings highlight the potential for OIC prescription medications to provide considerable relief for constipation symptom control, which may in turn reduce the need for lengthy and costly hospital stays and lead to important improvements in quality of life for the patient population with OIC. |
Introduction
Opioid-induced constipation (OIC) develops in approximately half of patients receiving opioid therapy for non-cancer pain [1]. As a side effect that typically persists for the duration of opioid treatment, OIC may result in a considerable patient burden, including impairment of daily activity and personal relationships, all of which contribute to poor patient quality of life (QoL) and well-being [2, 3]. In a multinational survey-based study of patients receiving opioid therapy, more than 60% of respondents reported experiencing constipation symptoms at least 4–6 times per week, with the majority having at least a moderate to great impact on QoL or overall well-being [4]. In addition, OIC is associated with a burden at the hospital level, including increased healthcare resource utilization (HRU; e.g., hospitalizations, emergency department [ED] visits) and healthcare costs [5,6,7]. Altogether, the negative impact of OIC may pose a significant burden on patients and the healthcare system [1, 8].
Laxatives (e.g., over-the-counter bisacodyl, sodium docusate, polyethylene glycol, etc.) are the first-line therapy for OIC, but only 46% of patients experience sufficient symptom management [1, 9]. Additionally, their lengthy time to laxation limits their use in the ED [10]. Alternatively, enemas and digital disimpaction may be performed in the ED, especially for severe cases [10, 11], despite consensus recommendations stating that these approaches are associated with invasiveness, discomfort, embarrassment, and healthcare burden for the patients [9]. Guideline-recommended treatment options for OIC include prescription medications (OIC-Rx), including lubiprostone or peripherally acting mu-opioid receptor antagonists (PAMORAs; methylnaltrexone, naloxegol, naldemedine) [12]. The PAMORA methylnaltrexone (subcutaneous [SC] or oral) has shown efficacy in treating OIC and may be of utility when administered in the ED because of its rapid time to laxation (often within 4 h for the SC formulation) [13,14,15,16,17]. In a meta-analysis of PAMORA clinical trials, patients treated with a PAMORA experienced larger improvements in spontaneous bowel movements from baseline, and higher response rates, versus those treated with placebo [18]. Accordingly, it is possible that this efficacy will translate into reduced HRU and healthcare cost savings, but there is no evidence available to date among patients with OIC in US clinical practice. Therefore, our purpose was to describe and compare HRU and healthcare costs in ED patients with OIC receiving an OIC-Rx versus those not receiving an OIC-Rx.
Methods
Data Source
This study was conducted using retrospective data (Q1 2016–Q3 2019) in a hospital-based ED encounters database. This database encompasses detailed inpatient services information from patients admitted to more than 700 US hospitals. Data elements include admission-level information (e.g., patient characteristics, primary and secondary admitting diagnoses), detailed day-of-service billing information during hospitalizations (e.g., inpatient procedures and medications used by day of stay), and discharge-level data (e.g., length of stay, discharge status). The database includes only hospitalization data and hospital outpatient visits, thereby limiting the observation to events occurring in the hospital setting.
Data are de-identified and comply with the requirements of the Health Insurance Portability and Accountability Act. Therefore, no institutional review board approval was needed.
Study Design and Sample Selection
A retrospective cohort design was used. Patients were included if they met the following criteria: (1) at least one ED encounter at any time, and were aged 18 years or older at the start of the ED encounter; (2) at least one diagnosis of constipation during the ED encounter (i.e., index ED encounter); (3) at least one indicator of OIC 6 months prior to (i.e., baseline period) or during the index ED encounter; (4) at least one index ED encounter on or after April 1, 2016; and (5) did not have a discharge status at the index ED encounter identified as expired. If hospitalized, (6) patients had an inpatient stay following the index ED encounter of at most 8 days. As there is no diagnosis code for OIC, an indicator of OIC was developed on the basis of the study medical experts’ input and was defined as having a diagnosis for constipation (primary diagnosis or secondary diagnosis where the primary diagnosis is abdominal pain or nausea and vomiting), in addition to a history of one or more of the following: (1) any OIC-Rx use during the index encounter or 6 months prior; and (2) a principal or secondary diagnosis for opioid use/abuse/dependency during the index encounter or 6 months prior. The 6-month baseline period was applied to allow for the identification of recent opioid use. The at most 8-day threshold applied to any inpatient stays that followed the index ED encounter was applied in collaboration with the study medical experts’ input to increase the probability that the index ED encounter was OIC-related, given that an OIC-related hospitalization is very unlikely to last 8 days or more.
Patients were classified into either the OIC-Rx cohort (patients who received a prescription medication approved to treat OIC; methylnaltrexone SC, naloxegol, naldemedine, lubiprostone) or the No OIC-Rx cohort (patients who did not receive OIC-Rx in the ED setting). Among patients in the OIC-Rx cohort, the subgroup receiving methylnaltrexone SC were identified, as most (more than 90%) patients in the OIC-Rx cohort were treated with methylnaltrexone SC. The index ED encounter was defined as the first observed ED encounter meeting the aforementioned sample selection criteria for the OIC-Rx cohort, or a randomly selected ED encounter from all potential index ED encounters for the No OIC-Rx cohort in the absence of OIC-Rx use as an anchor date for the index. The index encounter spanned from the ED admission date to the discharge date, with the following 30-day period defined as the 30-day post-discharge period.
Measures and Statistical Analysis
Descriptive statistics were presented, consisting of means, standard deviations (SD), and medians for continuous variables, and frequency counts and percentages for categorical variables.
Cohort Balancing
Entropy balancing was used to reweight the characteristics of patients included in the OIC-Rx and No OIC-Rx cohorts [19]. Patients in the No OIC-Rx cohort were reweighted such that the specified variables had the exact same mean and SD for continuous variables, or proportion for categorical variables, as those in the OIC-Rx cohort. Weights were normalized so that the sum of weights was equal to the number of patients in each cohort, thus maintaining the original sample size. Potential differences in more than 50 characteristics were adjusted for between the cohorts, including demographic (e.g., age, ethnicity), hospital (e.g., bed size, region), and ED encounter (e.g., OIC-related procedures) characteristics (Supplementary Table S1). Entropy balancing was also done for patients in the OIC-Rx cohort receiving methylnaltrexone SC and patients in the No OIC-Rx cohort.
Study Outcomes and Analysis
Study outcomes were measured during the index ED encounter and 30-day post-discharge period, including HRU and costs, and were compared between the OIC-Rx and No OIC-Rx cohorts using an intent-to-treat approach to assess the impact of OIC-Rx treatment use in the ED setting. HRU included discharge status from the ED (i.e., discharged home or admitted to an inpatient stay), number of inpatient days, length of inpatient stay, and proportion of patients with any re-encounter (inpatient or outpatient) during the 30-day post-discharge period. Costs included those associated with the index ED encounter and those incurred during the 30-day post-discharge period. Costs were adjusted to 2020 USD using the medical component of the Consumer Price Index and were reported from the hospital perspective [20].
Discharge status and proportion of patients with any re-encounter in the post-discharge period were compared between OIC-Rx and No OIC-Rx cohorts by weighted logistic regression models with robust standard errors, using a random effect at the hospital level (clusters), and reported as odd ratios (ORs), with 95% confidence intervals (CIs) and p values. Number of inpatient days, length of inpatient stay, and costs were compared between the cohorts by weighted ordinary least square regressions with robust standard errors, using a random effect at the hospital level (clusters), and reported as mean differences, with 95% CIs and p values.
Number of inpatient days and costs were also compared between the subgroup of patients in the OIC-Rx cohort who received methylnaltrexone SC and entropy-balanced patients in the No OIC-Rx cohort. Additionally, a separate stratification analysis was conducted to evaluate cost comparisons by bed size (fewer than 400 or at least 400 beds) and health insurance type (Medicare, Medicaid, or Commercial).
Cancer Subsample Analyses
The study outcomes were replicated and compared between the OIC-Rx and No OIC-Rx cohorts among a subsample of patients with a cancer diagnosis code (i.e., International Classification of Diseases, 10th revision, Clinical Modification [ICD-10 CM] codes for malignant neoplasms: C00-C96, D49) during the index ED encounter. Entropy balancing was used to reweight the characteristics of patients in the subsample. The results for the subsample analysis are described in the Supplementary material.
Results
Patient Characteristics
Of patients with OIC with an ED encounter, 11,135 (34%) who received an OIC-Rx in the ED, and 21,474 (66%) who did not, are included in the study (Fig. 1). Of patients in the OIC-Rx cohort, 10,330 (93%) received methylnaltrexone SC and 805 (7%) received another OIC-Rx (i.e., lubiprostone or naloxegol). After entropy balancing, the median age in both cohorts was 60 years, and 60% were female (Table 1). More than half (52%) of patients were covered by Medicare and 23% by Commercial insurance. The characteristics of patients in the methylnaltrexone SC subgroup were similar to those of the overall population (data not shown).
Identification of patients with OIC in the OIC-Rx and No OIC-Rx cohorts. ED emergency department, IP inpatient, OIC opioid-induced constipation. 1Data was available from Q1 2016 to Q3 2019. 2Most patients received a peripherally acting mu-opioid receptor antagonist (PAMORA), which was overwhelmingly methylnaltrexone SC injections (93% of patients in the overall OIC-Rx sample and 90% of patients in the cancer OIC-Rx sample). Few patients also received chloride channel activators, e.g., lubiprostone (for drug-induced constipation)
Healthcare Resource Utilization
Patients in the OIC-Rx cohort had a 0.7 inpatient day reduction per OIC ED encounter versus the No OIC-Rx cohort (0.3 vs. 0.9; mean difference [95% CI] − 0.7 [− 0.76, − 0.56]; p < 0.001; Fig. 2a). Those receiving an OIC-Rx had a 64% decreased odds of being hospitalized (11% vs. 26%; OR [95% CI] 0.36 [0.31, 0.41]; p < 0.001; Fig. 2b) and if hospitalized, a 1-day reduction in the length of stay versus the No OIC-Rx cohort (2.7 vs. 3.8; mean difference [95% CI] − 1.0 [− 1.22, − 0.84]; p < 0.001; Fig. 2a). During the post-discharge period, patients receiving an OIC-Rx had a 35% decreased odds of any re-encounter (34% vs. 44%; OR [95% CI] 0.65 [0.59, 0.71]; p < 0.001) versus the No OIC-Rx cohort (Fig. 2b).
Healthcare resource utilization for the OIC-Rx and No OIC-Rx cohorts: A inpatient days and length of stay, and B discharge from the ED and re-encounters. *Significant at the 5% level. ED emergency department, OIC opioid-induced constipation. 1Inpatient days was calculated among all patients in the cohort and length of inpatient stay was calculated among those with an inpatient stay as the difference between discharge date and admit date plus 1 day. 2Mean differences were estimated for continuous variables using weighted ordinary least squares regression models. A mean difference less than 0 indicates fewer inpatient days on average in OIC-Rx compared to No OIC-Rx. Odds ratios and 95% CIs were estimated for binary variables using weighted logistic regressions. An odds ratio less than 1 indicates a lower odds of OIC-Rx having the outcome than No OIC-Rx. 3Methylnaltrexone SC only results are reported among patients in the OIC-Rx cohort who received methylnaltrexone SC specifically
In the methylnaltrexone SC subgroup, patients in the OIC-Rx cohort had 0.7 fewer inpatient days per OIC ED encounter versus the No OIC-Rx cohort (0.3 vs. 0.9; mean difference [95% CI] − 0.7 [− 0.75, − 0.56]; p < 0.001; Fig. 2a).
Healthcare Costs
Overall, the OIC-Rx cohort had a $732 reduction in healthcare costs per OIC ED encounter versus the No OIC-Rx cohort ($1724 vs. $2455; mean difference [95% CI] − 732 [− 916, − 548]; p < 0.001; Fig. 3). Stratification analyses demonstrated that the cost reductions were generally higher in larger hospitals (i.e., at least 400 beds) and among patients with Medicare or Commercial health insurance (Fig. 3). In the Medicare population, the cost reductions per OIC ED encounter was $554 (p < 0.001) for hospitals with fewer than 400 beds and $1168 (p < 0.001) if 400 beds or more. In the Commercial population, the reduction was $568 (p = 0.001) and $1143 (p < 0.001), respectively.
Mean reduction in healthcare costs per OIC ED encounter in the OIC-Rx cohort compared to patients in the No OIC-Rx cohort. *Significant at the 5% level. 1Medicaid includes Medicaid, charity, indigent, and self-pay (i.e., Medicaid/Uninsured). 2Commercial includes workers compensation, direct employer contract, other government payors, and other. 3Methylnaltrexone SC only results are reported among patients in the OIC-Rx cohort who received methylnaltrexone SC specifically
During the 30-day post-discharge period, patients in the OIC-Rx cohort had a $421 reduction in costs associated with any re-encounters versus the No OIC-Rx cohort ($1527 vs. $1947; mean difference [95% CI] − 421 [− 707, − 135]; p = 0.004).
In the methylnaltrexone SC subgroup, patients in the OIC-Rx cohort had a $781 reduction in costs per OIC-ED encounter versus the No OIC-Rx cohort ($1603 vs. $2384; mean difference [95% CI] − 781 [− 963, − 599]; p < 0.001; Fig. 3). During the post-discharge period, the methylnaltrexone SC subgroup of the OIC-Rx cohort had a $392 reduction in costs associated with any re-encounters versus the No OIC-Rx cohort ($1545 vs. $1937; mean difference [95% CI] − 392 [− 687, − 96]; p = 0.009).
Modeling Impact of Methylnaltrexone SC or OIC-Rx Use on a Hospital
On the basis of an average of 36,050 annual ED encounters per hospital with any OIC encounters with methylnaltrexone SC use observed in the database, 51 of which were OIC ED encounters and 7 of which were OIC ED encounters where methylnaltrexone SC was received, patients from an average of 44 OIC ED encounters may be potential methylnaltrexone SC users. The potential impact of methylnaltrexone SC use versus no OIC-Rx use may thus result in an estimated cost saving of $1173 and a 0.7 inpatient day reduction per OIC ED encounter. In a simulation where 50% of patients without OIC-Rx used methylnaltrexone SC instead, there would be an estimated annual cost saving of $25,806 ($506 per OIC ED encounter) and an annual reduction of 15 inpatient days.
Similarly, on the basis of an average of 35,323 annual ED encounters per hospital with any OIC encounters with OIC-Rx use observed in the database, 50 of which were OIC ED encounters and 9 of which were OIC ED encounters where OIC-Rx was received, an average of 41 OIC ED encounters may be potential OIC-Rx users. The potential impact of OIC-Rx use versus no OIC-Rx use may result in an estimated cost saving of $1152 and a 0.7 inpatient day reduction per ED encounter. In a simulation where 50% of patients without OIC-Rx used OIC-Rx instead, there would be an estimated annual cost saving of $23,616 ($472 per OIC ED encounter) and an annual reduction of 14 inpatient days.
Discussion
In this real-world evaluation of patients with OIC in the ED setting, OIC-Rx use was associated with an increased odds of being discharged home from the ED, a reduction in inpatient days, and reduced odds of re-encounter in the 30-day post-discharge period versus no OIC-Rx use. These findings were largely driven by methylnaltrexone SC use, with over 90% of patients in the OIC-Rx cohorts receiving methylnaltrexone SC. The reduction in HRU found in this study translated to more than $1000 in total healthcare cost savings during the ED encounter and 30-day post-discharge periods.
Literature evaluating the impact of OIC-Rx use in the ED on HRU and costs in US real-world clinical practice is limited. OIC-Rx like methylnaltrexone SC has been shown to improve clinical outcomes, such as rescue-free bowel movement and laxation, in patients with OIC [21, 22]. In a previous chart review study of patients in the intensive care unit of a London hospital, critically ill patients (e.g., with pneumonia, pancreatitis, and cardiogenic and septic shock, among other conditions) with OIC treated with methylnaltrexone SC demonstrated improved and faster laxation (within 7.8 h vs. 96.0 h) and increased progression to full enteral feeding (100% vs. 50%) versus patients receiving conventional rescue therapy (i.e., stimulant laxatives) [22]. Furthermore, in a model-based analysis of methylnaltrexone clinical trial data, patients treated with methylnaltrexone experienced less time with constipation symptoms (38 days fewer) and had improved QoL versus patients treated with laxatives [23]. Meta-analytic evidence demonstrating significant clinical efficacy with the use of methylnaltrexone SC has suggested that the improved clinical efficacy may lead to reduced HRU, increased work productivity, and lower cost utilization, though prospective studies to evaluate these associations are warranted, especially in the context of the ED [21]. While reduced HRU and costs are aligned with our findings, work productivity could not be evaluated, and thus our results likely underestimate the true benefits of OIC-Rx from a patient perspective.
The improvements observed with OIC-Rx in prior studies [21,22,23] are particularly important given the large patient burden associated with OIC [3]. While opioids can provide significant relief and improve quality of life for patients suffering from chronic pain, they are also associated with adverse events like nausea, vomiting, and constipation [3, 24]. Many patients may find it challenging to balance effective pain relief through the use of opioids with their common side effects, including constipation [2]. Unfortunately, tolerance to opioid-induced gastrointestinal side effects like OIC rarely develops, often resulting in patients needing to modify or discontinue opioid therapy to relieve constipation [2, 3]. Consequently, patients who modify opioid therapy as a result of OIC report poorer QoL, more pain-related resource use (e.g., surgery, ED visits, hospitalizations), and lower treatment adherence than those who do not modify therapy [25]. If opioid therapy is sustained, the continual presence of OIC may also be associated with poorer patient QoL and impairment of daily activities and well-being [3]. More specifically, patients with OIC have been shown to experience impairments in work productivity, difficulties with social and intimate interactions, low self-esteem, and feelings of anxiety, depression, and embarrassment [2, 3]. Taken together, the emotional, symptom, and QoL burden experienced by patients with OIC translates to significantly higher rates of hospitalization and ED visits, as well as higher costs versus patients without OIC [5,6,7] pointing toward the clinical and economic importance of optimally managing OIC.
In light of the functional, social, and economic impact of OIC on patients, the decreased odds of being admitted to an inpatient stay, the reduction in inpatient days upon admittance, and the reduction of re-encounters in the 30-day post-discharge period we observed, the use of OIC-Rx may help reduce the hospital burden and improve patient QoL. Indeed, on an average hospital scale observed in our data, even if half of patients with OIC who would normally not receive OIC-Rx in the ED were to receive OIC-Rx, this would result in an annual reduction of 14 inpatient days and an associated cost saving of $23,616 per year. Altogether, these findings highlight the potential for OIC-Rx to provide considerable relief for constipation symptom control, which may in turn reduce the need for lengthy and costly hospital stays and lead to important improvements in QoL for the patient population with OIC.
Limitations
The study findings should be interpreted in the context of certain limitations. Given that this study was based in the ED setting, patients with OIC who did not visit the ED were not captured. Additionally, with the lack of a diagnosis code specific to OIC, the total population, and thus the true burden of OIC, are likely underestimated. Similarly, the total number of ED OIC encounters may be underestimated in our database, and hospitals included in the database may not be representative of all US hospitals. Considering the absence of a specific code for OIC, on the basis of the study medical experts’ input, the inclusion criteria requiring a primary or secondary diagnosis for constipation during the ED encounter and opioid use/abuse/dependency or OIC-Rx use during or preceding the ED encounter was used. However, this definition may not have been comprehensive to ensure that all ED encounters considered in the current study were due to OIC. The study was limited to clinical information available in the encounters-level database, so measures of efficacy (e.g., validated instrument scores, physician notes on symptom improvement), safety (e.g., adverse event reporting), reasons for patients receiving or not receiving OIC-Rx in the ED, and information outside the encounter level (e.g., over-the-counter laxatives, outpatient management) were not available or were incomplete. In addition, information on other OIC management approaches, such as enemas and digital disimpaction, are not well reported and thus their potential impact on outcomes could not be fully accounted for in our analyses. Lastly, among patients who were admitted on the same day as their ED encounter, the study database cannot distinguish between patients receiving OIC-Rx in the ED versus during their inpatient admission; in such cases, patients were assumed to receive OIC-Rx in the ED setting.
Conclusion
This retrospective, hospital encounter-based analysis of patients with OIC demonstrated that those who received OIC-Rx in the ED had a decreased odds of being hospitalized; and if hospitalized, patients had a reduction in the length of inpatient stay, fewer encounters in the 30-day post-discharge period, and associated cost savings for insurance agencies and healthcare providers. With the scarcity of real-world literature on OIC-Rx use in US clinical practice, further research is warranted to confirm the current results and the impact of OIC-Rx use on the patient and economic burden of OIC.
References
Farmer AD, Holt CB, Downes TJ, et al. Pathophysiology, diagnosis, and management of opioid-induced constipation. Lancet Gastroenterol Hepatol. 2018;3(3):203–12. https://doi.org/10.1016/S2468-1253(18)30008-6.
Andresen V, Banerji V, Hall G, et al. The patient burden of opioid-induced constipation: new insights from a large, multinational survey in five European countries. United Eur Gastroenterol J. 2018;6(8):1254–66. https://doi.org/10.1177/2050640618786145.
Argoff CE. Opioid-induced constipation: a review of health-related quality of life, patient burden, practical clinical considerations, and the impact of peripherally acting mu-opioid receptor antagonists. Clin J Pain. 2020;36(9):716–22. https://doi.org/10.1097/AJP.0000000000000852.
Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med. 2009;10(1):35–42. https://doi.org/10.1111/j.1526-4637.2008.00495.x.
Fernandes AW, Kern DM, Datto C, et al. Increased burden of healthcare utilization and cost associated with opioid-related constipation among patients with noncancer pain. Am Health Drug Benefits. 2016;9(3):160–70.
Olufade T, Kong AM, Princic N, et al. Comparing healthcare utilization and costs among Medicaid-insured patients with chronic noncancer pain with and without opioid-induced constipation: a retrospective analysis. Am Health Drug Benefits. 2017;10(2):79–86.
Wan Y, Corman S, Gao X, et al. Economic burden of opioid-induced constipation among long-term opioid users with noncancer pain. Am Health Drug Benefits. 2015;8(2):93–102.
Vallerand AH, Hendry S, Baldys E, et al. Analysis of patient-provider interactions regarding the burden and treatment of opioid-induced constipation in adults with chronic noncancer pain. Pain Med. 2019;20(5):889–96. https://doi.org/10.1093/pm/pny151.
Argoff CE, Brennan MJ, Camilleri M, et al. Consensus recommendations on initiating prescription therapies for opioid-induced constipation. Pain Med. 2015;16(12):2324–37. https://doi.org/10.1111/pme.12937.
Caffrey J, Pensa G. Who gets constipation? What are the causes? What is an evidence-based approach management? In: Graham A, Carlberg DJ, editors. Gastrointestinal emergencies. Cham: Springer; 2019. p. 185–187.
Niv G, Grinberg T, Dickman R, et al. Perforation and mortality after cleansing enema for acute constipation are not rare but are preventable. Int J Gen Med. 2013;6:323–8. https://doi.org/10.2147/IJGM.S44417.
Muller-Lissner S, Bassotti G, Coffin B, et al. Opioid-induced constipation and bowel dysfunction: a clinical guideline. Pain Med. 2017;18(10):1837–63. https://doi.org/10.1093/pm/pnw255.
Salix Pharmaceuticals, Inc. Highlights of Prescribing Information: RELISTOR (methylnaltrexone bromide). 2020;1–53.
Bull J, Wellman CV, Israel RJ, et al. Fixed-dose subcutaneous methylnaltrexone in patients with advanced illness and opioid-induced constipation: results of a randomized, placebo-controlled study and open-label extension. J Palliat Med. 2015;18(7):593–600. https://doi.org/10.1089/jpm.2014.0362.
Watkins JL, Eckmann KR, Mace ML, et al. Utilization of methylnaltrexone (Relistor) for opioid-induced constipation in an oncology hospital. P T. 2011;36(1):33–6.
Slatkin N, Thomas J, Lipman AG, et al. Methylnaltrexone for treatment of opioid-induced constipation in advanced illness patients. J Support Oncol. 2009;7(1):39–46.
Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;358(22):2332–43. https://doi.org/10.1056/NEJMoa0707377.
Nishie K, Yamamoto S, Yamaga T, et al. Peripherally acting mu-opioid antagonist for the treatment of opioid-induced constipation: systematic review and meta-analysis. J Gastroenterol Hepatol. 2019;34(5):818–29. https://doi.org/10.1111/jgh.14586.
Hainmueller J, Xu Y. ebalance: a stata package for entropy balancing. J Stat Softw. 2013;54(7):1–18.
Bureau of Labor Statistics. Consumer Price Index 2020. https://www.bls.gov/cpi/tables/supplemental-files/home.htm. Accessed 12 July 2021.
Mehta N, O’Connell K, Giambrone GP, et al. Efficacy of methylnaltrexone for the treatment of opiod-induced constipation: a meta-analysis and systematic review. Postgrad Med. 2016;128(3):282–9. https://doi.org/10.1080/00325481.2016.1149017.
Sawh SB, Selvaraj IP, Danga A, et al. Use of methylnaltrexone for the treatment of opioid-induced constipation in critical care patients. Mayo Clin Proc. 2012;87(3):255–9. https://doi.org/10.1016/j.mayocp.2011.11.014.
Earnshaw SR, Klok RM, Iyer S, et al. Methylnaltrexone bromide for the treatment of opioid-induced constipation in patients with advanced illness–a cost-effectiveness analysis. Aliment Pharmacol Ther. 2010;31(8):911–21. https://doi.org/10.1111/j.1365-2036.2010.04244.x.
Busse JW, Wang L, Kamaleldin M, et al. Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA. 2018;320(23):2448–60. https://doi.org/10.1001/jama.2018.18472.
Gupta S, Patel H, Scopel J, et al. Impact of constipation on opioid therapy management among long-term opioid users, based on a patient survey. J Opioid Manag. 2015;11(4):325–38. https://doi.org/10.5055/jom.2015.0282.
Acknowledgements
Funding
This study was funded by Bausch Health US, LLC, including the journal’s Rapid Service and Open Access fees. The study sponsor was involved in several aspects of the research, including the study design, the interpretation of data, and the development of the manuscript.
Medical Writing and Other Assistance
Medical writing support was provided by a professional medical writer, Christine Tam, an employee of Analysis Group, Inc. Support for this assistance was funded by Bausch Health US, LLC. The authors would like to thank Ankur Dashputre for technical review of the manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Patrick Gagnon-Sanschagrin, Jessica Maitland, and Annie Guérin contributed to study conception and design, collection and assembly of data, and data analysis and interpretation. W. Frank Peacock, George Joseph, and Neal Slatkin contributed to study conception and design, data analysis and interpretation. All authors reviewed and approved the final content of this manuscript.
Prior Presentation
Part of the material in this manuscript was presented at the 39th Annual Emergencies in Medicine Conference held on March 7–12, 2021 in Park City, Utah, USA as a poster presentation.
Disclosures
W. Frank Peacock received research grants from Abbott, Boehringer Ingelheim, Braincheck, CSL Behring, Daiichi-Sankyo, ImmunArray, Janssen, Ortho Clinical Diagnostics, Portola, Relypsa, and Roche; serves as a consultant for Abbott, AstraZeneca, Bayer, Beckman, Boehringer Ingelheim, Ischemia Care, LLC, ImmunArray, Instrument Labs, Janssen, Nabriva, Ortho Clinical Diagnostics, Relypsa, Roche, Quidel, Salix, and Siemens; has provided expert testimony for Johnson & Johnson; and has stock/ownership interests in AseptiScope Inc, Brainbox Inc, Comprehensive Research Associates LLC, Emergencies in Medicine LLC, and Ischemia Care, LLC. George Joseph and Neal Slatkin are employees of Bausch Health US, LLC, which funded the development and conduct of this study and manuscript. Patrick Gagnon-Sanschagrin, Jessica Maitland, and Annie Guérin are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Bausch Health US, LLC.
Compliance with Ethics Guidelines
Data are de-identified and comply with the requirements of the Health Insurance Portability and Accountability Act. Therefore, no institutional review board approval was needed.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available because they were used pursuant to a data use agreement.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Peacock, W.F., Slatkin, N., Gagnon-Sanschagrin, P. et al. Opioid-Induced Constipation: Cost Impact of Approved Medications in the Emergency Department. Adv Ther 39, 2178–2191 (2022). https://doi.org/10.1007/s12325-022-02090-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02090-9