Abstract
Introduction
Many treatment regimens have been evaluated in transplant-ineligible (TIE) patients with newly diagnosed multiple myeloma (NDMM). The objective of this study was to compare the efficacy of relevant therapies for the treatment of TIE patients with NDMM.
Methods
Progression-free survival (PFS) and overall survival (OS) from large randomised controlled trials (RCTs) evaluating different treatment options for TIE patients with NDMM were compared in a network meta-analysis (NMA). The NMA includes recent primary and long-term OS readouts from SWOG S0777, ENDURANCE, MAIA, and ALCYONE. Relevant trials were identified through a systematic literature review. Relative efficacy measures (i.e., hazard ratios [HRs] for PFS and OS) were extracted and synthesised in random-effects NMAs.
Results
A total of 122 publications describing 45 unique RCTs was identified. Continuous lenalidomide/dexamethasone (Rd) was selected as the referent comparator. Daratumumab-containing treatments (daratumumab/lenalidomide/dexamethasone [D-Rd], daratumumab/bortezomib/melphalan/prednisone [D-VMP]) and bortezomib/lenalidomide/dexamethasone (VRd) had the highest probabilities of being more effective than Rd continuous for PFS (HR: D-Rd, 0.53; D-VMP, 0.57, VRd, 0.77) and OS (HR: D-Rd, 0.68; VRd, 0.77, D-VMP, 0.78). D-Rd had the highest chance of being ranked as the most effective treatment with respect to PFS and OS. Results using a smaller network focusing on only those regimens that are relevant in Europe were consistent with the primary analysis.
Conclusions
These comparative effectiveness data may help inform treatment selection in TIE patients with NDMM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Many treatment regimens have been evaluated in transplant-ineligible (TIE) patients with newly diagnosed multiple myeloma (NDMM). |
The objective of this network meta-analysis (NMA) was to compare the efficacy of relevant therapies for the treatment of TIE patients with NDMM. |
This analysis incorporated clinical data not included in previous NMAs, and continuous lenalidomide/dexamethasone (Rd) was selected as the referent comparator. |
The results demonstrated that daratumumab/lenalidomide/dexamethasone (D-Rd), daratumumab/bortezomib/melphalan/prednisone, and bortezomib/lenalidomide/dexamethasone had the highest probabilities of being more effective than Rd continuous in improving PFS and OS in TIE patients with NDMM. Overall, D-Rd had the highest chance of being ranked as the most effective treatment with respect to both PFS and OS. Findings from a European NMA were consistent with the global NMA. |
Results of this NMA may help guide the choice of treatment for this patient population. |
Introduction
The treatment landscape of multiple myeloma (MM) has evolved considerably in recent years with the introduction of novel agents. Patients with newly diagnosed MM (NDMM) who are not considered suitable candidates for high-dose chemotherapy and autologous stem cell transplantation because of age or comorbidities are currently treated with combination therapies including steroids, alkylators, and novel agents [1, 2]. Bortezomib, melphalan, and prednisone (VMP), bortezomib, lenalidomide, and dexamethasone (VRd), and lenalidomide and dexamethasone (Rd) are considered standard of care (SOC) for transplant-ineligible (TIE) patients with NDMM [3,4,5]. While many new regimens have been tested in recent years for this patient population, few have been evaluated head to head with SOC other than VMP and Rd in large randomised controlled trials (RCTs).
In the absence of head-to-head comparisons versus all relevant comparators, a network meta-analysis (NMA) can use pooled treatment effects to estimate the relative efficacy of treatment regimens [6, 7]. An NMA can be utilised when more than two possible interventions are available for a specific indication that are linked through a network anchored in a common comparator.
Previous NMAs performed in this setting since 2019 have identified VRd, daratumumab in combination with VMP (D-VMP), and daratumumab in combination with Rd (D-Rd) as the most effective regimens in terms of progression-free survival (PFS) and/or overall survival (OS) [8,9,10,11,12,13,14,15]. The results from these analyses also emphasize the benefits obtained from the use of triplet or quadruplet regimens. Given the rapidly evolving treatment landscape, even recently published NMAs may lack the latest available clinical data in their analyses.
Here, we present an NMA designed to investigate the relative efficacy of relevant therapies for the treatment of TIE patients with NDMM [16, 17]. This analysis incorporates clinical data not included in previous NMAs, including the ENDURANCE trial [18] as well as extended follow-up from the SWOG S0777 [19], ALCYONE [20], and MAIA trials [21], which were published in recent years.
Methods
Systematic Literature Review
A systematic literature review (SLR) was used to identify RCTs evaluating therapies for the treatment of TIE patients with NDMM. Literature databases (PubMed, EMBASE®, the Cochrane Library, the American Society for Hematology, the American Society of Clinical Oncology, and the European Society for Medical Oncology), and ClinicalTrials.gov were searched for relevant studies. Additionally, Health Technology Assessment dossiers for the National Institute for Health and Care Excellence (NICE; UK), Federal Joint Committee (Germany), Canadian Agency for Drugs and Technologies in Health, Scottish Medicines Agency, and Pharmaceutical Benefits Advisory Committee (Australia) were reviewed for additional trials or data not captured in the SLR. Meta-analyses and literature reviews identified during screening were further reviewed for potential publications that were not identified through the initial search.
This review was conducted according to NICE guidelines [22] and used explicit criteria for inclusion of potential sources of evidence. The full eligibility criteria for the SLR are shown in the supplementary material (Table S1). Briefly, key RCTs were included that were conducted in TIE patients with NDMM that assessed the clinical outcomes of first-line treatments for MM and were published in the English language. Two reviewers independently selected studies at the title/abstract and full-text levels, with any disagreements resolved by a third reviewer. The SLR was initially performed on June 16, 2017, and rerun on March 25, 2021, to capture materials published between the two dates. There was no time restriction for the full-text publications; conference proceedings were restricted to those published from 2012. Additional meta-analyses/reviews and ClinicalTrials.gov were also searched for publications not included in the search engines up to June 2021.
Network Meta-analysis
A Bayesian NMA was conducted based on the results from the SLR. The NMA was performed using WinBUGS according to the NICE Decision Support Unit guidelines [23]. Three NMA assumptions (homogeneity, similarity, and consistency) were assessed across all studies. Reported hazard ratios (HRs) from relevant RCTs were applied in the NMA, assuming no violation of the proportional hazards assumption. All analyses were performed using fixed- and random-effects models. The choice between fixed- and random-effects models was based on deviance information criterion (DIC) score and/or the presence of observed heterogeneity in the network [24, 25]. If HRs and associated confidence intervals (CIs) were not reported but Kaplan-Meier curves with corresponding numbers of patients at risk were available, the HRs and CIs were estimated based on the Guyot methodology [26], as recommended by NICE and assuming no violation of proportional hazards. If HRs were reported with only P values, the CIs associated with the reported HRs were also estimated [27].
Outcomes for efficacy (PFS and OS) were compared across all relevant studies. A random-effects model was preferred over a fixed-effects model for OS and PFS because heterogeneity was observed in both networks of evidence. Additionally, the DIC score for these models was lower compared with the fixed-effects model. Results from all studies that included VMP were pooled, as matching-adjusted indirect comparison indicated noninferiority in PFS and OS outcomes regardless of bortezomib dose intensity [28]. A normal likelihood with identity link model was used for PFS. Rd continuous was selected as the referent comparator for the current analysis because it is approved and included in key treatment guidelines across regions [16, 17].
MM-015 [29], TMSG [30], HOVON 49 [31], NMSG [32], and GIMEMA [33] studies had maintenance therapy arms that could be separately included without causing a disconnect in the network and were not pooled with the nonmaintenance treatment arms. E1A06 [34] and HOVON87/NMSG18 [35] studies only had treatment arms allowing for maintenance treatment and as such the treatments from these studies were grouped under one label.
For evaluations of PFS and OS, an HR < 1 indicates that the treatment comparison favours the comparator versus Rd continuous. In total, 100,000 iterations were carried out for each analysis. The probability of a treatment being ranked first was calculated through a comparative analysis of all treatments and the number of times a treatment was found to be the best option from all iterations. OS was calculated using a Cox regression over reconstructed Kaplan-Meier data [26].
Not all patients in the SWOG S0777 (median age 63 years) [19, 36] and ENDURANCE (median age 65 years) [18] studies were TIE. Those studies enrolled patients with NDMM for whom immediate transplant was not intended and included a mix of patients who were TIE as well as patients who were transplant eligible (TE) but chose to decline or defer transplant. Patient age is one of the primary criteria used in assessing transplant eligibility. Based on the results of large randomised trials, autologous stem cell transplant is the preferred treatment among eligible patients under the age of 65 [37]. In the absence of data specifically from TIE patients in these studies, age-based subgroups for whom published data were available (patients aged ≥ 65 years in the SWOG S0777 [PFS and OS] and ENDURANCE [PFS only] trials) were selected to serve as proxies to represent TIE patients in the current analysis. In both trials, there was evidence of treatment-effect heterogeneity based on patient age (supplementary material, Table S2), and thus relying on the intent to treat (ITT) effect estimates in an NMA focused on interventions for TIE NDMM patients would be inappropriate due to the similarity assumption required for NMAs [38].
To evaluate regimens that are appropriate to patient management in Europe, a further analysis was performed using a simplified network comprising the main relevant comparators. Treatment regimens approved by the European Medicines Agency and/or recommended by key European treatment guidelines were included [17].
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Systematic Literature Review
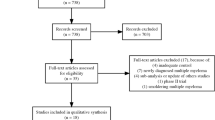
Overall, 122 publications describing 45 unique RCTs fulfilled the eligibility criteria (Fig. 1) and were analysed. Studies were conducted internationally, with sites in Africa, the Asia-Pacific region, Europe, Latin America, and North America. The number of patients enrolled ranged from 40 to 1623. Additional details from the studies identified with the SLR can be found in the supplementary material (Table S3).
Flow chart summarising the systematic literature review. ASCO American Society of Clinical Oncology, ASH American Society of Hematology, EHA European Hematology Association, ESMO European Society for Medical Oncology, MM multiple myeloma, RRMM relapsed/refractory MM, SLR systematic literature review
Network Meta-analysis
Progression-Free Survival
The evidence network for PFS contained 25 different treatment regimens and is shown in Fig. 2A. Compared with Rd continuous, PFS was improved with D-Rd (HR 0.53 [95% credible interval (CrI) 0.30–0.92]), D-VMP (HR 0.57; 95% CrI 0.20–1.59), and VRd (HR 0.77; 95% CrI 0.42–1.41; Fig. 3A). These treatment regimens also had the highest probability of being more effective than Rd continuous (98.4%, 89.4%, and 83.5%, respectively; Fig. 3A). D-Rd had the highest probability of being ranked first among all comparators regarding PFS (46.7%), followed by D-VMP (35.3%) and bortezomib/melphalan/prednisone/thalidomide induction with bortezomib/thalidomide maintenance (5.2%; Fig. 3B).
Evidence network for A PFS and B OS and C PFS and OS using main relevant comparators in Europea. aBlue colour indicates EHA-ESMO–recommended treatments. CMP carfilzomib/melphalan/prednisone, CPR cyclophosphamide/prednisone/lenalidomide, CTD cyclophosphamide/thalidomide/dexamethasone, D-Rd daratumumab/lenalidomide/dexamethasone, D-VMP daratumumab/bortezomib/melphalan/prednisone, DEX dexamethasone, DEX-IFN dexamethasone/interferon alfa 2b, EHA-ESMO European Hematology Association-European Society for Medical Oncology, KRd carfilzomib/lenalidomide/dexamethasone, M-DEX melphalan/dexamethasone, MP melphalan/prednisone, MPR melphalan/prednisone/lenalidomide, MPR-R melphalan/prednisone/lenalidomide as induction, and lenalidomide as maintenance, MPT melphalan/prednisone/thalidomide, MPT-T melphalan/prednisone/thalidomide as induction, and thalidomide as maintenance, NCCN National Comprehensive Cancer Network, OS overall survival, Pembro-Rd pembrolizumab/lenalidomide/dexamethasone, PFS progression-free survival, Rd cont lenalidomide/dexamethasone, continuous, Rd9 lenalidomide/dexamethasone 9 cycles, Rd18 lenalidomide/dexamethasone 18 cycles, TD thalidomide/dexamethasone, VD bortezomib/dexamethasone, VMP bortezomib/melphalan/prednisone, VMP-S bortezomib/melphalan/prednisone/siltuximab, VMPT-VT bortezomib/melphalan/prednisone/thalidomide as induction, and bortezomib/thalidomide as maintenance, VRd bortezomib/lenalidomide/dexamethasone, VTD bortezomib/thalidomide/dexamethasone
Progression-free survival. A Forest plot of PFS HRs of treatments versus Rd continuous by efficacy and probability of being better than Rd continuous, and B rankogram presenting the probability of being ranked first for PFS. CMP carfilzomib/melphalan/prednisone, cont continuous, CPR cyclophosphamide/prednisone/lenalidomide, Crl LL credible interval lower limit, Crl UL credible interval upper limit, CTD cyclophosphamide/thalidomide/dexamethasone, D-Rd daratumumab/lenalidomide/dexamethasone, D-VMP daratumumab/bortezomib/melphalan/prednisone, DEX dexamethasone, DEX-IFN dexamethasone/interferon alfa 2b, HR hazard ratio, KRd carfilzomib/lenalidomide/dexamethasone, M-DEX melphalan/dexamethasone, MP melphalan/prednisone, MPR melphalan/prednisone/lenalidomide, MPR-R melphalan/prednisone/lenalidomide as induction, and lenalidomide as maintenance, MPT melphalan/prednisone/thalidomide, MPT-T melphalan/prednisone/thalidomide as induction, and thalidomide as maintenance, Pembro-Rd pembrolizumab/lenalidomide/dexamethasone; PFS, progression-free survival, Rd lenalidomide/dexamethasone, Rd9 lenalidomide/dexamethasone 9 cycles, Rd18 lenalidomide/dexamethasone 18 cycles, TD thalidomide/dexamethasone, VD bortezomib/dexamethasone, VMP bortezomib/melphalan/prednisone, VMP-S bortezomib/melphalan/prednisone/siltuximab, VMPT-VT bortezomib/melphalan/prednisone/thalidomide as induction, and bortezomib/thalidomide as maintenance, VRd bortezomib/lenalidomide/dexamethasone, VTD bortezomib/thalidomide/dexamethasone
Overall Survival
The evidence network for OS is shown in Fig. 2B and contained 23 different treatment regimens. OS was improved with D-Rd (HR 0.68; 95% CrI 0.48–0.96), VRd (HR 0.77; 95% CrI 0.48–1.23), and D-VMP (HR 0.78; 95% CrI 0.41–1.49) compared with Rd continuous (Fig. 4A). D-Rd, VRd, and D-VMP had the highest probability of being more effective than Rd continuous (98.2%, 87.6%, and 78.0%, respectively; Fig. 4A). The regimens with the highest probability of being ranked first among all comparators in terms of OS were D-Rd (45.9%), VRd (23.1%), and D-VMP (22.6%; Fig. 4B).
Overall survival. A Forest plot of OS HRs of treatments versus Rd continuous by efficacy and probability of being better than Rd continuous and B rankogram presenting the probability of being ranked first for OS. CMP carfilzomib/melphalan/prednisone, CPR cyclophosphamide/prednisone/lenalidomide, Crl LL credible interval lower limit, Crl UL credible interval upper limit, CTD cyclophosphamide/thalidomide/dexamethasone, D-Rd daratumumab/lenalidomide/dexamethasone, D-VMP daratumumab/bortezomib/melphalan/prednisone, DEX dexamethasone, DEX-IFN dexamethasone/interferon alfa 2b, HR hazard ratio, M-DEX melphalan/dexamethasone, MP melphalan/prednisone, MPR melphalan/prednisone/lenalidomide, MPR-R melphalan/prednisone/lenalidomide as induction, and lenalidomide as maintenance, MPT melphalan/prednisone/thalidomide, MPT-T melphalan/prednisone/thalidomide as induction, and thalidomide as maintenance, OS overall survival, Pembro-Rd pembrolizumab/lenalidomide/dexamethasone, Rd cont lenalidomide/dexamethasone, continuous, Rd9 lenalidomide/dexamethasone 9 cycles, Rd18 lenalidomide/dexamethasone 18 cycles, TD thalidomide/dexamethasone, VD bortezomib/dexamethasone, VMP bortezomib/melphalan/prednisone, VMPT-VT bortezomib/melphalan/prednisone/thalidomide as induction, and bortezomib/thalidomide as maintenance, VRd bortezomib/lenalidomide/dexamethasone, VTD bortezomib/thalidomide/dexamethasone
Evidence Network of Relevant Comparators for Europe
A simplified evidence network containing the main relevant comparators for Europe for PFS and OS is shown in Fig. 2C and contained ten unique treatment regimens.
Progression-Free Survival
The regimens with improved PFS compared with Rd continuous were D-Rd (HR 0.53; 95% CrI 0.43–0.66), D-VMP (HR 0.58; 95% CrI 0.37–0.93), and VRd (HR 0.77; 95% CrI 0.55–1.08; Fig. 5A). These regimens also had the highest probability of being more effective than Rd continuous (100%, 98.9%, and 93.2%, respectively; Fig. 5A). D-Rd had the highest probability of being ranked first in terms of PFS, (62%) followed by D-VMP (35%) and VRd (2%; Fig. 5B).
Progression-free survival (using simplified evidence network of main relevant comparators in Europe). A Forest plot of PFS HRs of treatments versus Rd continuous by efficacy and probability of being better than Rd continuous and B rankogram presenting probability of being ranked first in PFS. CMP carfilzomib/melphalan/prednisone, CPR cyclophosphamide/prednisone/lenalidomide, Crl LL credible interval lower limit, Crl UL credible interval upper limit, CTD cyclophosphamide/thalidomide/dexamethasone, D-Rd daratumumab/lenalidomide/dexamethasone, D-VMP daratumumab/bortezomib/melphalan/prednisone, DEX dexamethasone, DEX-IFN dexamethasone/interferon alfa 2b, HR hazard ratio, MP melphalan/prednisone, MPR-R melphalan/prednisone/lenalidomide as induction, and lenalidomide as maintenance, MPT melphalan/prednisone/thalidomide, PFS progression-free survival, Rd cont lenalidomide/dexamethasone, continuous, Rd18 lenalidomide/dexamethasone 18 cycles, VMP bortezomib/melphalan/prednisone, VRd bortezomib/lenalidomide/dexamethasone
Overall Survival
The regimens with improved OS compared with Rd continuous were D-Rd (HR 0.68; 95% CrI 0.54–0.86), VRd (HR 0.77; 95% CrI 0.52–1.14), and D-VMP (HR 0.79; 95% Crl 0.50–1.23; Fig. 6A). The regimens with the highest probability of being more effective than Rd continuous with respect to OS included D-Rd (99.9%), VRd (90.1%), and D-VMP (85.5%; Fig. 6A). Similarly, D-Rd had the highest chance of being ranked first with respect to OS, (53%) followed by VRd (24%) and then D-VMP (23%; Fig. 6B).
Overall survival (using simplified evidence network of main relevant comparators in Europe). A Forest plot of OS HRs of treatments versus Rd continuous by efficacy and probability of being better than Rd continuous and B rankogram presenting probability of being ranked first in OS. CMP carfilzomib/melphalan/prednisone, CPR cyclophosphamide/prednisone/lenalidomide, Crl LL credible interval lower limit, Crl UL credible interval upper limit, CTD cyclophosphamide/thalidomide/dexamethasone, D-Rd daratumumab/lenalidomide/dexamethasone, D-VMP daratumumab/bortezomib/melphalan/prednisone, DEX dexamethasone, DEX-IFN dexamethasone/interferon alfa 2b, HR hazard ratio, MP melphalan/prednisone, MPR-R melphalan/prednisone/lenalidomide as induction, and lenalidomide as maintenance, MPT melphalan/prednisone/thalidomide, OS overall survival, Rd cont lenalidomide/dexamethasone, continuous, Rd18 lenalidomide/dexamethasone 18 cycles, VMP bortezomib/melphalan/prednisone, VRd bortezomib/lenalidomide/dexamethasone
Discussion
As novel treatment regimens become available for patients with TIE NDMM, it will be necessary to assess their comparative efficacy. NMAs provide a platform to compare treatment outcomes across these trials, allowing evaluation of therapies that have not yet been tested head to head in a clinical trial setting. The current study featured an SLR conducted according to NICE guidelines and a Bayesian NMA to evaluate the most relevant efficacy endpoints (PFS, OS) for > 20 different treatment regimens using the most recent publications of the included trials. To our knowledge, the current NMA is the first to include the ENDURANCE trial [18], which compared carfilzomib/lenalidomide/dexamethasone (KRd) versus VRd in patients with NDMM, and it includes the longest available follow-up for the SWOG S0777 [19], ALCYONE [20], and MAIA [21] studies (84, 40.1, and 56.2 months, respectively).
In this NMA, the daratumumab-containing combination therapies (D-Rd and D-VMP) and VRd were consistently ranked as being more effective than Rd continuous and had the highest probability of being ranked first among all the comparators evaluated in the analysis with regards to PFS and OS. Results were consistent between global and European networks and did not vary according to the SOC in each region.
VRd is recommended for the treatment of TIE patients with NDMM by the US and European treatment guidelines [16, 17], making it a relevant treatment comparator for daratumumab-containing regimens. The efficacy and safety of VRd versus Rd continuous were investigated in the SWOG S0777 trial [19], which included both TIE and TE NDMM patients. However, only ~ 50% of the patient population in the SWOG S0777 [19] trial was considered TIE. The median patient age was 63 years, which is younger than in other trials included here (median ages 67 to 79 years). Due to the potential for treatment-effect heterogeneity based on patient age, including the ITT population from the SWOG S0777 study would be inappropriate because of the similarity assumption required for NMAs and could have potentially introduced uncertainty and caused difficulty in drawing meaningful conclusions [38]. Therefore, the HRs for PFS and OS were estimated from the subset of patients in SWOG S0777 aged ≥ 65 years, which was used as a proxy to represent TIE patients.
Although KRd is not currently an approved regimen for NDMM, it is a recommended treatment option for TIE patients with NDMM in the US NCCN guidelines [16]. The ENDURANCE study comparing KRd versus VRd included a combination of TIE and TE patients [18]. The median patient age was 65 years, similar to the SWOG 0777 study. A subset of patients aged ≥ 65 years from this trial was also used to represent TIE patients in the current NMA.
We found that both daratumumab-containing regimens evaluated (D-Rd and D-VMP) and VRd consistently had better PFS than Rd continuous; this finding was also seen in the simplified network of comparators relevant for Europe despite the different SOC options. These results are consistent with those of previous NMAs, including Cao et al. [9], Ramasamy et al. [13], and Xu et al. [12] (D-Rd versus Rd PFS HRs, 0.57, 0.57, and 0.55, respectively). Cao et al. also observed an advantage for D-VMP versus Rd (PFS HR, 0.59) [9], whereas the other studies were favourable but with different point estimators (PFS HRs, 0.73 and 0.71, respectively) [12, 13]. This divergence in benefit is potentially attributable to the different data cut-offs for ALCYONE and VISTA used in the analyses. In addition, since Cao et al. [9] evaluated the full ITT population from the SWOG S0777 trial, their results may not accurately reflect the more vulnerable TIE patients. Ramasamy et al. [13] performed a sensitivity analysis including age-adjusted data from SWOG S0777 that demonstrated results similar to their primary analysis.
Our analyses suggest that for OS, D-Rd, D-VMP, and VRd are more favourable than Rd continuous, which is especially compelling given that the most up to date and mature data available from the included RCTs were incorporated. These results are concordant with those of Xu et al. for the D-Rd versus Rd comparison, but differ for the D-VMP versus Rd comparison [12]. Potential explanations for the difference include different HRs at the cut-offs used for ALCYONE and VISTA; however, our results are also influenced by the additional studies included in our network, including a loop of melphalan and prednisone (MP) combined with agents like lenalidomide or thalidomide (MPT), which revealed some inconsistencies in relative efficacy. This may increase the uncertainty over MP, and therefore may have an impact on VMP versus MPT.
Strengths of the current NMA were the inclusion of the latest available data from the MAIA (D-Rd versus Rd) and ALCYONE (D-VMP versus VMP) studies, allowing for the analysis of OS in addition to PFS. Our study also has several limitations. First, some trials did not report HRs with corresponding CIs for PFS, so those values were extracted by the Guyot methodology [26]. Although this methodology is well established, it is possible that these extracted values do not exactly reflect HRs and CIs. Second, the VRd data included in the analysis for PFS and OS were based on a subset of patients aged ≥ 65 years in the SWOG S0777 study as a proxy for TIE patients; however, in SWOG S0777, ~ 50% of patients were considered TIE and only 43% of patients were aged ≥ 65 years [19]. Similarly, the KRd data included in the PFS analysis were also from patients aged ≥ 65 years from the ENDURANCE study and were included as representative of TIE patients. Third, the impact of maintenance therapy on OS and PFS was not considered in this analysis. This and the lack of adjustment for baseline heterogeneity may be confounding factors. Finally, while our meta-analysis provides evidence on the relative clinical effectiveness of 25 distinct treatment regimens in the average patient with TIE NDMM, it does not account for all clinical factors that may be relevant for appropriate treatment selection, e.g., patient age, cytogenetic risk, or renal function, which may be important sources of treatment effect heterogeneity in patients with TIE NDMM [39].
Conclusions
In the absence of head-to-head RCTs, NMAs allow for the estimation of the comparative effectiveness of different treatments. The present NMA incorporated the most recently published data evaluating SOC treatments from RCTs with more mature data including the daratumumab-containing regimens from the ALCYONE and MAIA trials. The results demonstrated that, compared with other relevant treatment options, D-Rd, D-VMP, and VRd are most effective in improving PFS and OS in TIE patients with NDMM. Overall, D-Rd had the highest chance of being ranked as the most effective treatment with respect to both PFS and OS. Findings from the European NMA were consistent with the global NMA. Results of this study may help guide choice of treatment for this patient population.
Change history
28 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12325-022-02211-4
References
Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet. 2015;385:2197–208.
Palumbo A, Rajkumar SV, San Miguel JF, Larocca A, Niesvizky R, Morgan G, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32:587–600.
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–17.
Palumbo A, Bringhen S, Larocca A, Rossi D, Di Raimondo F, Magarotto V, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. J Clin Oncol. 2014;32:634–40.
Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380:2104–15.
van Beurden-Tan CHY, Franken MG, Blommestein HM, Uyl-de Groot CA. Systematic literature review and network meta-analysis of treatment outcomes in relapsed and/or refractory multiple myeloma. J Clin Oncol. 2017;35:1312–9.
Botta C, Ciliberto D, Rossi M, Staropoli N, Cuce M, Galeano T, et al. Network meta-analysis of randomized trials in multiple myeloma: efficacy and safety in relapsed/refractory patients. Blood Adv. 2017;1:455–66.
Blommestein HM, van Beurden-Tan CHY, Franken MG, Uyl-de Groot CA, Sonneveld P, Zweegman S. Efficacy of first-line treatments for multiple myeloma patients not eligible for stem cell transplantation: a network meta-analysis. Haematologica. 2019;104:1026–35.
Cao Y, Wan N, Liang Z, Xie J, Wang S, Lin T, et al. Treatment outcomes in patients with newly diagnosed multiple myeloma who are ineligible for stem-cell transplantation: systematic review and network meta-analysis. Clin Lymphoma Myeloma Leuk. 2019;19:e478–88.
Piechotta V, Jakob T, Langer P, Monsef I, Scheid C, Estcourt LJ, et al. Multiple drug combinations of bortezomib, lenalidomide, and thalidomide for first-line treatment in adults with transplant-ineligible multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2019;2019:CD013487.
Sekine L, Ziegelmann PK, Manica D, Pithan CDF, Sosnoski M, Morais VD, et al. Upfront treatment for newly diagnosed transplant-ineligible multiple myeloma patients: a systematic review and network meta-analysis of 14,533 patients over 29 randomized clinical trials. Crit Rev Oncol Hematol. 2019;143:102–16.
Xu W, Li D, Sun Y, Ran X, Wang B, Wu W, et al. Daratumumab added to standard of care in patients with newly diagnosed multiple myeloma: a network meta-analysis. Eur J Haematol. 2019;103:542–51.
Ramasamy K, Dhanasiri S, Thom H, Buchanan V, Robinson S, D’Souza VK, et al. Relative efficacy of treatment options in transplant-ineligible newly diagnosed multiple myeloma: results from a systematic literature review and network meta-analysis. Leuk Lymphoma. 2019;61:668–79.
Gil-Sierra MD, Gimeno-Ballester V, Fenix-Caballero S, Alegre-Del Rey EJ. Network meta-analysis of first-line treatments in transplant-ineligible multiple myeloma patients. Eur J Haematol. 2020;105:56–65.
Giri S, Aryal MR, Yu H, Grimshaw A, Pathak R, Huntington SP, et al. Efficacy and safety of frontline regimens for older transplant-ineligible patients with multiple myeloma: a systematic review and meta-analysis. J Geriatr Oncol. 2020;11:1285–92.
Kumar SK, Callander NS, Adekola K, Anderson L, Baljevic M, Campagnaro E, et al. Multiple myeloma, Version 3.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2020;18:1685–717.
Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and Follow-up. Hemasphere. 2021;5: e528.
Kumar SK, Jacobus SJ, Cohen AD, Weiss M, Callander N, Singh AK, et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21:1317–30.
Durie BGM, Hoering A, Sexton R, Abidi MH, Epstein J, Rajkumar SV, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 2020;10:53.
Mateos MV, Cavo M, Blade J, Dimopoulos MA, Suzuki K, Jakubowiak A, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet. 2020;395:132–41.
Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis NJ, et al. Overall survival results with daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: phase 3 MAIA study. In: Presented at European Hematology Association 2021.
Dias S, Welton NJ, Sutton AJ, Ades AE. Evidence synthesis for decision making 1: introduction. Med Decis Making. 2013;33:597–606.
Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38:200–11.
Spiegelhalter D. Some DIC slides. Cambridge: MRC Biostatistics Unit; 2006.
Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. London: NICE Decision Support Unit Technical Support Documents; 2014.
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9.
Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ. 2011;343:d2090.
San Miguel J, Mateos MV, Goldschmidt H, Sonneveld P, Dimopoulos M, Heeg B, et al. Impact of modified dose schedule of bortezomib, melphalan, and prednisone (VMP) for previously untreated, transplant-ineligible patients with multiple myeloma (MM): a matching-adjusted indirect comparison. Blood. 2018;132:3553.
Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366:1759–69.
Beksac M, Haznedar R, Firatli-Tuglular T, Ozdogu H, Aydogdu I, Konuk N, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2011;86:16–22.
Wijermans P, Schaafsma M, Termorshuizen F, Ammerlaan R, Wittebol S, Sinnige H, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. J Clin Oncol. 2010;28:3160–6.
Waage A, Gimsing P, Fayers P, Abildgaard N, Ahlberg L, Bjorkstrand B, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116:1405–12.
Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112:3107–14.
Stewart AK, Jacobus S, Fonseca R, Weiss M, Callander NS, Chanan-Khan AA, et al. Melphalan, prednisone, and thalidomide vs melphalan, prednisone, and lenalidomide (ECOG E1A06) in untreated multiple myeloma. Blood. 2015;126:1294–301.
Zweegman S, van der Holt B, Mellqvist UH, Salomo M, Bos GM, Levin MD, et al. Melphalan, prednisone, and lenalidomide versus melphalan, prednisone, and thalidomide in untreated multiple myeloma. Blood. 2016;127:1109–16.
Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389:519–27.
Belotti A, Ribolla R, Cancelli V, Crippa C, Bianchetti N, Ferrari S, et al. Transplant eligibility in elderly multiple myeloma patients: prospective external validation of the international myeloma working group frailty score and comparison with clinical judgment and other comorbidity scores in unselected patients aged 65–75 years. Am J Hematol. 2020;95:759–65.
Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: application and practice using Stata. Epidemiol Health. 2017;39: e2017047.
Larocca A, Mina R, Offidani M, Liberati AM, Ledda A, Patriarca F, et al. First-line therapy with either bortezomib-melphalan-prednisone or lenalidomide-dexamethasone followed by lenalidomide for transplant-ineligible multiple myeloma patients: a pooled analysis of two randomized trials. Haematologica. 2020;105:1074–80.
Acknowledgements
Funding
The analysis was funded by Janssen Global Services, LLC. The journal’s rapid service and open access fees were funded by Janssen Global Services, LLC.
Medical writing and editing assistance
Editorial and medical writing support was provided by Karen Pemberton, PhD, of Eloquent Scientific Solutions and was funded by Janssen Global Services, LLC.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
All authors contributed equally to the study design and data interpretation. SB performed the systematic literature search, data collection, and analysis. ZY updated the network meta-analysis. All authors contributed to writing the manuscript, approved the final version, decided to publish this report, and vouch for data accuracy and completeness.
Disclosures
Thierry Facon served on advisory committees for Amgen, Celgene, Janssen, Karyopharm, Oncopeptides, Roche, Sanofi, and Takeda; and speakers’ bureaus for Celgene, Janssen, and Takeda. Jesús San-Miguel received honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, Roche, and Sanofi. Meletios A. Dimopoulos received honoraria from Amgen, BeiGene, Bristol-Myers Squibb, Janssen-Cilag, and Takeda and consulted for and/or served in an advisory role for Amgen, BeiGene, Bristol-Myers Squibb, Janssen-Cilag, and Takeda. Maria-Victoria Mateos received honoraria from Amgen, AbbVie, Adaptive Biotechnologies, Celgene, GlaxoSmithKline, Janssen-Cilag, Roche, and Takeda and served in a consulting and/or advisory role for Amgen, AbbVie, Celgene, GlaxoSmithKline, Janssen-Cilag, Pfizer, Regeneron, Roche/Genentech, and Takeda. Michele Cavo received honoraria from Amgen, Bristol-Myers Squibb, Celgene, and Janssen and served in a consulting and/or advisory role for AbbVie, Amgen, Celgene, and Janssen. Sophie van Beekhuizen and Zijiao Yuan are employees of Ingress Health. Jianming He, Eric Ammann, Annette Lam, and João Mendes are employees of Janssen and may own company stock. Shaji Kumar received research funding from Takeda, Janssen, and Celgene and has consulted for Janssen and Celgene.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Facon, T., San-Miguel, J., Dimopoulos, M.A. et al. Treatment Regimens for Transplant-Ineligible Patients With Newly Diagnosed Multiple Myeloma: A Systematic Literature Review and Network Meta-analysis. Adv Ther 39, 1976–1992 (2022). https://doi.org/10.1007/s12325-022-02083-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02083-8