Abstract
Introduction
Teneligliptin/canagliflozin combination tablets, which combine a dipeptidyl peptidase-4 (DPP-4) inhibitor (teneligliptin) and a sodium-glucose cotransporter 2 (SGLT2) inhibitor (canagliflozin), are a treatment option for type 2 diabetes mellitus (T2DM) in Japan. This post-marketing surveillance evaluated the real-world safety and effectiveness of teneligliptin/canagliflozin combination tablets, and changes in self-reported adherence to oral antihyperglycaemic agents.
Methods
Japanese patients with T2DM who were prescribed the combination tablets for the first time between December 2017 and June 2018 were registered and followed up for 12 months. Safety and effectiveness were assessed in terms of adverse drug reactions (ADRs) and the changes in haemoglobin A1c (HbA1c) and body weight from baseline to 12 months with the last observation carried forward, respectively. Adherence was assessed using the Morisky Medication Adherence Scale 8.
Results
Overall, 821 patients were eligible for the analyses, including 733 who were prescribed the combination tablets for 12 months. ADRs and serious ADRs were reported in 4.38% and 0.85% of patients, respectively. Gastrointestinal disorders (0.97%) were the most common class of ADRs. No new safety concerns were identified beyond those described in the Japanese package insert. The changes in HbA1c and body weight from baseline to 12 months were − 0.43 ± 0.93% and − 1.29 ± 5.57 kg, respectively. The reductions in HbA1c at 12 months tended to be greater among patients who switched from either DPP-4 inhibitors (− 0.71 ± 0.89%) or SGLT2 inhibitors (− 0.51 ± 1.00%) relative to patients who switched from both (− 0.22 ± 0.88%). The decrease in body weight was greatest among patients who switched from DPP-4 inhibitors. An improvement in self-reported adherence to oral antihyperglycaemic agents occurred after switching to the combination tablets.
Conclusion
Teneligliptin/canagliflozin combination tablets were effective and associated with an improvement in adherence without new safety concerns in Japanese patients with T2DM in real-world clinical practice.
Trial Registration
JapicCTI-173778.
Plain Language Summary
Teneligliptin/canagliflozin combination tablets are used as an option for the treatment of type 2 diabetes mellitus in Japan. We performed this surveillance to obtain data on the frequency of side effects (adverse drug reactions) and effectiveness (in terms of changes in haemoglobin A1c and body weight) in Japanese patients treated with teneligliptin/canagliflozin combination tablets in real-world clinical practice. We also asked patients to evaluate their adherence to oral antihyperglycaemic agents as part of their prescribed therapies. We collected data for up to 12 months. We detected no new safety concerns, other than those already described in the Japanese package insert for the combination tablets. In terms of effectiveness, we observed improvements in both haemoglobin A1c and body weight over 12 months of treatment. Furthermore, self-reported adherence to oral antihyperglycaemic agents improved after treatment with the combination tablets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Teneligliptin/canagliflozin combination tablets were recently approved in Japan for the treatment of type 2 diabetes mellitus (T2DM), but there are limited data regarding the safety and effectiveness of these combination tablets when prescribed in real-world settings. |
We performed this post-marketing surveillance to obtain information regarding the real-world safety, effectiveness, and adherence among Japanese patients with T2DM who switched to these combination tablets. |
What was learned from the study? |
There were no new safety concerns for the teneligliptin/canagliflozin combination tablets, other than those already described in the Japanese package insert. Switching to the combination tablets was associated with improvements in HbA1c, body weight, and adherence to the prescribed antihyperglycaemic therapies. |
Introduction
The initial treatment of type 2 diabetes mellitus (T2DM) involves diet and exercise therapies; however, patients with inadequate glycaemic control require antihyperglycaemic agents. The individual patient’s condition, complications, mechanisms of action of the agents and other factors are considered when choosing the antihyperglycaemic agent [1, 2]. If blood glucose levels cannot be sufficiently controlled with one oral antihyperglycaemic agent, a combination of drugs with different mechanisms of action is necessary. However, the resulting pill burden in patients taking multiple drugs may impact their treatment adherence and may contribute to their inability to maintain glycaemic control [3]. Fixed-dose combination tablets, which comprise two antihyperglycaemic agents, provide a simplified treatment regimen and may improve medication adherence [4,5,6].
Dipeptidyl peptidase-4 (DPP-4) and sodium-glucose cotransporter 2 (SGLT2) inhibitors are two classes of drugs for T2DM. DPP-4 inhibitors, such as teneligliptin, are widely used in Japan. It was estimated that DPP-4 inhibitors were prescribed to about 57% of patients with T2DM in Japan in 2019 [7]. This high use of DPP-4 inhibitors is at least partly related to the metabolic/genetic characteristics of Asian patients, which include impaired β-cell function without marked insulin resistance [8, 9]. SGLT2 inhibitors such as canagliflozin have been marketed for several years. They lower glucose levels and exert pleiotropic effects, including weight loss, reduction in blood pressure, improvement in liver function, and cardioprotective and renoprotective effects [10, 11].
Because DPP-4 inhibitors and SGLT2 inhibitors lower blood glucose levels via independent mechanisms, they are well suited for administration in combination [12,13,14]. Indeed, several clinical trials have demonstrated the efficacy and safety of co-administering canagliflozin and teneligliptin as separate tablets [15,16,17]. Importantly, no pharmacokinetic interactions were observed when administering both drugs as separate tablets [18]. Thus, combination tablets comprising 20 mg teneligliptin and 100 mg canagliflozin (CANALIA® Combination Tablets; Mitsubishi Tanabe Pharmaceutical Corporation/Daiichi Sankyo Company, Ltd.; hereafter, the combination tablets) were developed and approved in Japan in 2017. The combination tablets are expected to provide a treatment option for T2DM with a lower pill burden compared with the individual drugs, and improve glycaemic control and adherence.
In real-world clinical practice, the combination tablets are likely to be administered to T2DM patients across a diverse range of background characteristics. However, the clinical trials of combined teneligliptin and canagliflozin therapy enrolled relatively small numbers of patients with narrow eligibility criteria. Furthermore, the trials did not evaluate the effects of switching to the combination tablets on adherence to oral antihyperglycaemic agents because they did not use the combination tablets; instead, patients took teneligliptin and canagliflozin as separate tablets. In consideration of these factors, this Japanese post-marketing surveillance (PMS) was designed to evaluate the safety and effectiveness of the combination tablets prescribed in real-world clinical practice, across a broader patient population than was eligible for clinical trials, with a follow-up of up to 12 months. In addition, we investigated the effects of switching to the combination tablets on self-reported adherence to oral antihyperglycaemic agents.
Methods
Ethics
This PMS was approved by the Ministry of Health, Labour and Welfare of Japan and was conducted by Mitsubishi Tanabe Pharma Corporation in compliance with Good Post-Marketing Study Practice in Japan, which does not require the collection of informed consent or ethical approval from participating institutions. All patients who participated in this PMS provided written informed consent. All data were collected anonymously. The PMS was registered on Japic Clinical Trials Information (JapicCTI-173778).
Patients and Treatments
Patients who were prescribed the combination tablets for the first time between December 2017 and June 2018 were to be registered and observed for up to 12 months in real-world clinical practice, with a follow-up period of December 2017 to December 2019. The registration and follow-up of patients in the PMS were conducted at the discretion of the physicians at 197 institutions in Japan.
The combination tablets were to be prescribed to patients who were deemed candidates for treatment with teneligliptin and canagliflozin in accordance with the Japanese package insert [19], usually once daily before or after breakfast. The combination tablets could be administered together with other antihyperglycaemic agents. The dose of the combination tablets could not be adjusted due to the formulation (20 mg teneligliptin/100 mg canagliflozin), but the administration of other antihyperglycaemic agents could be adjusted by the physician at any time, in accordance with their approved labels. All treatment decisions, including the prescription of the combination tablets and concomitant agents, and lifestyle modifications, were at the physician’s discretion.
Data Collection
Electronic case report forms were used to collect patient data at 3 months (including baseline data/patient characteristics and data at 1 month), 6 months and 12 months after starting the combination tablets and at the time of discontinuation. The data included patient characteristics, treatment status, adverse events (AEs), haemoglobin A1c (HbA1c), body weight, blood pressure, laboratory tests (e.g. aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase, and serum creatinine), and self-reported adherence to oral antihyperglycaemic agents [Morisky Medication Adherence Scale 8 (MMAS-8)] [20,21,22,23]. The estimated glomerular filtration rate (eGFR) was retrieved from medical records or calculated using serum creatinine levels, age and sex [24]. All AEs were recorded by the physicians by referring to their patients’ reports, medical records, interviews, and laboratory test data.
Data Analysis
Safety was evaluated in terms of adverse drug reactions (ADRs). ADRs were defined as AEs for which a causal relationship with the combination tablets could not be ruled out, i.e. related or unknown. Furthermore, ADRs or AEs that have previously been associated with the use of DPP-4 inhibitors or SGLT2 inhibitors [19, 25,26,27,28] were defined and evaluated as ADRs or AEs of special interest, as follows: hypoglycaemia-related ADRs, genital infections, urinary tract infections, ADRs associated with polyuria or pollakiuria, ADRs associated with volume depletion (thrombosis/embolism, including dehydration and cerebral infarction), ADRs associated with ketone body increased, ADRs associated with lower limb amputation (including venous thromboembolism), renal disorders, hepatic disorders, gastrointestinal disorders (including intestinal obstruction and pancreatitis acute), skin and subcutaneous tissue disorders (including pemphigoid), fracture, ADRs associated with body weight decreased, QT prolongation, and interstitial pneumonia. Malignant tumours that were reported as AEs were denoted as AEs of special interest. All AEs and ADRs were classified according to Medical Dictionary for Regulatory Activities/Japanese edition (MedDRA/J) version 22.1.
The effectiveness of the combination tablets was evaluated in terms of HbA1c and body weight, including changes from baseline to each visit for patients with available data, and from baseline to 12 months, with missing data imputed using the last observation carried forward (LOCF) method.
Self-reported adherence to multiple medications was evaluated using Morisky Widget MMAS-8© Software comprising the MMAS-8 [20,21,22,23]. This is an eight-item questionnaire where items 1–7 are recorded as yes/no responses and item 8 is recorded on a five-point scale. The maximum score is 8 points, and adherence is classified into three categories: low (< 6 points), medium (≥ 6 to < 8 points) or high (= 8 points). The change in adherence score from baseline to 12 months with LOCF was classified as worsened (the adherence score decreased by at least one category), no change (no change in the adherence score category) or improved (adherence score increased by at least one category).
The safety analysis set (comprising case report forms collected from patients in whom safety was assessed, excluding those with a contract violation, enrolment violation, duplicate cases, unevaluated AEs, or administration of the combination tablets was not confirmed) was used for analyses of patient demographics, AEs, ADRs and laboratory test data. The effectiveness analysis set (excluding patients in whom effectiveness outcomes were not evaluated or patients administering the combination tablets for unapproved purposes) was used for analyses of HbA1c, body weight and adherence to oral antihyperglycaemic agents. Data were analysed descriptively as the mean ± standard deviation or number and percentage of patients, as appropriate. The statistical tests were conducted for reference purposes. Paired t tests were used to evaluate the changes in HbA1c, body weight, laboratory test data, blood pressure and eGFR from baseline to 12 months. Missing data were inserted using the LOCF method. The Wilcoxon signed-rank test was used to compare the changes in adherence categories between baseline and 12 months with LOCF. SAS v.9.4 (TS1M2; SAS Institute, Cary, NC, USA) was used for all analyses.
Results
Patients
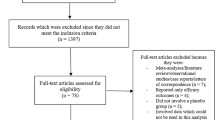
Among the 847 patients initially registered, 821 were included in the safety analysis set and 808 in the effectiveness analysis set (Fig. 1). Overall, 733 of 821 patients were prescribed the combination tablets for ≥ 12 months. The observation was discontinued in 89 patients for various reasons, including an AE/ADR (n = 19), the patient stopped visiting the clinic (n = 19), the patient transferred to another clinic (n = 16), the patient's decision (n = 11), ineffective/insufficient effect (n = 10), recovered/improved symptoms (n = 7), or other reasons (n = 11); multiple reasons were possible.
Among 821 eligible patients, 66.6% were male and 33.4% were female, and the mean age, duration of T2DM, body mass index (BMI) and eGFR prior to starting the combination tablets were 61.8 years, 9.83 years, 26.82 kg/m2 and 75.86 mL/min/1.73 m2, respectively (Table 1). Comorbidities included diabetic complications in up to 20.3% of patients, hypertension in 56.2% and dyslipidaemia in 49.3% (Table 1). Adherence to oral antihyperglycaemic agents before starting the combination tablets was classified as low in 22.5%, medium in 30.3%, and high in 25.0% of patients.
The prior medications including DPP-4 inhibitors, SGLT2 inhibitors, and other concomitant antihyperglycaemic agents are summarised in Table S1 (Supplementary Material). DPP-4 inhibitors, SGLT2 inhibitors, or both were used in 29.7%, 16.7%, and 44.3% of patients, respectively, before starting the combination tablets. Biguanides were the most commonly prescribed concomitant antihyperglycaemic agents during the surveillance period, followed by sulfonylureas. In patients previously treated with both DPP-4 inhibitors and SGLT2 inhibitors, the prior treatment was switched to the combination tablets ‘to improve adherence’, ‘to reduce drug costs’ and ‘to reduce the number of drugs taken’ in > 50% of patients (Fig. 2). Among patients who were previously treated with DPP-4 inhibitors or SGLT2 inhibitors, the prior therapy was switched to the combination tablets ‘to improve glycaemic control’ in ≥ 80% of patients. For one-fourth or one-fifth of patients who switched from DPP-4 inhibitors or SGLT2 inhibitors, the purpose of switching to the combination tablets was ‘to improve adherence’ or ‘to reduce the number of drugs taken’.
Table S2 (Supplementary Material) presents the patient characteristics according to prior use of DPP-4 inhibitors and/or SGLT2 inhibitors. Among these patients, the subgroup of patients who switched from SGLT2 inhibitors had the highest percentage of patients aged < 65 years and the highest mean BMI.
Safety
Among 821 patients prescribed the combination tablets, 36 (4.38%) experienced a total of 46 ADRs, which included eight serious ADRs in seven patients (0.85%) (Table 2). There were no clear differences in the incidences of ADRs and serious ADRs among patients who switched from DPP-4 inhibitors, SGLT2 inhibitors, or both (Table 2). The most common ADR was constipation, which occurred in four patients (0.49%), followed by blood creatinine decreased in three patients (0.37%) and hepatic function abnormal, renal impairment and blood pressure decreased in two patients (0.24%) each (Table S3 in the Supplementary Material). Serious ADRs were hypoglycaemia, decreased appetite, lacunar infarction, cardiac failure congestive, diverticulum intestinal haemorrhagic, vomiting, pemphigus, and patella fracture in one patient each. The outcomes of the 46 ADRs were classified as recovered in 26 (56.52%), recovering in 12 (26.09%), not recovered in 6 (13.04%) and unknown/not recorded in 2 (4.35%); no deaths or sequelae were reported. There were no unresolved serious ADRs.
The ADRs and AEs of special interest are listed in Table 3. The most common ADRs of special interest were gastrointestinal disorders (n = 8), such as constipation, followed by renal impairment (n = 6) and skin and subcutaneous tissue disorders (n = 5). For patients who switched from DPP-4 inhibitors, SGLT2 inhibitors, or both, the numbers of patients with these ADRs were as follows: gastrointestinal disorders (n = 1, 2, and 5, respectively), renal impairment (n = 3, 0, and 3, respectively), and skin and subcutaneous tissue disorders (n = 2, 1, and 1, respectively). The gastrointestinal disorders were constipation (n = 1) among patients who switched from DPP-4 inhibitors, and constipation and bowel movement irregularity (n = 1, each) among patients who switched from SGLT2 inhibitors; and constipation (n = 2), diverticulum intestinal haemorrhagic, vomiting, and diarrhoea (n = 1, each) among patients who switched from both DPP-4 inhibitors and SGLT2 inhibitors.
The changes in blood pressure and laboratory test data from baseline to 12 months in the overall population and according to the prior use of DPP-4 inhibitors and/or SGLT2 inhibitors are presented in Table S4 (Supplementary Material). The reductions in mean values of systolic and diastolic blood pressures, aspartate aminotransferase and alanine aminotransferase were numerically greater in patients who switched from DPP-4 inhibitors than in the other groups of patients, except for alanine aminotransferase at 12 months with LOCF.
Effectiveness
In the overall population, the mean HbA1c decreased over time from baseline. The mean change from baseline was − 0.43% at 12 months with LOCF (Fig. 3a). The reductions in the mean HbA1c from baseline were maintained through to 12 months in patients who switched from DPP-4 inhibitors (− 0.71% at 12 months with LOCF), SGLT2 inhibitors (− 0.51%), or both (− 0.22%) (Fig. 3b), with a numerically greater reduction in patients who switched from DPP-4 inhibitors or SGLT2 inhibitors. The changes in HbA1c among patients who switched to the combination tablets from teneligliptin, canagliflozin, or both as separate tablets are shown in Fig. S1 (Supplementary Material); the results were consistent with those observed by drug class.
Changes in HbA1c over time in overall patients (a) and in patients who switched from DPP-4 inhibitors and/or SGLT2 inhibitors (b). Values are mean ± standard deviation. DPP-4 dipeptidyl peptidase-4, HbA1c haemoglobin A1c, LOCF last observation carried forward, mo months, n number of patients, SGLT2 sodium-glucose cotransporter 2
In the overall population, the mean body weight decreased from baseline by − 1.29 kg at 12 months with LOCF (Fig. S2a in the Supplementary Material). The mean changes from baseline to 12 months with LOCF were − 1.91, − 1.38, and − 0.98 kg in patients who switched from DPP-4 inhibitors, SGLT2 inhibitors, and both, respectively (Fig. S2b in the Supplementary Material). Changes in body weight after switching to the combination tablets from teneligliptin, canagliflozin, or both as separate tablets (Fig. S2c in the Supplementary Material) were consistent with those observed by drug class.
Self-Reported Adherence
We investigated the changes in self-reported adherence measured using the MMAS-8. Table S5 (Supplementary Material) shows the characteristics of patients with low (MMAS-8 score < 6), medium (≥ 6 to < 8) and high (8) adherence at baseline. Patients with low adherence tended to be younger and to have higher HbA1c relative to patients with medium or high adherence.
Adherence at 12 months with LOCF showed trends towards an improvement (Fig. 4a), with an improvement by at least one category in 47.8% of patients with low adherence and 32.8% of patients with medium adherence at baseline. Among patients with high adherence at baseline, adherence was maintained for 74.0% and worsened in 24.5%. Improvements in adherence were also observed in patients who switched from both DPP-4 inhibitors and SGLT2 inhibitors to the combination tablets (Fig. 4b). We further explored the changes in adherence among patients who switched from DPP-4 inhibitors (Fig. 4c) or SGLT2 inhibitors (Fig. 4d) to the combination tablets. As illustrated in these figures, adherence also improved among patients who switched from DPP-4 inhibitors or SGLT2 inhibitors.
Transition in the MMAS-8 adherence score according to the baseline score in overall patients (a) and in patients who switched from both DPP-4 inhibitors and SGLT2 inhibitors (b), DPP-4 inhibitors (c), or SGLT2 inhibitors (d). The numbers of patients are given in parentheses. Worsened: the adherence score decreased by at least one category from baseline; unchanged: no change in the adherence score from baseline; improved: the adherence score increased by at least one category from baseline. LOCF last observation carried forward, MMAS-8 Morisky Medication Adherence Scale 8
Consistent trends were seen in patients who switched from teneligliptin, canagliflozin, or both as separate tablets to the combination tablets (Fig. S3 in the Supplementary Material), with improvements among patients divided according to their baseline adherence.
Discussion
This PMS was conducted to evaluate the safety, effectiveness, and adherence among Japanese patients who switched to teneligliptin/canagliflozin combination tablets in real-world clinical practice. We detected no new safety concerns other than those already included in the Japanese package insert for the combination tablets. Improvements in glycaemic control and self-reported adherence were seen in patients who started the combination tablets. The results of this PMS indicate that the combination tablets may be a useful option for the treatment of T2DM.
In addition to hypoglycaemia, which is a common ADR for antihyperglycaemic agents, there are several ADRs requiring special attention when prescribing teneligliptin or canagliflozin: for teneligliptin, these include gastrointestinal disorders (e.g. constipation), pemphigoid, and acute pancreatitis, etc.; and for canagliflozin, these include volume depletion, polyuria and pollakiuria, genital infections, urinary tract infections, and ketoacidosis, etc [15,16,17, 19, 27,28,29,30,31,32,33,34,35,36]. Most of the ADRs reported in this PMS were already listed in the Japanese package insert [19]. Several ADRs (hyperlipidaemia, dysgeusia, cardiac failure congestive, back pain, neck pain, and chest pain) that had not been reported in prior studies occurred in one patient each in this PMS. So far, we have not been able to identify a clear association between these ADRs and the combination tablets. Therefore, we consider that the results of this PMS have not identified any new safety concerns beyond the ADRs already listed in the Japanese package insert for this product [19]. Nevertheless, because several known ADRs were reported in this PMS, the prescribing physician should pay attention to the occurrence of these ADRs by carefully monitoring the patient's condition, while considering the Japanese package insert [19] and recommendations regarding the proper use of DPP-4 inhibitors and SGLT2 inhibitors [37,38,39,40].
In patients who previously used either DPP-4 inhibitors or SGLT2 inhibitors, HbA1c decreased over the 12-month period after switching to the combination tablets. These results may reflect the glucose-lowering effect of adding a new component as the combination tablets. In addition, the magnitudes of the decreases in body weight, blood pressure, and hepatic enzymes were greater in patients who switched from DPP-4 inhibitors to the combination tablets relative to the changes in patients who switched from SGLT2 inhibitors, or from both SGLT2 inhibitors and DPP-4 inhibitors. It has been reported that SGLT2 inhibitors have multifaceted effects, including weight loss, reduction in blood pressure and hepatoprotective effects, beyond their established glucose-lowering effects [10, 11]. Therefore, the changes seen in patients who switched to the combination tablets from DPP-4 inhibitors were likely due to the effect of adding canagliflozin (as part of the combination tablets) to their treatment regimen.
Self-reported adherence was also evaluated in this PMS. At baseline, the proportion of patients aged < 65 years and the mean HbA1c level were numerically greater among patients with low adherence compared with patients with medium or high adherence. In earlier studies, adherence was lower in patients aged ≤ 54 years [41] or < 65 years old [42] than in the corresponding subgroups of older patients. Furthermore, HbA1c levels are generally higher in patients with low adherence [43, 44]. Supporting those earlier studies, we found that low adherence is associated with younger age and worse glycaemic control. Regarding the association between adherence and age, in particular, it is possible that working outside may be associated with a higher frequency of missed doses in younger patients, and that greater adherence in older patients may be driven by the support from family members or caregivers [45].
Combination tablets with multiple active ingredients can improve adherence by virtue of reducing the number of drugs taken at the same time [4,5,6]. Results of this PMS indicate that adherence improved in patients who switched from both DPP-4 inhibitors and SGLT2 inhibitors to the combination tablets. Unexpectedly, the improvement in adherence in patients who switched from either DPP-4 inhibitors or SGLT2 inhibitors was comparable with that in patients who switched from both DPP-4 inhibitors and SGLT2 inhibitors. Changes in the number of concomitant agents or the frequency of administration, either when starting the combination tablets or during the observation period, may have influenced patient adherence. In fact, some patients discontinued another concomitant agent or required a lower daily dosing frequency after switching from twice-daily DPP-4 inhibitors. Other possible reasons could be the improvement in glycaemic control after switching from DPP-4 inhibitors or from SGLT2 inhibitors to the combination tablets, or a decrease in body weight attributable to canagliflozin in patients who switched from DPP-4 inhibitors to the combination tablets; these factors may have contributed to greater patient awareness of diabetes treatment [46, 47]. As a factor unique to this PMS, it is also feasible that periodic interviews on adherence may have increased the physician–patient communication, which may have helped motivate the patients to adhere to their medications [48, 49]. Thus, the improvement in adherence was likely due to multiple factors, not just to a reduction in the number of medications taken.
Trends for improvements in HbA1c and body weight were found in patients who switched from concomitant use of both DPP-4 inhibitors and SGLT2 inhibitors. These phenomena can not be explained by the addition of a new component as part of the combination tablets. Instead, we suspect that the improvement in adherence observed in these patients may contribute to these findings, as explained in the paragraph above. Thus, switching to the combination tablets may provide additional benefits to patients in terms of the therapeutic effect, not just greater convenience.
We must also consider a possible association between adherence and the occurrence of ADRs. Although these relationships could not be evaluated due to the limited number of patients who experienced ADRs in this PMS, we cannot exclude the possibility that greater drug exposure due to improved adherence increases the likelihood of ADRs. Moreover, it has been reported that the occurrence of ADRs may decrease adherence [50]. Therefore, these possibilities should be considered when prescribing the combination tablets.
There are several limitations of this PMS that should be acknowledged. Particular limitations were the absence of a control group, missing data, and differences in the numbers of patients in the subgroup analyses by prior therapy/adherence. We cannot exclude the possibilities that there were unreported ADRs/serious ADRs, changes in concomitant therapies, lifestyle modifications and changes in family or social support during the observation period. These factors may introduce some bias and should be considered when interpreting the results. Clinically unreasonable values were entered for some patients, which may skew some results. Furthermore, we cannot exclude the possibility that the changes in some parameters were related to the previous medications, including the duration of prior therapy before starting the combination tablets. In addition, MMAS-8, which was used in this PMS, is not an objective assessment of adherence because it does not count the remaining number of tablets. Furthermore, the MMAS-8 assesses adherence to antihyperglycaemic agents; the individual items are not specific to the teneligliptin/canagliflozin combination tablets. It is possible that different results would have been obtained if we had used alternative questionnaires to evaluate adherence or if the number of prescriptions/pills dispensed had been counted.
In conclusion, teneligliptin/canagliflozin combination tablets were effective and associated with an improvement in self-reported adherence to the prescribed antihyperglycaemic agents, without new safety concerns in Japanese patients with T2DM in real-world clinical practice.
References
Araki E, Tanaka A, Inagaki N, Ito H, Ueki K, Murohara T, et al. Diagnosis, prevention, and treatment of cardiovascular diseases in people with type 2 diabetes and prediabetes—a consensus statement jointly from the Japanese Circulation Society and the Japan Diabetes Society. Circ J. 2020;85(1):82–125. https://doi.org/10.1253/circj.CJ-20-0865.
Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 Update to: Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487–93. https://doi.org/10.2337/dci19-0066.
Guerci B, Chanan N, Kaur S, Jasso-Mosqueda JG, Lew E. Lack of treatment persistence and treatment nonadherence as barriers to glycaemic control in patients with type 2 diabetes. Diabetes Ther. 2019;10(2):437–49. https://doi.org/10.1007/s13300-019-0590-x.
Han S, Iglay K, Davies MJ, Zhang Q, Radican L. Glycemic effectiveness and medication adherence with fixed-dose combination or coadministered dual therapy of antihyperglycemic regimens: a meta-analysis. Curr Med Res Opin. 2012;28(6):969–77. https://doi.org/10.1185/03007995.2012.684045.
Hutchins V, Zhang B, Fleurence RL, Krishnarajah G, Graham J. A systematic review of adherence, treatment satisfaction and costs, in fixed-dose combination regimens in type 2 diabetes. Curr Med Res Opin. 2011;27(6):1157–68. https://doi.org/10.1185/03007995.2011.570745.
Lokhandwala T, Smith N, Sternhufvud C, Sörstadius E, Lee WC, Mukherjee J. A retrospective study of persistence, adherence, and health economic outcomes of fixed-dose combination vs. loose-dose combination of oral anti-diabetes drugs. J Med Econ. 2016;19(3):203–12. https://doi.org/10.3111/13696998.2015.1109518.
Yagi N, Komiya I, Arai K, Oishi M, Fukumoto Y, Shirabe S, et al. Current status of oral antidiabetic drug prescribing patterns based on the body mass index for Japanese type 2 diabetes mellitus patients and yearly changes in diabetologists’ prescribing patterns from 2002 to 2019 (JDDM61). J Diabetes Investig. 2021. https://doi.org/10.1111/jdi.13621.
Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Investig. 2015;6(5):495–507. https://doi.org/10.1111/jdi.12305.
Yabe D, Seino Y, Fukushima M, Seino S. Beta cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diabetes Rep. 2015;15(6):602. https://doi.org/10.1007/s11892-015-0602-9.
Kashiwagi A, Maegawa H. Metabolic and hemodynamic effects of sodium-dependent glucose cotransporter 2 inhibitors on cardio-renal protection in the treatment of patients with type 2 diabetes mellitus. J Diabetes Investig. 2017;8(4):416–27. https://doi.org/10.1111/jdi.12644.
Mudaliar S, Polidori D, Zambrowicz B, Henry RR. Sodium-glucose cotransporter inhibitors: effects on renal and intestinal glucose transport: from bench to bedside. Diabetes Care. 2015;38(12):2344–53. https://doi.org/10.2337/dc15-0642.
Cho KY, Nomoto H, Nakamura A, Kawata S, Sugawara H, Takeuchi J, et al. Favourable effect of the sodium-glucose co-transporter-2 inhibitor canagliflozin plus the dipeptidyl peptidase-4 inhibitor teneligliptin in combination on glycaemic fluctuation: an open-label, prospective, randomized, parallel-group comparison trial (the CALMER study). Diabetes Obes Metab. 2020;22(3):458–62. https://doi.org/10.1111/dom.13879.
Fushimi Y, Obata A, Sanada J, Iwamoto Y, Mashiko A, Horiya M, et al. Effect of combination therapy of canagliflozin added to teneligliptin monotherapy in Japanese subjects with type 2 diabetes mellitus: a retrospective study. J Diabetes Res. 2020;2020:4861681. https://doi.org/10.1155/2020/4861681.
Rosenstock J, Perl S, Johnsson E, García-Sánchez R, Jacob S. Triple therapy with low-dose dapagliflozin plus saxagliptin versus dual therapy with each monocomponent, all added to metformin, in uncontrolled type 2 diabetes. Diabetes Obes Metab. 2019;21(9):2152–62. https://doi.org/10.1111/dom.13795.
Kadowaki T, Inagaki N, Kondo K, Nishimura K, Kaneko G, Maruyama N, et al. Efficacy and safety of teneligliptin added to canagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a multicentre, randomized, double-blind, placebo-controlled, parallel-group comparative study. Diabetes Obes Metab. 2018;20(2):453–7. https://doi.org/10.1111/dom.13079.
Kadowaki T, Inagaki N, Kondo K, Nishimura K, Kaneko G, Maruyama N, et al. Efficacy and safety of canagliflozin as add-on therapy to teneligliptin in Japanese patients with type 2 diabetes mellitus: results of a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2017;19(6):874–82. https://doi.org/10.1111/dom.12898.
Kadowaki T, Inagaki N, Kondo K, Nishimura K, Kaneko G, Maruyama N, et al. Long-term safety and efficacy of canagliflozin as add-on therapy to teneligliptin in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2018;20(1):77–84. https://doi.org/10.1111/dom.13038.
Kinoshita S, Kondo K. Evaluation of pharmacokinetic and pharmacodynamic interactions of canagliflozin and teneligliptin in Japanese healthy male volunteers. Expert Opin Drug Metab Toxicol. 2015;11(1):7–14. https://doi.org/10.1517/17425255.2015.982531.
Mitsubishi Tanabe Pharma Corporation. CANALIA® combination tablets. Package insert, revised July 2021. https://medical.mt-pharma.co.jp/di/file/dc/cnl.pdf. Accessed September 1, 2021 (in Japanese).
Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care. 2009;15(1):59–66.
Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10(5):348–54. https://doi.org/10.1111/j.1751-7176.2008.07572.x.
Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. J Clin Epidemiol. 2011;64(3):255–7. https://doi.org/10.1016/j.jclinepi.2010.09.002(discussion 8–63).
Clifford S, Perez-Nieves M, Skalicky AM, Reaney M, Coyne KS. A systematic literature review of methodologies used to assess medication adherence in patients with diabetes. Curr Med Res Opin. 2014;30(6):1071–85. https://doi.org/10.1185/03007995.2014.884491.
Kanda E, Kashihara N, Matsushita K, Usui T, Okada H, Iseki K, et al. Guidelines for clinical evaluation of chronic kidney disease: AMED research on regulatory science of pharmaceuticals and medical devices. Clin Exp Nephrol. 2018;22(6):1446–75. https://doi.org/10.1007/s10157-018-1615-x.
Deacon CF. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2020;16(11):642–53. https://doi.org/10.1038/s41574-020-0399-8.
McGill JB, Subramanian S. Safety of sodium-glucose co-transporter 2 inhibitors. Am J Cardiol. 2019;124(Suppl 1):S45–52. https://doi.org/10.1016/j.amjcard.2019.10.029.
Mitsubishi Tanabe Pharma Corporation. CANAGLU® 100 mg tablets. Package insert, revised June 2019. https://medical.mt-pharma.co.jp/di/file/dc/can_a.pdf. Accessed 1 Sept 2021 (in Japanese).
Mitsubishi Tanabe Pharma Corporation. TENELIA® 20 mg/ 40 mg tablets. Package insert, revised July 2021. https://medical.mt-pharma.co.jp/di/file/dc/tnl.pdf. Accessed 1 Sept 2021 (in Japanese).
Kadowaki T, Kondo K. Efficacy and safety of teneligliptin in combination with pioglitazone in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2013;4(6):576–84. https://doi.org/10.1111/jdi.12092.
Kadowaki T, Kondo K. Efficacy and safety of teneligliptin added to glimepiride in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study with an open-label, long-term extension. Diabetes Obes Metab. 2014;16(5):418–25. https://doi.org/10.1111/dom.12235.
Kadowaki T, Kondo K, Sasaki N, Miyayama K, Yokota S, Terata R, et al. Efficacy and safety of teneligliptin add-on to insulin monotherapy in Japanese patients with type 2 diabetes mellitus: a 16-week, randomized, double-blind, placebo-controlled trial with an open-label period. Expert Opin Pharmacother. 2017;18(13):1291–300. https://doi.org/10.1080/14656566.2017.1359259.
Ceriello A, De Nigris V, Iijima H, Matsui T, Gouda M. The unique pharmacological and pharmacokinetic profile of teneligliptin: implications for clinical practice. Drugs. 2019;79(7):733–50. https://doi.org/10.1007/s40265-019-01086-0.
Kadowaki T, Marubayashi F, Yokota S, Katoh M, Iijima H. Safety and efficacy of teneligliptin in Japanese patients with type 2 diabetes mellitus: a pooled analysis of two Phase III clinical studies. Expert Opin Pharmacother. 2015;16(7):971–81. https://doi.org/10.1517/14656566.2015.1032249.
Inagaki N, Harashima SI, Iijima H. Canagliflozin for the treatment of type 2 diabetes: a comparison between Japanese and non-Japanese patients. Expert Opin Pharmacother. 2018;19(8):895–908. https://doi.org/10.1080/14656566.2018.1473378.
Kadowaki T, Haneda M, Ito H, Sasaki K, Matsukawa M, Yamada Y. Long-term, real-world safety and efficacy of teneligliptin: a post-marketing surveillance of more than 10,000 patients with type 2 diabetes in Japan. Adv Ther. 2020;37(3):1065–86. https://doi.org/10.1007/s12325-019-01189-w.
Inagaki N, Nangaku M, Sakata Y, Sasaki K, Mori-Anai K, Iwasaki T, et al. Real-world safety and effectiveness of canagliflozin treatment for type 2 diabetes mellitus in Japan: SAPPHIRE, a long-term, large-scale post-marketing surveillance. Adv Ther. 2021. https://doi.org/10.1007/s12325-021-01984-4.
Committee on the Proper Use of Incretin-related Drugs (GLP-1 Receptor Agonists and DPP-4 Inhibitors). Recommendations for appropriate use of incretin-related drugs (GLP-1 receptor agonists and DPP-4 inhibitors), revised September 29, 2011. http://www.fa.kyorin.co.jp/jds/uploads/photos/797.pdf. Accessed June 15, 2021 (in Japanese).
Committee on the Proper Use of SGLT2 Inhibitors. Recommendations on the proper use of SGLT2 inhibitors, revised December 25, 2020. http://www.fa.kyorin.co.jp/jds/uploads/recommendation_SGLT2.pdf. Accessed June 15, 2021 (in Japanese).
Committee on the Proper Use of SGLT2 Inhibitors. Recommendations on the proper use of SGLT2 inhibitors. J Diabetes Investig. 2020;11(1):257–61. https://doi.org/10.1111/jdi.13160.
Yabe D, Seino Y. Dipeptidyl peptidase-4 inhibitors and sulfonylureas for type 2 diabetes: friend or foe? J Diabetes Investig. 2014;5(5):475–7. https://doi.org/10.1111/jdi.12229.
Curkendall SM, Thomas N, Bell KF, Juneau PL, Weiss AJ. Predictors of medication adherence in patients with type 2 diabetes mellitus. Curr Med Res Opin. 2013;29(10):1275–86. https://doi.org/10.1185/03007995.2013.821056.
Tunceli K, Zhao C, Davies MJ, Brodovicz KG, Alexander CM, Iglay K, et al. Factors associated with adherence to oral antihyperglycemic monotherapy in patients with type 2 diabetes. Patient Prefer Adherence. 2015;9:191–7. https://doi.org/10.2147/ppa.S71346.
Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27(12):2800–5. https://doi.org/10.2337/diacare.27.12.2800.
Schectman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care. 2002;25(6):1015–21. https://doi.org/10.2337/diacare.25.6.1015.
de Oliveira REM, Ueta JM, Franco LJ. Variables associated with adherence to the treatment of type 2 diabetes mellitus among elderly people. Diabetol Int. 2021. https://doi.org/10.1007/s13340-021-00518-1.
Glasgow RE, Fisher EB, Anderson BJ, LaGreca A, Marrero D, Johnson SB, et al. Behavioral science in diabetes. Contributions and opportunities. Diabetes Care. 1999;22(5):832–43. https://doi.org/10.2337/diacare.22.5.832.
Pi-Sunyer FX. The impact of weight gain on motivation, compliance, and metabolic control in patients with type 2 diabetes mellitus. Postgrad Med. 2009;121(5):94–107. https://doi.org/10.3810/pgm.2009.09.2056.
Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med. 2002;17(4):243–52. https://doi.org/10.1046/j.1525-1497.2002.10905.x.
Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care. 1998;21(10):1644–51. https://doi.org/10.2337/diacare.21.10.1644.
Jarab AS, Almrayat R, Alqudah S, Thehairat E, Mukattash TL, Khdour M, et al. Predictors of non-adherence to pharmacotherapy in patients with type 2 diabetes. Int J Clin Pharm. 2014;36(4):725–33. https://doi.org/10.1007/s11096-014-9938-5.
Acknowledgements
We are grateful to all of the physicians and patients involved in this post-marketing surveillance. We also wish to thank T. Goto (Mitsubishi Tanabe Pharma Corporation) and Y. Watanabe (Takumi Information Technology Inc.) for conducting the statistical analyses, and K. Arakawa, K. Ueta, and A. Takahashi (Mitsubishi Tanabe Pharma Corporation) for insightful discussions. The MMAS-8 scoring and coding presented in the article was done using the electronic Morisky Widget MMAS-8© Software. The Morisky Widget MMAS-8© Software is protected by U.S. copyright laws (registration number: TX 8-816-517). Permission is required to use the Morisky Widget MMAS-8© software and was obtained for this research. A license agreement is available from MMAS Research LLC, 1501 Spring Hill Rd, Petaluma, CA 94952, USA, or by email (strubow@morisky.org).
Funding
This PMS was funded by Mitsubishi Tanabe Pharma Corporation and Daiichi Sankyo Company, Ltd.. Mitsubishi Tanabe Pharma Corporation funded the journal publication fees, including the rapid service fee and open access.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Takashi Kadowaki, Nobuya Inagaki, and Hirotaka Watada contributed to data interpretation and provided medical advice. Kazuyo Sasaki, Kazumi Mori-Anai, and Tatsuki Teranishi contributed to the conception of the surveillance and data interpretation. Tomohisa Iwasaki contributed to the analyses and data interpretation. All authors contributed to manuscript development and approved the final manuscript.
Medical Writing Assistance
The authors thank Nicholas D. Smith (EMC K.K.) for medical writing support, which was funded by Mitsubishi Tanabe Pharma Corporation.
Prior Presentation
Results of this PMS were presented as abstracts and posters at the 62nd (2019, May 23–25; Sendai), 63rd (2020, October 5–16; Virtual conference) and 64th (2021, May 20–22; Kanazawa) Annual Meetings of the Japan Diabetes Society.
Disclosures
Takashi Kadowaki has received lecture fees from MSD K.K., Astellas Pharma Inc., AstraZeneca K.K., Abbott Japan LLC, Sanofi K.K., Terumo Corporation, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Eli Lilly Japan K.K., Nippon Boehringer Ingelheim Co., Ltd., Takeda Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd.; manuscript fees from Takeda Pharmaceutical Co., Ltd.; research funds from AstraZeneca K.K., Daiichi Sankyo Company, Ltd., Takeda Pharmaceutical Co., Ltd.; research grants from Astellas Pharma Inc., Kissei Pharmaceutical Co., Ltd., Sanofi K.K., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Taisho Pharma Co., Ltd., Daiichi Sankyo Company, Ltd., Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd.; and endowed departments from MSD K.K., Novo Nordisk Pharma Ltd., Kowa Company Ltd., Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Asahi Mutual Life Insurance Company.
Nobuya Inagaki has received research funds from Terumo Corporation, Drawbridge Inc., and Asken Inc.; lecture fees from Kowa Company Ltd., MSD K.K., Astellas Pharma Inc., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Sumitomo Dainippon Pharma Co., Ltd., Sanofi K.K., and Eli Lilly Japan K.K.; and research grants from Kissei Pharmaceutical Co., Ltd., Sanofi K.K., Daiichi Sankyo Company, Ltd., Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Japan Tobacco Inc., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Astellas Pharma Inc., MSD K.K., Ono Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Novartis Pharma K.K., and Life Scan Japan.
Hirotaka Watada has received lecture fees from MSD K.K., Takeda Pharmaceutical Co., Ltd., Sanofi K.K., Ono Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., AstraZeneca K.K., Astellas Pharma Inc., Sanwa Kagaku Kenkyusho Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Kyowa Kirin Co., Ltd., Terumo Corporation; research funds from Sanofi K.K., Kowa Company Ltd., Nippon Boehringer Ingelheim Co., Ltd.; research grants from Kissei Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Taisho Pharma Co., Ltd., Abbott Japan LLC, Novo Nordisk Pharma Ltd., Daiichi Sankyo Company, Ltd., Astellas Pharma Inc., Kyowa Kirin Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceutical Co., Ltd.; and endowed departments from MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceutical Co., Ltd., Kowa Company Ltd., Takeda Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., and Soiken Holdings Inc.
Kazuyo Sasaki, Kazumi Mori-Anai, Tomohisa Iwasaki, and Tatsuki Teranishi are employees of Mitsubishi Tanabe Pharma Corporation.
Compliance with Ethics Guidelines
This PMS was approved by the Ministry of Health, Labour and Welfare of Japan and was conducted by Mitsubishi Tanabe Pharma Corporation in compliance with Good Post-Marketing Study Practice in Japan, which does not require the collection of informed consent or ethical approval from participating institutions. All patients who participated in this PMS provided written informed consent. All data were collected anonymously. The surveillance was registered on Japic Clinical Trials Information (JapicCTI-173778).
Data Availability
The datasets generated during and/or analysed in this PMS are not publicly available to protect patient confidentiality but may be obtained from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kadowaki, T., Inagaki, N., Watada, H. et al. Real-World Evidence of Treatment with Teneligliptin/Canagliflozin Combination Tablets for Type 2 Diabetes Mellitus: A Post-Marketing Surveillance in Japan. Adv Ther 39, 1642–1658 (2022). https://doi.org/10.1007/s12325-021-02038-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-02038-5