Abstract
Introduction
CD19-directed chimeric antigen receptor T cells (CAR T) are approved for treatment of adults with relapsed/refractory diffuse large B cell lymphoma (DLBCL) following at least two lines of therapy.
Methods
This study describes real-world treatment patterns after CAR T in adults with DLBCL. It includes adults diagnosed with DLBCL in IBM MarketScan Commercial and Medicare Supplemental healthcare claims databases administered CAR T between 2017 and 2019 (index event) and at least 6 months of continuous health plan enrollment pre-index. Kaplan-Meier methods were used to estimate risk and time to first subsequent treatment after CAR T, as a proxy for CAR T failure.
Results
Among 129 patients meeting study criteria, most (123; 95.4%) were hospitalized during CAR T therapy. Median length of stay was 17 (25th–75th percentile, 13–22) days. Estimated 6-month risk of subsequent treatment was 36.2% (95% confidence interval [CI] 27.1–45.8%). During median follow-up of 195 (25th–75th percentile, 102–362) days, median time to the first line of therapy after CAR T, accounting for censoring, was 378 days (95% CI 226, not reached). Among 48 patients who received another therapy after CAR T, 58.3% received immunotherapy, 50.0% radiation therapy, 25.0% chemotherapy, 25.0% targeted therapy, and 12.5% hematopoietic stem cell transplant.
Conclusions
Among real-world patients with DLBCL treated with CAR T, the risk of not achieving a durable response is considerable; additional, effective options for DLBCL salvage treatment are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Little is known regarding how real-world patients who do not respond to chimeric antigen receptor T cells (CAR T) or do not achieve a durable response are managed following CAR T therapy failure. |
What was learned from the study? |
Most CAR T therapy for diffuse large B cell lymphoma (DLBCL) occurs in the hospital, with a median length of stay of 17 days. |
The risk of not achieving a durable response is considerable, with a 6-month probability of subsequent treatment of ~ 35%. |
The variety of treatments administered suggests no standard of care after CAR T. |
Additional, effective options for DLBCL salvage treatment are needed. |
Introduction
CD19-directed chimeric antigen receptor T cell (CAR T) therapies axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel were approved in the US in 2017, 2018, and 2021, respectively, for the treatment of adult patients with relapsed/refractory diffuse large B cell lymphoma (DLBCL) following at least two lines of therapy. These approvals were based on single-arm open-label clinical trials that reported overall response rates of 82%, 52%, and 68%, respectively, among patients with DLBCL [1,2,3]. As is standard for pivotal trials of new therapies, restrictive inclusion and exclusion criteria for enrollment were used, and the trials were conducted in tightly controlled clinical settings with close monitoring and follow-up. A recent analysis found that participants in clinical trials evaluating CAR T were younger than patients receiving CAR T in real-world settings, and all but one of the clinical trials excluded participants with comorbid conditions that might interfere with treatment or assessments [4].
While real-world evidence is accumulating on the safety and effectiveness of CAR T therapy in routine clinical practice [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27], most of these studies may not be generalizable because they represent the experience of a single center or a few academic centers and/or they reported on the experience of few patients (i.e., < 50). Moreover, most of these studies did not report how patients were treated after CAR T failure. Thus, little is known regarding how patients who do not respond to CAR T or do not achieve a durable response are managed following CAR T therapy failure. Thus, the objective of this study was to describe real-world treatment patterns after CAR T therapy among patients with DLBCL in the US, using a nationwide claims database.
Methods
Data
This retrospective cohort study used claims data for CAR T administration from the IBM MarketScan® Commercial and Medicare Supplemental healthcare claims databases between January 1, 2017, and December 31, 2019. IBM MarketScan® is an employer-sponsored insurance claims database, including approximately 20 million insured patients and their dependents annually in the US, with a complete longitudinal record of inpatient/outpatient services and prescription drug claims covered under fee-for-service and capitated health plans. MarketScan data are deidentified; thus, ethics committee approval was not required.
Study Population
Adults with DLBCL who received CAR T therapy were identified. The index date was the date of first CAR T administration, based on at least one procedure code for administration of CAR T therapy (inpatient, central vein: XW043C3; inpatient, peripheral vein: XW033C3; or outpatient: 0540T). Each patient was also required to be ≥ 18 years of age on the index date and have at least 6 months of continuous health plan enrollment pre-index. This 6-month baseline period ensured enough longitudinal data were available to describe baseline characteristics and recent treatments. Patients were required to have at least one diagnosis code for DLBCL (ICD-10-CM: C83.3x) during the baseline period and were excluded if they had a baseline diagnosis of acute lymphoblastic leukemia (ICD-10-CM: C91.0x), which is another approved indication for tisagenlecleucel.
Outcomes
Patients were followed from the index date until insurance plan disenrollment or December 31, 2019, whichever came first. Any of the following treatments (identified using national drug codes [NDCs] and procedure codes) after CAR T were a proxy for CAR T failure: radiation therapy; chemotherapy (e.g., bendamustine, gemcitabine); targeted therapy (e.g., ibrutinib); immunotherapy (e.g., rituximab); transplant (hematopoietic stem cell transplant; or a second round of CAR T. We assumed that patients receiving cyclophosphamide and fludarabine therapy up to 7 days after the first CAR T claim were receiving lymphodepleting chemotherapy; thus, administration of either of these medications within this time frame was not counted as first-line therapy after CAR T. We also assumed that CAR T-related claims or stem cell transplants up to 14 days after the index date were not a second round of CAR T or a stem cell transplant, respectively.
Lines of therapy after CAR T therapy were defined as follows. The first line of therapy included the first post-CAR T treatment plus any treatment initiated up to 60 days after the first treatment’s start date. The second line of therapy was defined as treatments initiated after the first line of therapy and included all treatments initiated up to 60 days after first treatment in the second line of therapy’s start date. If a patient received a transplant, this was considered a separate line of therapy.
Variables
Patient characteristics (age, sex, insurance type, US census region, and year of CAR T administration) were assessed on the index date. Location of CAR T administration was categorized as inpatient or outpatient, and duration of hospitalization was assessed from admission and discharge dates for CAR T administered during an inpatient stay. We identified DLBCL treatments based on treatment guidelines in effect in December 2019 (the end of the study period) from the American Cancer Society (https://www.cancer.org/cancer/non-hodgkin-lymphoma/treating/chemotherapy.html) and the National Comprehensive Cancer Network [28]. Baseline treatment (within 6 months pre-index) with radiation therapy, chemotherapy, targeted therapy, immunotherapy, or transplant (as defined above) was identified as follows:
-
Chemotherapy: bendamustine, bleomycin, carboplatin, chlorambucil, cisplatin, cladribine, cytarabine, doxorubicin, etoposide, gemcitabine, ifosfamide, methotrexate, mitoxantrone, oxaliplatin, pentostatin, pralatrexate, procarbazine, vincristine, vinorelbine
-
Targeted therapy: acalabrutinib, belinostat, bortezomib, copanlisib, duvelisib, ibrutinib, idelalisib, romidepsin, venetoclax
-
Immunotherapy: alemtuzumab, brentuximab vedotin, ibritumomab, lenalidomide, obinutuzumab, ofatumumab, pembrolizumab, polatuzumab vedotin, rituximab, thalidomide
-
Hematopoietic stem cell transplant
-
Radiation therapy
Statistical Analyses
Counts and frequencies for categorical variables and mean (standard deviation [SD]) or median (25th–75th percentile) for continuous variables are reported for patient characteristics and prior treatments. Among patients receiving a treatment following CAR T, counts and frequencies are also reported for individual treatments as well as first and second line of therapy after CAR T. Kaplan-Meier methods are used to estimate cumulative risks of subsequent treatment, with 95% confidence intervals (95% CI).
Results
Patient characteristics
We identified 129 adults with DLBCL receiving CAR T in the US from 2017 to 2019 (Table 1). Mean (SD) patient age at the index date was 55.7 (11.1) years, and 34.1% of patients were female. Most patients (86.8%) were commercially insured. By year, 3.1%, 41.9%, and 55.0% of patients received CAR T in 2017, 2018, and 2019, respectively. Most patients (95.3%) were hospitalized during CAR T administration; the median length of stay was 17 (25th–75th percentile, 13–22) days.
Prior Treatments
Most patients (69.0%) were treated with radiation therapy or systemic treatment at baseline: radiation therapy (25.6%), chemotherapy (45.7%), targeted therapy (11.6%), immunotherapy (51.9%), or transplant (5.4%). The most used individual systemic treatment within 6 months before CAR T was rituximab (45.7%) (Supplementary Material Table S1).
Treatments after CAR T
Median follow-up after CAR T administration was 195 (25th–75th percentile 102–362) days. The 6-month risk of receiving another line of therapy after CAR T was 36.2% (95% CI: 27.1%, 45.8%) and the 12-month risk was 47.9% (95% CI 37.0%, 58.9%) (Fig. 1). The median time to initiation of the first line of therapy after CAR T, accounting for censoring, was 378 (95% CI: 226, not reached) days.
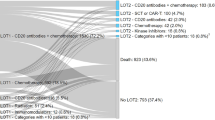
Among the 48 patients who were treated after CAR T, median time between treatments was 100 (25th–75th percentile, 53–144) days. Subsequent treatments initiated included radiation therapy (50.0%), chemotherapy (25.0%), immunotherapy (58.3%), targeted therapy (25.0%), or hematopoietic stem cell transplant (12.5%) (Table 2). The most common first lines of therapy after CAR T were ibrutinib in five patients (12.8%), pembrolizumab in five patients (12.8%), lenalidomide plus rituximab in four patients (10.3%), lenalidomide alone in three patients (7.7%), and rituximab alone in three patients (7.7%); all other treatments or combinations of treatments were used by only one or two patients (Table 3). Nine patients received a second line of therapy during follow-up, including pembrolizumab monotherapy (2 patients [22.2%]), transplant (2 patients [22.2%]), gemcitabine plus oxaliplatin (± rituximab) (2 patients [22.2%]), and other combinations (each 1 patient [11.1%]) (Supplementary Material Table S2).
Discussion
This is one of only a few studies to assess treatment patterns after CAR T therapy among real-world patients with DLBCL. In this study, we analyzed data from a large claims database and reported on real-world treatment patterns for adults in the US with DLBCL who received CAR T between 2017 and 2019, across a variety of practice settings nationwide. Our analysis resulted in two significant findings. First, most patients were treated in the inpatient setting and had a prolonged hospitalization. Second, many patients received salvage therapy after CAR T, and there was no consistent pattern of therapy used in this setting, suggesting that there is no standard of care following CAR T failure.
Recent analyses of claims data reported similar findings to our study regarding the percentage of patients receiving CAR T in an inpatient setting and length of hospitalization. An analysis of 177 Medicare patients (mean, 70 years of age; 59% male) between October 2017 and September 2018 found that 94% received CAR T in the inpatient setting. Among patients administered CAR T in the inpatient setting, the reported median length of stay was 16 (25th–75th percentile, 11–21) days [29]. Similarly, patients receiving CAR T at two Mayo Clinic centers between 2018 and 2020 were hospitalized for a median for 14 days [25]. An analysis of 485 patients treated with CAR T in a hospital setting in France between 2019 and 2020 reported even longer median length of hospitalization, ranging from 24 to 26 days [30]. In an analysis of claims from four US databases, 88–100% of patients in each database were hospitalized during CAR T receipt and median length of stay for CAR T administration ranged from 14 to 17 (range 1–91) days. Mean total cost of care for CAR T administration (inpatient, outpatient, and pharmacy) was considerable, ranging from $353,642 to $525,772 across the four databases [31].
We observed that subsequent treatment following CAR T administration, a proxy for CAR T failure, was common in our study. The probability of receiving an additional line of therapy was > 35% at 6 months and almost 50% at 12 months. Our analysis did not include patients whose disease progressed but who were not subsequently treated, or patients who died, and thus does not capture all patients who failed CAR T. In addition, current treatment guidelines for B cell lymphoma from the National Comprehensive Cancer Network (NCCN) recommend enrolling a patient with DLBCL in a clinical trial if a CAR T has already been given [32], but we were not able to identify patients who went on to receive an investigational agent. Thus, the true CAR T failure rate is likely higher than what we observed in this study. Our results are consistent with an analysis of administrative data with many of the same limitations as this study; that analysis identified 145 patients receiving 3 or more lines of therapy for DLBCL between 2017 and early 2020, including 25 patients who received CAR T as third-line therapy [21]. Among CAR T-treated patients, over a median follow-up time of 8 months, 48.0% (12/25) required a fourth-line therapy.
Among the 48 patients who were subsequently treated after CAR T in our analysis, we found that the median time between treatments was 100 (25th–75th percentile, 53–144) days. These findings are consistent with a recent analysis of CAR T use in 205 patients with DLBCL across four US administrative claims databases that reported that, among patients who were subsequently treated, median time to subsequent therapy after CAR T ranged from 98 to 107 days across databases [31]. To our knowledge, no published real-world study has assessed time to next treatment using Kaplan-Meier methods.
Time to next treatment was assessed in the pivotal trial of axicabtagene ciloleucel, ZUMA-1 [1]. Median time to next anticancer therapy in that trial was 8.7 (range 0.3–53.8) months [33]. The median time to the first line of therapy after CAR T was considerably longer in our study (median 378 days), likely because of differences in how the endpoint was operationalized; in ZUMA-1, time to next treatment was defined as time to initiation of new anticancer therapy (except stem cell transplantation but likely including other investigational treatments) or all-cause mortality.
Patients who were treated following CAR T in our analysis received a diverse range of treatments and combinations of treatments. Most patients received treatments that seemed to be individualized, with no clear pattern in the treatments used. A single-center study from Germany reported that 15 patients experienced relapse/progression at a median of 82 (range 9–269) days after CAR T [24]. These patients received 19 salvage treatments after CAR T failure, including targeted therapy (10 treatments), immunotherapy (4), chemotherapy (3), or radiation therapy (2). A single-center analysis of 53 patients in the US with relapsed or refractory aggressive B cell lymphomas treated with CAR T between 2017 and 2020 reported that 49% (26/53) of patients were subsequently treated and that post-CAR T progression therapy commonly involved clinical trial enrollment or use of novel agents or supportive care, with no clear standard of care [26]. A two-center analysis of 34 patients treated with CAR T between 2018 and 2020 in the US found that, among the 12 patients receiving additional salvage therapy, radiotherapy (7/12), polatuzumab/bendamustine/rituximab (3/12) (Pola-BR), and pembrolizumab (3/12) were the most common [25].
There is currently no therapy approved for patients who have failed CAR T. Moreover, current NCCN treatment guidelines suggest a variety of treatment options for patients in second or later relapse who previously received CAR T, including clinical trial participation, alternative second-line chemotherapy (but the guidelines note patients are unlikely to derive benefit from this approach), palliative radiation therapy, or best supportive care [32]. Our findings support that there is no clear standard of care following CAR T failure and underscores the need for additional, effective treatment options after CAR T failure in adults with DLBCL.
Our findings must be interpreted in the context of a few limitations. We used subsequent treatment initiation as a proxy for CAR T failure and could not capture use of investigational treatments, disease progression, or death. While we are likely to have underestimated the CAR T failure rate, a recent study suggests that most patients who progress on CAR T receive a subsequent therapy; 12/15 patients who progressed on CAR T were subsequently retreated [25]. In our analysis, no information was available in claims regarding number of prior lines of therapy, disease severity, or characteristics of treatment centers (e.g., size, teaching affiliation, prior CAR T experience). Over the study period, information on type of CAR T received was missing for > 90% of patients because the procedure codes for specific CAR T therapies that were recently added were not yet in use and thus outcomes by type of CAR T received could not be examined. We could not determine treatment indication and assumed that any use of cyclophosphamide or fludarabine up to 7 days after the index date was lymphodepleting chemotherapy. As such, patients initiating CAR T combination treatment (e.g., CAR T + rituximab) would be misclassified as initiating a subsequent treatment. We also assumed that all CAR T-related claims or stem cell transplants up to 14 days after the index date were not a second round of CAR T or a stem cell transplant, respectively. It is possible, but unlikely, that these other therapies were given as salvage treatments. The study period, from 2017 to 2019, represented early real-world experience with CAR T therapy in a limited population that may represent a subset of patients with more severe disease and with limited follow-up to observe subsequent treatments. Moreover, the landscape for DLBCL is changing rapidly. Several therapies for DLBCL have been approved recently, including polatuzumab vedotin, selinexor, tafasitamab, and loncastuximab tesirine, which may change how patients are treated after CAR T. Moreover, outpatient administration of CAR T may become more frequent as research has found that CAR T may be safely administered in an outpatient setting [34].
Conclusions
In summary, most CAR T administrations for DLBCL occurred in the hospital setting, and the median length of stay during CAR T administration was substantial, at more than 2 weeks. The risk of requiring additional therapy within 6 months after CAR T was high, supporting the need for additional, effective salvage treatment strategies for patients with DLBCL who do not respond to CAR T or do not achieve a durable response.
References
Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR-T cell therapy in refractory large B cell lymphoma. N Engl J Med. 2017;377:2531–44.
Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56.
Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52.
Apaydin EA, Richardson AS, Baxi S et al. Differences in lymphoma patients between chimeric antigen receptor T-cell therapy trials and the general population. Clin Exp Med. 2021.
Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol. 2020;38:3119–28.
Jacobson CA, Hunter BD, Redd R, et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38:3095–106.
Vercellino L, Di Blasi R, Kanoun S, et al. Predictive factors of early progression after CAR-T cell therapy in relapsed/refractory diffuse large B cell lymphoma. Blood Adv. 2020;4:5607–15.
Kittai AS, Huang Y, Gordon M, et al. Comorbidities predict inferior survival in patients receiving chimeric antigen receptor T cell therapy for diffuse large B cell lymphoma: a multicenter analysis. Transplant Cell Ther. 2021;27:46–52.
Pinnix CC, Gunther JR, Dabaja BS, et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma. Blood Adv. 2020;4:2871–83.
Sermer D, Batlevi C, Palomba ML, et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020;4:4669–78.
Sesques P, Ferrant E, Safar V, et al. Commercial anti-CD19 CAR T cell therapy for patients with relapsed/refractory aggressive B cell lymphoma in a European center. Am J Hematol. 2020;95:1324–33.
Holtzman NG, Xie H, Bentzen S, et al. Immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy for lymphoma: predictive biomarkers and clinical outcomes. Neuro Oncol. 2021;23:112–21.
Abbasi A, Peeke S, Shah N, et al. Axicabtagene ciloleucel CD19 CAR-T cell therapy results in high rates of systemic and neurologic remissions in ten patients with refractory large B cell lymphoma including two with HIV and viral hepatitis. J Hematol Oncol. 2020;13:1.
Schaefer A, Saygin C, Maakaron J, et al. Cytopenias after chimeric antigen receptor t-cells (CAR-T) infusion; patterns and outcomes. Transplant Cell Ther. 2019;25(3 Suppl):S171.
Lin RJ, Lobaugh SM, Pennisi M, et al. Impact and safety of chimeric antigen receptor T-cell therapy in older, vulnerable patients with relapsed/refractory large B cell lymphoma. Haematologica. 2021;106:255–8.
Vitale C, Strati P. CAR-T cell therapy for B-cell non-hodgkin lymphoma and chronic lymphocytic leukemia: clinical trials and real-world experiences. Front Oncol. 2020;10:849.
Khurana A, Hathcock M, Habermann TM, et al. Lines of therapy before autologous stem cell transplant and CAR-T affect outcomes in aggressive non-Hodgkin’s lymphoma. Am J Hematol. 2021;96:E386–9.
Shapiro LC, Mustafa J, Lombardo A, et al. Safety of axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma in an elderly intercity population. Bone Marrow Transplant. 2021;56:1761–3.
Mian A, Wei W, Winter AM, et al. Outcomes and factors impacting use of axicabtagene ciloleucel in patients with relapsed or refractory large B-cell lymphoma: results from an intention-to-treat analysis. Leuk Lymphoma. 2021;62:1344–52.
Casadei B, Argnani L, Guadagnuolo S, et al. Real world evidence of CAR-T cell therapies for the treatment of relapsed/refractory B cell non-Hodgkin lymphoma: a monocentric experience. Cancers (Basel). 2021;13:4789.
Xie J, Wu A, Liao L, et al. Characteristics and treatment patterns of relapsed/refractory diffuse large B-cell lymphoma in patients receiving >/=3 therapy lines in post-CAR-T era. Curr Med Res Opin. 2021;37:1789–98.
Schaefer A, Huang Y, Kittai A, et al. Cytopenias after CD19 chimeric antigen receptor T-cells (CAR-T) therapy for diffuse large B-cell lymphomas or transformed follicular lymphoma: a single institution experience. Cancer Manag Res. 2021;13:8901–6.
Hamadani M, Gopal AK, Pasquini M, et al. Allogeneic transplant and CAR-T therapy after autologous transplant failure in DLBCL: a noncomparative cohort analysis. Blood Adv. 2022;6:486–94.
Dreger P, Dietrich S, Schubert ML, et al. CAR-T cells or allogeneic transplantation as standard of care for advanced large B-cell lymphoma: an intent-to-treat comparison. Blood Adv. 2020;4:6157–68.
Forero-Forero JV, Lengerke-Diaz PA, Moreno-Cortes E et al. Predictors and management of relapse to Axicabtagene Ciloleucel in patients with aggressive B-cell lymphoma. Hematol Oncol Stem Cell Ther. 2021.
Ghafouri S, Fenerty K, Schiller G, et al. Real-world experience of axicabtagene ciloleucel and tisagenlecleucel for relapsed or refractory aggressive B-cell lymphomas: a single-institution experience. Clin Lymphoma Myeloma Leuk. 2021;21:861–72.
Shadman M, Pasquini MC, Ahn KW et al. Autologous transplant versus chimeric antigen receptor T-cell therapy for relapsed DLBCL in partial remission. Blood. 2021.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: B-Cell Lymphomas. Version 6.2019. November 26, 2019.
Kilgore KM, Mohammadi I, Schroeder A, Teigland C, Purdum A, Shah GL. Medicare patients receiving chimeric antigen receptor T-cell therapy for non-Hodgkin lymphoma: a first real-world look at patient characteristics, healthcare utilization and costs. Blood. 2019;134(Suppl1):793 (abstract).
Huguet M, Raimond V, Kaltenbach E, Augusto V, Perrier L. How much does the hospital stay for infusion of anti-CD19 CAR-T cells cost to the French National Health Insurance? Bull Cancer. 2021;108:1170–80.
Keating SJ, Gu T, Jun MP, Pelletier C, McBride A. Health care resource utilization (HCRU) and total costs of care (TCOC) among patients (pts) with diffuse large B-cell lymphoma (DLBCL) treated with chimeric antigen receptor (CAR) T-cell therapies in the United States: an analysis of four claims databases. J Clin Oncol. 2020;38(29 Suppl):76 (abstract).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: B-Cell Lymphomas. Version 4.2021. May 28, 2021.
Jacobson C, Locke FL, Ghobadi A, et al. Long-term survival and gradual recovery of B-cells in patients with refractory large B-cell lymphoma treated with axicabtagene ciloleucel (axi-cel). Blood. 2020;136(Suppl1):40–2 (abstract).
Myers GD, Verneris MR, Goy A, Maziarz RT. Perspectives on outpatient administration of CAR-T cell therapy in aggressive B-cell lymphoma and acute lymphoblastic leukemia. J Immunother Cancer. 2021;9:e002056.
Acknowledgements
Funding
Regeneron Pharmaceuticals, Inc., sponsored and designed the study, funded the medical writing support, and supported the journal’s Rapid Service and Open Access Fees.
Medical Writing and Editorial Assistance
Jonathan Latham, a medical writer supported by funding from Regeneron Pharmaceuticals, Inc., provided drafts and editorial assistance to the authors during preparation of this manuscript.
Prior Presentation
Portions of this work were previously presented in the form of an abstract at the American Society of Clinical Oncology Annual Meeting, May 29–31, 2020, and as a presentation at the 36th ICPE Annual Conference, September 16–17, 2020.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Jessica J. Jalbert, Ning Wu, Chieh-I Chen, Srikanth Ambati, Wenzhen Ge, and Jon E Arnason. Formal analysis: Ning Wu. Investigation, writing, review, and editing: Jessica J. Jalbert, Ning Wu, Chieh-I Chen, Srikanth Ambati, Wenzhen Ge, and Jon E Arnason.
Disclosures
Jessica J. Jalbert, Ning Wu, Chieh-I Chen, Srikanth Ambati, and Wenzhen Ge are employees and stockholders of Regeneron Pharmaceuticals, Inc. Jon E. Arnason has received advisory board fees from Regeneron and Juno/Celgene.
Compliance with Ethics Guidelines
MarketScan data are deidentified; thus, ethics committee approval was not required.
Data Availability
Data for this study, which were obtained from a proprietary source, are not available for sharing.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jalbert, J.J., Wu, N., Chen, CI. et al. Real-World Treatment Patterns After CD19-Directed CAR T Cell Therapy Among Patients with Diffuse Large B Cell Lymphoma. Adv Ther 39, 2630–2640 (2022). https://doi.org/10.1007/s12325-022-02087-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02087-4