Abstract
Since 1955, the only available H1 antihistamines for intravenous administration have been first-generation formulations and, of those, only intravenously administered (IV) diphenhydramine is still approved in the USA. Orally administered cetirizine hydrochloride, a second-generation H1 antihistamine, has been safely used over-the-counter for many years. In 2019, IV cetirizine was approved for the treatment of acute urticaria. In light of this approval, this narrative review discusses the changing landscape of IV antihistamines for the treatment of histamine-mediated conditions. Specifically, IV antihistamines will be discussed as a treatment option for acute urticaria and angioedema, as premedication to prevent infusion reactions related to anticancer agents and other biologics, and as an adjunct treatment for anaphylaxis and other allergic reactions. Before the development of IV cetirizine, randomized controlled trials of IV antihistamines for these indications were lacking. Three randomized controlled trials have been conducted with IV cetirizine versus IV diphenhydramine in the ambulatory care setting. A phase 3 trial of IV cetirizine 10 mg versus IV diphenhydramine 50 mg was conducted in 262 adults who presented to the urgent care/emergency department with acute urticaria requiring antihistamines. For the primary efficacy endpoint, defined as change from baseline in a 2-h patient-rated pruritus score, non-inferiority of IV cetirizine to IV diphenhydramine was demonstrated (score − 1.6 vs − 1.5, respectively; 95% CI − 0.1, 0.3). Compared with IV diphenhydramine, IV cetirizine demonstrated fewer adverse effects including less sedation, a significantly shorter length of stay in the treatment center, and fewer returns to the treatment center at 24 and 48 h. Similar findings were demonstrated in another phase 2 acute urticaria trial and in a phase 2 trial assessing IV cetirizine for pretreatment for infusion reactions in the oncology/immunology setting. IV cetirizine is associated with similar patient outcomes, fewer adverse effects, and increased treatment center efficiency than IV diphenhydramine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intravenously administered (IV) antihistamines are a treatment option for acute urticaria and angioedema, and an adjunct therapy for anaphylaxis and other allergic reactions in ambulatory care settings. |
Diphenhydramine is the only first-generation H1 antihistamine still FDA approved for intravenous use, and cetirizine is the first second-generation H1 antihistamine approved for intravenous use. |
In randomized controlled trials with direct comparison between IV antihistamines, IV cetirizine prevented infusion reactions in patients receiving anticancer treatments and was non-inferior to IV diphenhydramine for the treatment of acute urticaria, with less sedation and fewer adverse effects than IV diphenhydramine. |
Introduction
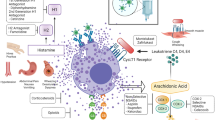
Histamine is found in mast cells and basophils throughout the body and has a wide range of effects [1]. The release of stored histamine granules from these cells can be triggered by various stimuli [2]. H1 antihistamines became available for clinical use in the 1940s and their anti-allergic effects have been well recognized since the 1950s [3]. The effects of specific antihistamines depend on their target receptor subtype. There are four histamine receptor subtypes (H1, H2, H3, and H4), each of which has a different expression pattern in cells throughout the body [4]. H1 receptors are expressed on central nervous system (CNS) neurons, endothelial cells, white blood cells, and smooth muscle cells [2]. Binding of histamine to H1 receptors leads to symptoms of an acute allergic reaction such as itching, sneezing, and increased vascular permeability [2]. Thus, H1 antihistamines are a standard treatment for histamine-mediated allergic reactions (i.e., allergic rhinitis, urticaria) [5,6,7].
Many of the first-generation H1 antihistamines (including chlorpheniramine, diphenhydramine, and hydroxyzine) are still in use. However, the first-generation H1 antihistamines cross the blood–brain barrier and bind to CNS H1 receptors, which can lead to drowsiness, sedation, fatigue, decreased cognition, and other CNS functions [8]. There may also be a small risk of dementia in patients with high exposure to first-generation H1 antihistamines [9]. In addition, the first-generation H1 antihistamines bind non-selectively to muscarinic, serotonin, and alpha-adrenergic receptors, which can cause adverse effects such as dry mouth and dizziness [3]. The effects of first-generation H1 antihistamine on the CNS and non-H1 receptors have sometimes been leveraged to treat motion sickness, nausea, anxiety, and parkinsonism [10, 11]. In the early 1980s, second-generation H1 antihistamines became available and in the USA include cetirizine, fexofenadine, loratadine, desloratadine, and levocetirizine. In contrast to the first-generation formulations, the second-generation H1 antihistamines are relatively non-sedating or less sedating because they have limited ability to cross the blood–brain barrier [4]. Also unlike the first-generation formulations, the second-generation H1 antihistamines have a low affinity for non-H1 receptors [4].
H1 antihistamines have been available over-the-counter in oral, topical, and ocular formulations for many years. Intravenously administered (IV) formulations are used in settings where other IV drugs are already being administered (i.e., as premedication to prevent allergic reactions to chemotherapy or biologics), when oral formulations are not usable (i.e., patients with nausea or who are unconscious), or when a rapid onset of action is needed to treat serious allergic reactions.
Since 1955, the only available IV H1 antihistamines have been first-generation formulations. In the USA, diphenhydramine is currently the only first-generation H1 antihistamine still approved by the US Food and Drug Administration (FDA) for intravenous use, although other IV first-generation H1 antihistamines are still used in other countries. IV diphenhydramine has several labeled indications (Table 1) [11]. In 2019, IV cetirizine hydrochloride (Quzyttir®, TerSera Therapeutics LLC, Deerfield, IL) was approved for acute urticaria and is the only second-generation H1 antihistamine approved by the FDA for intravenous use [12]. This narrative review discusses the changing landscape of IV H1 antihistamines for the treatment of histamine-related conditions in light of the first approval of a second-generation IV H1 antihistamine. The content of this review article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Pharmacology
Cetirizine hydrochloride is a metabolite of the first-generation H1 antihistamine hydroxyzine and exerts its effects by selectively inhibiting peripheral H1 receptors [11, 12]. In healthy volunteers, peak concentrations of cetirizine injected over a period of 1.0 to 1.5 min [12] were reached at 3.6 min with the 5 mg IV dose and 1.8 min with the 10 mg IV dose [12]. The half-life of cetirizine in healthy volunteers is 8.3 h [12].
There is variability in the literature regarding the pharmacokinetics of IV diphenhydramine in different age groups and little information is available in the prescribing information [13,14,15]. Time to peak concentration was not reported in any of the studies [13,14,15], and onset of action is simply described as “rapid” in the prescribing information [11]. In healthy adults, IV diphenhydramine 50 mg has an elimination half-life of 8.5 h [14].
The potency of a drug can be measured by its Ki value, which is reflective of the binding affinity of the compound to its target receptor or enzyme. The smaller the Ki value, the less of the compound is needed to produce the desired clinical result. The Ki value is 6 nM for cetirizine and 9.6–16 nM for diphenhydramine, indicating that cetirizine is pharmacodynamically more potent than diphenhydramine [16,17,18].
Treatment of Acute Urticaria
H1 antihistamines are a recommended treatment for acute urticaria, which can manifest as wheals, angioedema, or both [7]. In the emergency department (ED) or urgent care (UC) setting, an IV H1 antihistamine may be used to provide faster relief of acute urticaria symptoms than an orally administered H1 antihistamine, for patients unable to take oral formulations, and/or for patients with more serious or generalized urticaria. Two trials have been conducted to date evaluating the role of IV cetirizine in the treatment of acute urticaria. A phase 2 multicenter, randomized, double-blind trial of IV cetirizine 10 mg versus IV diphenhydramine 50 mg was conducted in 33 adults who presented with acute urticaria (with or without angioedema) that required H1 antihistamines in EDs or UC centers [19]. The trial had two primary efficacy endpoints, the first being the extent of physician-assessed urticaria/erythema score reduction (percentage of body area affected and the intensity of redness on a scale of 0 = none to 3 = severe) and the second being pruritus severity score (physician-assessed and patient-rated on a scale of 0 = no pruritus to 3 = severe pruritus). The change from baseline for a composite score that combined the two primary endpoints was not significantly different between cetirizine and diphenhydramine at either 1 h or 2 h post treatment or at discharge (p ≥ 0.68). Patients receiving cetirizine had a shorter amount of time at the treatment center compared with those receiving diphenhydramine (1.7 h vs 2.3 h, respectively) and fewer returned to the treatment center within 24 h (n = 0 and n = 2, respectively; Table 2). A similar, phase 3 trial evaluated the non-inferiority of IV cetirizine 10 mg versus IV diphenhydramine 50 mg in 262 adults who presented with acute urticaria (with or without angioedema) that required H1 antihistamines in EDs or UC centers [20]. For the primary efficacy endpoint of change in patient-rated pruritus score at baseline to 2 h after treatment administration, the mean change with IV cetirizine was − 1.6 and with IV diphenhydramine it was − 1.5, with the treatment difference of 0.1 (95% CI − 0.1, 0.3) between the two groups meeting the non-inferiority criteria (Table 2). The key secondary endpoints of time spent in the treating facility and percentage of patients needing to return to a treatment center were significantly different between the two groups, indicating better clinical outcomes with IV cetirizine (Table 2). Patients receiving IV cetirizine spent a mean of 1.7 h at the center compared with 2.1 h for patients receiving IV diphenhydramine (p = 0.005). Of patients receiving IV cetirizine, 3.9% and 5.5% needed to return to a treatment center within 24 and 48 h, respectively, compared with 11.1% and 14.1% of patients receiving IV diphenhydramine (p ≤ 0.04). A significantly lower percentage of patients receiving IV cetirizine needed additional rescue medications (i.e., epinephrine, corticosteroids, etc.) than patients receiving IV diphenhydramine (15.0% vs 27.4%, respectively; p = 0.02).
No other phase 3 trials of IV diphenhydramine for acute urticaria have been conducted.
Treatment of Angioedema
Histamine-mediated angioedema is caused by the same underlying pathologic mechanism as wheals but occurs deeper in the skin. Angioedema is a common reason for ED visits [21] and can be life-threatening if the airway is involved [22, 23]. H1 antihistamines, along with corticosteroids, are first-line treatment for histamine-mediated angioedema without anaphylaxis [23]. However, emergency medicine workgroups recommend IV H1 antihistamines when time-critical therapy is needed [23]. H1 antihistamines are also recommended as adjunct therapy to epinephrine when the angioedema manifests with other signs/symptoms consistent with anaphylaxis [23,24,25]. However, H1 antihistamines are not effective for bradykinin-related angioedema (i.e., hereditary angioedema or angiotensin-converting enzyme inhibitor-induced angioedema) [26, 27].
There are no phase 3 trials of IV diphenhydramine specifically for the treatment of angioedema. In the phase 3 non-inferiority trial of IV cetirizine versus IV diphenhydramine, 12% of the patients in each treatment group had urticaria and angioedema [20]. In a post hoc subgroup analysis of patients with urticaria and angioedema, for the primary endpoint of the change in patient-rated pruritus score from baseline to 2 h after treatment administration, the mean (SD) change with IV cetirizine was − 1.5 (1.1) and with IV diphenhydramine was − 1.4 (0.9) [28].
Treatment and Pretreatment of Infusion Reactions
Anticancer Agents
Infusion reactions (IR) to anticancer agents, which include chemotherapy drugs and monoclonal antibodies (mAbs), can be immune-mediated or non-immune-mediated [29]. Non-immune-mediated reactions can still be pseudo-allergic, meaning the reaction resembles a true allergic reaction with direct mast cell histamine degranulation [30]. For acute onset IRs that have respiratory symptoms and/or hypotension, the IR should be treated as either anaphylaxis, in which case epinephrine should be administered (see “IV Antihistamine as Adjunct Therapy for Anaphylaxis” section), or cytokine release syndrome [29]. In these cases, clinical practice guidelines recommend a combination of IV H1 (diphenhydramine 50 mg) and H2 (ranitidine 50 mg) antihistamines as part of the management algorithm [29, 31].
Pretreatment with H1 antihistamines, usually in combination with glucocorticoids and/or antipyretics, is recommended to prevent IR related to some anticancer drugs (Table 3) [32,33,34,35,36,37]. Chemotherapy drugs of the taxane class (e.g., paclitaxel, docetaxel) in particular should always be premedicated with a combination of an H1 antihistamine, H2 antihistamine, and dexamethasone [32]. Use of premedication (in conjunction with a slowed infusion rate) drops the incidence of hypersensitivity reactions with paclitaxel from 30% down to a severe hypersensitivity reaction rate of approximately 2–4% [32, 38].
IV diphenhydramine has been part of the premedication regimen for anticancer agents for decades [39, 40]. However, there have been no randomized controlled trials (RCTs) specifically evaluating the impact of IV diphenhydramine on IRs related to anticancer agents. One recently completed phase 2, randomized, double-blind, multicenter trial in 34 adults with cancer or immune disorders compared the incidence of IRs related to treatment with an anti-CD20 (rituximab, its biosimilar, or obinutuzumab) or paclitaxel (first-cycle infusion, re-treatment after 6 months, or in patients with persistent reactions while on maintenance or retreatment) after premedication with either IV cetirizine 10 mg or IV diphenhydramine 50 mg [41]. Overall, 2/17 (11.8%) patients pretreated with IV cetirizine and 3/17 (17.6%) patients pretreated with IV diphenhydramine experienced an infusion reaction (Table 2). Patients receiving IV cetirizine had a shorter mean time of 24 min spent at the treatment center than those receiving IV diphenhydramine (4.3 h vs 4.7 h, respectively) and had a lower sedation score at all time points, including discharge (0.1 vs 0.4; Table 2).
Other Biologics
Many biologics have been developed that are designed to neutralize cytokines or other targets and it can be assumed that all of them have the potential to cause hypersensitivity reactions. The Joint Task Force on Practice Parameters of the American College of Allergy, Asthma, and Immunology and the American Academy of Allergy, Asthma, and Immunology on Drug Allergy suggests that readministration strategies of a biologic after an immediate-type hypersensitivity reaction may include premedication upon next infusion [30]. Currently, all biologics are delivered either intravenously or subcutaneously. Thus, there is a potential role for IV H1 antihistamines as pretreatment for any IV biologics if pretreatment is warranted and there are no contraindications. Commonly used biologics that currently require pretreatment with an H1 antihistamine include infliximab, cetuximab, and rituximab (Table 3).
Iodinated Contrast Media
Use of nonionic low-osmolarity iodinated contrast media has replaced ionic contrast media to reduce the number and severity of associated adverse drug reactions. Nevertheless, nonionic low-osmolarity contrast media can directly cause histamine release and allergic-like reactions can still occur [42,43,44]. Routine premedication is not recommended, but a 12-h to 13-h oral premedication may be considered for patients with previous allergic-like or unknown-type reactions to contrast media of the same class [45]. For those patients, an IV premedication regimen may be considered if they have not already been premedicated with oral formulations and cannot easily reschedule, or if the patient is being treated in an emergency or inpatient setting and a 12-h to 13-h oral premedication could adversely impact their care [45]. To date, there has been no RCT evaluating the impact of IV H1 antihistamines on infusion reactions to contrast media. However, the American College of Radiology (ACR) manual on contrast media indicates that supplemental administration of a non-selective antihistamine, administered either orally or intravenously 1 h before administration of contrast medium, may reduce the frequency of urticaria, angioedema, and respiratory symptoms [45]. The ACR manual notes that second-generation H1 antihistamines cause less drowsiness and may be beneficial for patients who need to drive themselves home [45].
IV Antihistamine as Adjunct Therapy for Anaphylaxis
Anaphylaxis is a severe hypersensitivity reaction that can be life-threatening [46]. Given that many of the manifestations of anaphylaxis are mediated by histamine, logic dictates that H1 antihistamines would be a therapeutic option [47]. However, H1 antihistamines do not prevent the serious complications of anaphylaxis (e.g., airway obstruction, hypotension, and shock), do not prevent biphasic reactions, nor do they target other mediators of anaphylaxis, such as platelet-activating factor and tryptase [48, 49]. Thus, epinephrine should always be the primary treatment [49, 50]. IV H1 antihistamine still has potential benefits for the treatment of anaphylaxis-associated urticaria or itching but should not be administered until after epinephrine is given [11, 50]. The Centers for Disease Control (CDC) suggests IV diphenhydramine and IV cetirizine as adjunct therapy for vaccine-related anaphylaxis, including for COVID-19 vaccinations [51, 52]. It should be noted that IV diphenhydramine can cause or exacerbate hypotension, which could be detrimental in anaphylactic patients who may already have unstable blood pressure [53, 54]. Hypotension has not been observed with IV cetirizine to date. Despite their decades-long use as an adjunct to anaphylaxis, there have been no RCTs evaluating the effect of H1 antihistamines on anaphylaxis.
Safety
Adverse Events
The most common adverse events (AEs) with IV cetirizine (all incidence no greater than 1%) are dysgeusia, headache, paresthesia, presyncope, dyspepsia, feeling hot, and hyperhidrosis [12]. The most common AEs with IV diphenhydramine are sedation, sleepiness, dizziness, disturbed coordination, epigastric distress, and thickening of bronchial secretions [11]. In the phase 3 RCT of IV cetirizine versus IV diphenhydramine for acute urticaria, 1 (0.8%) patient receiving IV cetirizine experienced treatment-related AEs (dysgeusia, paresthesia, and sensation of warmth) and 9 (6.7%) patients receiving IV diphenhydramine experienced treatment-related AEs, the most common of which were dizziness (n = 5) and nausea (n = 3) (Table 2) [20].
Because the first-generation H1 antihistamines bind to H1 receptors in the CNS, they can cause sedation, drowsiness, and impairment of cognitive function. Sedation can also occur with IV cetirizine, but typically to a lesser extent. In the phase 3 RCT of IV cetirizine versus IV diphenhydramine for acute urticaria, the change in mean patient-rated sedation scores from baseline were significantly less with IV cetirizine than with IV diphenhydramine at the 1-h, 2-h, and discharge assessments (Fig. 1; p ≤ 0.04 for all time points) [20].

Reprinted from Ann Emerg Med, Abella et al., Intravenous cetirizine versus intravenous diphenhydramine for the treatment of acute urticaria: a phase III randomized controlled noninferiority trial, pages 489–500, 2020, with permission from Elsevier [20]
Patient-rated sedation score and change by visit. Intention-to-treat population.
The CNS effects of H1 antihistamines, particularly sedation, have several implications in the infusion or ED setting. In patients with layrngeal edema, sedation can further complicate airway protection. Furthermore, the sedative effects can be indirectly dangerous to the patient. Many patients drive themselves to their appointments, a task that requires mental alertness. Under the sedative and impaired cognitive function effects of the H1 antihistamines, patients driving home from the appointments may be at increased risk of an accident. A review conducted by the US Department of Transportation determined that there was “overwhelming” evidence that the first-generation H1 antihistamines impaired some performance skills (e.g., “divided attention”, “vigilance”, and “tracking”) but that while the second-generation H1 antihistamines show a low incidence of performance skill impairment, they may cause sedation and skill impairment for some individuals [55]. Use of antihistamines has also been shown to place individuals at a higher risk of work-related injuries [56]. In a driving simulator study conducted by the University of Iowa, driver test results were worse in patients who had received a 50 mg dose of diphenhydramine than drivers who had a blood alcohol level of 0.1% [57]. As such, the prescribing information for IV diphenhydramine states that “patients should be warned about engaging in activities requiring mental alertness such as driving a car or operating appliances, machinery, etc.” [11]. The prescribing information for IV cetirizine states that patients should “exercise due caution when driving a car or operating potentially dangerous machinery” [12]. Thus, patients experiencing sedation, dizziness, or drowsiness may need to stay longer in the infusion center or ED for their own safety. This can have a secondary effect on the treatment center in that fewer patients may be treated if patients are in infusion chairs for longer in order to monitor these potential adverse effects.
Drug–Drug Interactions
No clinically significant drug interactions have been found with orally administered cetirizine, although a 400 mg dose of theophylline caused a 16% decrease in the clearance of orally administered cetirizine [12]. Diphenhydramine is metabolized by the CYP2D6 enzyme in the cytochrome P450 pathway [58]; multiple drug interactions exist with diphenhydramine, including a significant interaction with metoprolol [59]. Diphenhydramine has additive effects with alcohol or other CNS depressants, which can increase drowsiness [11]. Concomitant use of monoamine oxidase inhibitors can prolong and intensify the anticholinergic effects of antihistamines [11].
Contraindications, Warnings, and Precautions
The only contraindication for IV cetirizine is hypersensitivity to the cetirizine hydrochloride or its ingredients, levocetirizine, or hydroxyzine (Table 4) [12, 60]. IV diphenhydramine is contraindicated for use in neonates or premature infants, in nursing mothers, for use as a local anesthetic, and when the patient has hypersensitivity to diphenhydramine hydrochloride and other antihistamines of similar chemical structure (Table 4) [11, 60].
Both IV cetirizine and IV diphenhydramine have warnings about the occurrence of sedation, driving or operating potentially dangerous equipment, and concurrent use with alcohol or CNS depressants (Table 4) [11, 12, 60]. IV diphenhydramine has several additional warnings and precautions (Table 4) [11, 60].
IV Antihistamines in Special Patient Populations
Older Adults
In the phase 3 RCT of IV cetirizine versus IV diphenhydramine for acute urticaria, post hoc sub-analyses indicated that IV cetirizine was as efficacious in the primary efficacy outcome in patients at least 65 years of age (n = 18) versus those less than 65 years of age (n = 244) [20]. The increases in mean sedation scores in patients at least 65 years of age were smaller in those receiving IV cetirizine than in those receiving IV diphenhydramine at 1 h, 2 h, and discharge [20]. The mean (SD) time in the treatment center for patients at least 65 years of age was 1.4 h (0.5) for IV cetirizine versus 3.0 h (1.6) for patients receiving IV diphenhydramine (p = 0.03 between groups), and the number of patients returning to the treatment center within 24 h was 0/9 in the IV cetirizine arm versus 3/9 in the IV diphenhydramine arm.
In the phase 2 infusion reaction pretreatment RCT of IV cetirizine versus IV diphenhydramine, of the 21 patients at least 65 years of age, 1/9 (11.1%) patient pretreated with IV cetirizine and 2/12 (16.7%) patients pretreated with IV diphenhydramine experienced an infusion reaction [41]. The mean (SD) sedation score at discharge was 0.1 (0.3) in patients receiving IV cetirizine and 0.4 (0.7) in patients receiving IV diphenhydramine. Patients receiving IV cetirizine had a shorter mean time of 30 min spent at the treatment center than those receiving IV diphenhydramine (4.4 h vs 4.9 h, respectively).
In a study of orally administered diphenhydramine, the half-life was approximately 70% longer in older adults (13.5 h) than young adults (9.2 h) and clearance rates were more than twice as slow in older adults (11.7 mL/min/kg) than young adults (23.3 mL/min/kg) [15].
The American Geriatrics Society discourages the use of diphenhydramine in older adults because of its anticholinergic (e.g., confusion, dry mouth) effects [61].
Children
IV cetirizine is approved in children as young as 6 months of age, on the basis of extrapolation of efficacy and safety data from the phase 3 trial for acute urticaria and orally administered cetirizine data [12].
In a study of orally administered diphenhydramine, the half-life was approximately 70% shorter in children (5.4 h) than young adults (9.2 h) and clearance rates were almost twice as fast in children (49.2 mL/min/kg) than young adults (23.3 mL/min/kg) [15].
Cost
IV cetirizine is a brand name drug, whereas IV diphenhydramine is available as a generic drug, which will affect the direct cost and drug charges. However, there are significant total overall cost implications and patient outcome implications when choosing to use a first-generation H1 antihistamine when a second-generation H1 antihistamine exists. Indirect costs of first-generation H1 antihistamines such as time spent in the treatment center, need to return to treatment center within 1–2 days after initial visit, need for additional rescue medication, increased staff resources related to longer stays, lack of health system payment for revisits, and the effect on health system throughput will significantly impact the overall costs. The phase 3 RCT of IV cetirizine versus IV diphenhydramine for acute urticaria showed significantly less time in the treatment center, a smaller percentage of patients returning for treatment within 24 or 48 h, and less rescue medication use with IV cetirizine (Table 2) [20]. A budget impact model taking into account direct costs, indirect costs, and revenues associated with IV cetirizine versus IV diphenhydramine for the treatment of acute urticaria in the ED in the USA concluded that adoption of IV cetirizine has a positive budget impact for an estimated 50,000 or 100,000 ED visits per year [62]. The drivers of the positive budget impact for IV cetirizine were shorter duration of visit, lower 24-h return visits, and higher drug revenue. Another indirect cost consideration, albeit not simply financial in nature, is the liability of accidents from the CNS and sedating effects of IV diphenhydramine.
Conclusions
IV diphenhydramine has been in use for many years. Cetirizine is the only second-generation H1 antihistamine approved for intravenous use. Compared with IV diphenhydramine, IV cetirizine has an improved safety profile including less sedation, fewer contraindications, fewer warnings and precautions, and less risk in the elderly population. This improved safety profile, along with IV cetirizine’s longer duration of action, resulted in comparable patient outcomes to IV diphenhydramine with a positive impact on health care systems in RCTs. Although IV cetirizine is currently only approved for acute urticaria, H1 antihistamines are first-line or adjunct treatments for other conditions, and it may be an option when intravenous administration is preferred.
References
Akdis C, Jutel M, Akdis M. Regulatory effects of histamine and histamine receptor expression in human allergic immune responses. Chem Immunol Allergy. 2008;94:67–82.
Akdis CA, Blaser K. Histamine in the immune regulation of allergic inflammation. J Allergy Clin Immunol. 2003;112(1):15–22.
Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011;128(6):1139-50.e4.
Bernstein J, Leslie T. Antihistamines. In: Grammer LC, Greenberger PA, editors. Patterson’s allergic diseases. 8th ed. Philadelphia: Wolters Kluwer Health; 2018. p. 719–34.
Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133(5):1270–7.
Dykewicz MS, Wallace DV, Amrol DJ, et al. Rhinitis 2020: a practice parameter update. J Allergy Clin Immunol. 2020;146(4):721–67.
Zuberbier T, Abdul Latiff AH, Abuzakouk M, et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2021. https://doi.org/10.1111/all.15090.
Church MK, Maurer M, Simons FER, et al. Risk of first-generation H1-antihistamines: a GA2LEN position paper. Allergy. 2010;65(4):459–66.
Richardson K, Fox C, Maidment I, et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ. 2018;361:k1315.
Vistaril® (hydroxyzine pamoate) capsules and oral suspension. New York, NY: Pfizer Labs; 2014.
Diphenhydramine hydrochloride injection. Lake Forest, IL: Hospira, Inc.; 2019.
Quzyttir™ (cetirizine hydrochloride injection). Deerfield, IL: TerSera Therapeutics LLC; 2020.
Berlinger WG, Goldberg MJ, Spector R, Chiang CK, Ghoneim M. Diphenhydramine: kinetics and psychomotor effects in elderly women. Clin Pharmacol Ther. 1982;32(3):387–91.
Blyden GT, Greenblatt DJ, Scavone JM, Shader RI. Pharmacokinetics of diphenhydramine and a demethylated metabolite following intravenous and oral administration. J Clin Pharmacol. 1986;26(7):529–33.
Simons KJ, Watson WT, Martin TJ, Chen XY, Simons FE. Diphenhydramine: pharmacokinetics and pharmacodynamics in elderly adults, young adults, and children. J Clin Pharmacol. 1990;30(7):665–71.
Krystal AD, Richelson E, Roth T. Review of the histamine system and the clinical effects of H1 antagonists: basis for a new model for understanding the effects of insomnia medications. Sleep Med Rev. 2013;17(4):263–72.
Ghoneim OM, Legere JA, Golbraikh A, Tropsha A, Booth RG. Novel ligands for the human histamine H1 receptor: synthesis, pharmacology, and comparative molecular field analysis studies of 2-dimethylamino-5-(6)-phenyl-1,2,3,4-tetrahydronaphthalenes. Bioorg Med Chem. 2006;14(19):6640–58.
Gillard M, Van Der Perren C, Moguilevsky N, Massingham R, Chatelain P. Binding characteristics of cetirizine and levocetirizine to human H(1) histamine receptors: contribution of Lys(191) and Thr(194). Mol Pharmacol. 2002;61(2):391–9.
Data on File. ETTAU-02 Clinical study report. TerSera Therapeutics; 2015.
Abella BS, Berger WE, Blaiss MS, et al. Intravenous cetirizine versus intravenous diphenhydramine for the treatment of acute urticaria: a phase III randomized controlled noninferiority trial. Ann Emerg Med. 2020;76(4):489–500.
Zilberberg MD, Jacobsen T, Tillotson G. The burden of hospitalizations and emergency department visits with hereditary angioedema and angioedema in the United States, 2007. Allergy Asthma Proc. 2010;31(6):511–9.
Pall AH, Lomholt AF, von Buchwald C, Bygum A, Rasmussen ER. Clinical features and disease course of primary angioedema patients in a tertiary care hospital. J Asthma Allergy. 2020;13:225–36.
Moellman JJ, Bernstein JA, Lindsell C, et al. A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21(4):469–84.
James C, Bernstein JA. Current and future therapies for the treatment of histamine-induced angioedema. Expert Opin Pharmacother. 2017;18(3):253–62.
Long BJ, Koyfman A, Gottlieb M. Evaluation and management of angioedema in the emergency department. West J Emerg Med. 2019;20(4):587–600.
Baram M, Kommuri A, Sellers SA, Cohn JR. ACE inhibitor-induced angioedema. J Allergy Clin Immunol Pract. 2013;1(5):442–5.
Busse PJ, Christiansen SC, Riedl MA, et al. US HAEA medical advisory board 2020 guidelines for the management of hereditary angioedema. J Allergy Clin Immunol Pract. 2021;9(1):132-50 e3.
Data on file. ETTAU-03. https://clinicaltrials.gov/ct2/show/NCT02023164. TerSera Therapeutics; 2020.
Roselló S, Blasco I, García Fabregat L, Cervantes A, Jordan K. Management of infusion reactions to systemic anticancer therapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2017;28(suppl_4):iv100–18.
Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–73.
Ovarian cancer including fallopian tube cancer and primary peritoneal cancer: NCCN Clinical Practice Guidelines in Oncology. Version 2.2020. National Comprehensive Cancer Network. 2021. https://www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed 15 Oct 2021.
Taxol (paclitaxel) injection. Princeton, NJ: Bristol-Myers Squibb Company; 2011.
Adcetris™ (brentuximab vedotin) for injection. Bothell, WA: Seattle Genetics, Inc.; 2012.
Erbitux® (cetuximab) injection, for intravenous use. Indianapolis, IN: Eli Lilly and Company; 2020.
Darzalex (daratumumab) injection, for intravenous use. Horsham, PA: Janssen Biotech, Inc.; 2020.
Arzerra® (ofatumumab) injection, for intravenous use. East Hanover, NJ: Novartis Pharmaceutical Corporation; 2016.
Rituxan® (rituximab) injection, for intravenous use. South San Franciso, CA: Genentech, Inc.; 2018.
Lee C, Gianos M, Klaustermeyer WB. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann Allergy Asthma Immunol. 2009;102(3):179–87.
ten Bokkel Huinink WW, Eisenhauer E, Swenerton K, Canadian-European Taxol Cooperative Trial Group. Preliminary evaluation of a multicenter, randomized comparative study of TAXOL (paclitaxel) dose and infusion length in platinum-treated ovarian cancer. Cancer Treat Rev. 1993;19(Suppl C):79–86.
Kintzel PE. Prophylaxis for paclitaxel hypersensitivity reactions. Ann Pharmacother. 2001;35(9):1114–7.
Holmes JP, Peguero J, Campbell Garland R, et al. Intravenous cetirizine vs intravenous diphenhydramine for the prevenstion of hypersensitivity infusion reactions: results of an exploratory phase 2 study. J Infus Nurs. 2021. https://doi.org/10.1097/NAN.0000000000000444.
Katayama H, Yamaguchi K, Kozuka T, et al. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175(3):621–8.
Callahan MJ, Poznauskis L, Zurakowski D, Taylor GA. Nonionic iodinated intravenous contrast material-related reactions: incidence in large urban children’s hospital–retrospective analysis of data in 12,494 patients. Radiology. 2009;250(3):674–81.
Salem DN, Findlay SR, Isner JM, Konstam MA, Cohen PF. Comparison of histamine release effects of ionic and non-ionic radiographic contrast media. Am J Med. 1986;80(3):382–4.
ACR manual on contrast media. American College of Radiology. 2021. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed 15 Oct 2021.
Cardona V, Ansotegui IJ, Ebisawa M, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 2020;13(10):100472.
Tomasiak-Lozowska MM, Klimek M, Lis A, Moniuszko M, Bodzenta-Lukaszyk A. Markers of anaphylaxis—a systematic review. Adv Med Sci. 2018;63(2):265–77.
Fineman SM. Optimal treatment of anaphylaxis: antihistamines versus epinephrine. Postgrad Med. 2014;126(4):73–81.
Campbell RL, Li JT, Nicklas RA, et al. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599–608.
Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082–123.
CDC. General best practice guidelines for immunization: Best practices guidance of the Advisory Committee on Immunization Practices. Preventing and managing adverse reactions. Centers for Disease Control and Prevention, Atlanta, GA. 2020. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/adverse-reactions.pdf. Accessed 15 Oct 2021.
CDC. Interim considerations: Preparing for the potential management of anaphylaxis after COVID-19 vaccination. Centers for Disease Control and Prevention, Atlanta, GA. 2021. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html. Accessed 15 Oct 2021.
vanSonnenberg E, Neff CC, Pfister RC. Life-threatening hypotensive reactions to contrast media administration: comparison of pharmacologic and fluid therapy. Radiology. 1987;162(1 Pt 1):15–9.
Brown AF. Therapeutic controversies in the management of acute anaphylaxis. J Accid Emerg Med. 1998;15(2):89–95.
Moskowitz H, Jeavons Wilkinson C. Antihistamines and driving-related behavior: a review of the evidence for impairment. Report DOT HS 809 714. US Department of Transportation. 2004. https://one.nhtsa.gov/people/injury/research/antihistamines/Antihistamines%20Web/pages/1%20Introduction.htm. Accessed 15 Oct 2021.
Gilmore TM, Alexander BH, Mueller BA, Rivara FP. Occupational injuries and medication use. Am J Ind Med. 1996;30(2):234–9.
Weiler JM, Bloomfield JR, Woodworth GG, et al. Effects of fexofenadine, diphenhydramine, and alcohol on driving performance. A randomized, placebo-controlled trial in the Iowa driving simulator. Ann Intern Med. 2000;132(5):354–63.
Sharma A, Hamelin BA. Classic histamine H1 receptor antagonists: a critical review of their metabolic and pharmacokinetic fate from a birds eye view. Curr Drug Metab. 2003;4(2):105–29.
Hamelin BA, Bouayad A, Méthot J, et al. Significant interaction between the nonprescription antihistamine diphenhydramine and the CYP2D6 substrate metoprolol in healthy men with high or low CYP2D6 activity. Clin Pharmacol Ther. 2000;67(5):466–77.
Baker DE. Cetirizine hydrochloride injection. Hosp Pharm. 2020. https://doi.org/10.1177/0018578720910386.
2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4): 674–94.
Bloudek LM, Ho LK, Kapur N, Brent LD. Budget impact of intravenous cetirizine hydrochloride for the treatment of acute urticaria in the United States emergency department setting. American Society of Health-System Pharmacists midyear clinical meeting; 2020; Virtual meeting.
Acknowledgements
Funding
The journal's Rapid Service fee was funded by TerSera Therapeutics.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial assistance were provided by Erin P. Scott, PhD, of Scott Medical Communications, LLC. This assistance was funded by TerSera Therapeutics.
Authorship
All named authors meet the International Committee of Medial Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Michael Blaiss contributed to the conception and critical revision of this article. Jonathan A. Bernstein, Adam Kessler, Jesse M. Pines, Carlos A. Camargo, Jr., Paula Fulgham, Ryan Haumschild, Kristin Rupp, Timothy Tyler, and Joseph Moellman critically revised the article.
Disclosures
Michael Blaiss has served as a consultant for TerSera Therapeutics, Pfizer, Merck, Covis Pharma, GSK, ALK, Sanofi, and Regeneron. Jonathan A. Bernstein has served as a consultant and/or speaker and/or investigator for Sanofi Genzyme-Regeneron, AstraZeneca, Novartis, Genentech, Shire/Takeda, CSL Behring, Pharming, Biocryst, Merck, Kalvista, Ionis, GlaxoSmithKline, and ALK. Carlos A. Camargo, Jr. has served as a consultant for Bryn Pharma and Kaleo. Paula Fulgham has been a speaker for Celgene, Novartis, Eli Lilly, Merck, BMS, AbbVie, Karyopharm, Amgen, and Sanofi Genzyme. Ryan Haumschild has participated in an advisory board for TerSera. Adam Kessler has served as a consultant and speaker for Zoll Circulation, Inc. Joseph Moellman has served as a consultant for TerSera Therapeutics and CSL Behring. Jesse M. Pines has served as a consultant for CSL Behring, Medtronic, Abbott Point-of-Care, and Novo Nordisk. Kristin Rupp has nothing to disclose. Timothy Tyler has served as a consultant for TerSera.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Blaiss, M.S., Bernstein, J.A., Kessler, A. et al. The Role of Cetirizine in the Changing Landscape of IV Antihistamines: A Narrative Review. Adv Ther 39, 178–192 (2022). https://doi.org/10.1007/s12325-021-01999-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01999-x