Abstract
Introduction
Certain genetic features in chronic lymphocytic leukemia (CLL) are associated with inferior outcomes after chemoimmunotherapy (CIT). This retrospective study evaluated treatment patterns and clinical outcomes of patients with CLL, stratified into high-risk and non-high-risk groups, who received first-line ibrutinib or CIT therapy.
Methods
High-risk group included confirmed presence of del(17p), del(11q), unmutated IGHV, TP53 mutations, or complex karyotype. Weighted high-risk ibrutinib and CIT groups were compared for treatment effects using inverse probability of treatment weighting. Hazard ratios [95% CI] (HR) for time to next treatment (TTNT) were analyzed using Kaplan–Meier curves.
Results
Bendamustine/rituximab was the most common CIT regimen initiated for high-risk patients. During the available follow-up (median 34–35 months), 74.7% of the weighted high-risk ibrutinib group received only one line of treatment, compared with 47.2% of the weighted high-risk CIT group. The most common second-line treatment was ibrutinib for those in the CIT groups and venetoclax for the ibrutinib groups. The weighted high-risk ibrutinib group had a significantly longer TTNT (median not reached) than the weighted high-risk CIT group (median 34.4 months) and was 54% less likely to start a new treatment (HR 0.5 [0.3–0.6], P < 0.010). Among CIT-treated groups, high-risk patients had significantly shorter median TTNT than non-high-risk patients (P < 0.010). However, within the ibrutinib-treated groups, the median TTNT was similar between high-risk and non-high-risk patients (HR 2.2 [1.0–5.0]; P = 0.060).
Conclusion
This study found that first-line single-agent ibrutinib therapy was associated with significantly longer TTNT than CIT regimens in real-world patients with high-risk CLL. The results support the use of ibrutinib in high-risk patients.
Infographic

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Patients with high-risk chronic lymphocytic leukemia (CLL), characterized by cytogenetic/molecular abnormalities, are associated with poor prognosis when treated with chemoimmunotherapy; however, it continues to be used in real-world practice. |
There is limited real-world evidence on clinical outcomes in high-risk and non-high-risk patients with CLL receiving first-line treatment. |
This study describes patient characteristics, treatment patterns, and clinical outcomes among high-risk and non-high-risk patients with CLL receiving first-line chemoimmunotherapy (CIT) or ibrutinib treatment in the real-world setting. |
What was learned from the study? |
High-risk patients on first-line ibrutinib had a longer time to the next line of therapy than those on first-line chemoimmunotherapy. |
Ibrutinib therapy provided sustained clinical benefit regardless of risk status, which is consistent with clinical trial results and supports its use in the first-line setting. |
Digital Features
This article is published with digital features, including an infographic, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.16964926.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common type of leukemia in adults in the USA and Europe [1,2,3]. It occurs primarily in older adults at a male-to-female ratio of 1.7:1 [4]. The disease course is highly variable and associated with heterogeneous cytogenetic characteristics. Deletion 13q, the most common chromosomal alteration found in more than 50% of patients with CLL, is associated with favorable prognosis if it is the sole genetic abnormality [5]. Other cytogenetic features, including chromosomal 17p or 11q deletion, TP53 mutations, unmutated immunoglobulin heavy-chain variable region (IGHV), and complex karyotype, are associated with shorter time from diagnosis to treatment initiation and worse prognosis [5,6,7,8]. It is reported that, in the treatment-naïve setting, the presence of del(17p), which eliminates the tumor suppressor gene TP53, is found in up to 7% of patients, and the del(11q) is found in up to 18% [5]. Unmutated IGHV gene, which does not change over time, is present in approximately 50% of patients with CLL, and complex karyotype is present in 14–34% of untreated patients [6, 9]. In addition to poor prognosis, patients with certain genetic features, such as 17p or 11q deletion, TP53 mutations, and unmutated IGHV, have inferior response to chemotherapy and chemoimmunotherapy (CIT) [6, 8,9,10].
Several targeted agents have been investigated with promising activity in patients with high-risk genetic characteristics, including B cell receptor-targeted small-molecule inhibitors, cyclin-dependent kinase inhibitors, and anti-apoptotic protein B cell lymphoma 2 antagonists [11, 12]. Ibrutinib, a once-daily small-molecule inhibitor of Bruton’s tyrosine kinase (BTK), was initially approved in the USA in February 2014 and in Europe in October 2014 for the treatment of relapsed/refractory CLL. Subsequently, ibrutinib was approved for first-line treatment of all CLL in the USA and Europe in 2016. In randomized clinical trials, ibrutinib has demonstrated significant benefits in overall survival and progression-free survival over chemo- and/or immunotherapy in patients with previously untreated or relapsed/refractory CLL [13,14,15,16]. The treatment guidelines recommend single-agent ibrutinib as a Category 1 preferred first-line treatment for treatment-naïve patients without del(17p)/TP53 mutation and Category 1 preferred treatment for relapsed/refractory patients [17]. Single-agent ibrutinib is also a Category 2A preferred first-line treatment and Category 1 preferred second-line treatment for patients with del(17p)/TP53 mutation.
There is limited real-world evidence on CLL treatments in patients with different cytogenetic risk features. We conducted a retrospective chart review to analyze treatment patterns and clinical outcomes in patients with CLL who received either ibrutinib or CIT as first-line therapy, stratified into high-risk and non-high-risk groups according to cytogenetic features.
Methods
Study Design
This was a retrospective, multisite, medical chart review study of treatment patterns and clinical outcomes in patients with CLL, stratified by genetic risk status, who received either ibrutinib or CIT as the first-line treatment. The study was approved by the New England Institutional Review Board, a WIRB Copernicus Group Company (#120190010).
High-risk patients were those with confirmed presence of at least one of the following genetic abnormalities: del(17p), del(11q), unmutated IGHV, TP53 mutations, and complex karyotype (defined as having at least three chromosomal abnormalities) [18]. Non-high-risk patients included those with confirmed absence of all aforementioned abnormalities (excluding complex karyotype). As complex karyotype is not routinely tested in clinical practice, if this information was not available for any patients, they were categorized as non-high risk if they had none of the del(17p), del(11q), unmutated IGHV, or TP53 mutations.
Data were collected from March 2019 to July 2019, after the approval by the New England Institutional Review Board, a WIRB Copernicus Group Company (#12019010). Oncologists and/or hematologists throughout the USA were identified in a national physician database and invited to participate. Physicians who had treated or managed at least 10 patients with CLL in the past 12 months were randomly sampled from the database to represent different geographic areas and practice settings. These physicians were invited for participation if they were not currently acting as a consultant or clinical investigator for a company involved in marketing or producing of oncology drugs. Participating physicians received honoraria.
Patients with CLL who had been treated by the participating physicians with either ibrutinib or CIT as first-line therapy were identified, and relevant data were extracted to an electronic or paper case report form. The collected data were de-identified and included no protected health information. The extracted data encompassed the period from a patient’s diagnosis of CLL through the patient’s most recent visit or the end of the data collection period (December 31, 2018), whichever occurred first. If a patient died before December 31, 2018, the patient was censored.
Study Population
The patients included were adults 18 years of age or older, had a confirmed diagnosis of CLL, received first-line treatment for CLL with either single-agent ibrutinib or a CIT regimen consistent with the treatment guidelines at the time [19], and had an index date between February 1, 2014 and December 31, 2016 (the index period). The index date was defined as the initiation of first-line treatment for CLL. The index period was chosen to encompass at least 2 years of data accumulation after the initial US approval of ibrutinib. The CIT regimens were any combinations of an immunotherapy agent among alemtuzumab, obinutuzumab, ofatumumab, and rituximab and one or more chemotherapy agents among bendamustine, chlorambucil, cyclophosphamide, cytarabine, doxorubicin, fludarabine, oxaliplatin, pentostatin, vincristine, and cladribine.
Patients were excluded from the chart review if they were enrolled in a cancer clinical trial after the date of CLL diagnosis or if their medical history was incomplete from diagnosis through the most recent visit (or death). Complete medical history included available CLL diagnosis details, first-line and subsequent treatments, procedures, tests, and follow-up information.
Outcomes and Statistics
The outcomes evaluated were treatment patterns and the time to next treatment (TTNT), summarized using descriptive statistics for each of the following patient groups: (1) high-risk treated with ibrutinib, (2) non-high-risk treated with ibrutinib, (3) high-risk treated with CIT, and (4) non-high-risk treated with CIT.
An assessment was conducted before treatment group comparisons to confirm that a balance could be achieved between the groups for confounders. This balance, termed “equipoise,” is a method frequently used to assess patient similarity and the feasibility of conducting unbiased comparative research between two naturalistic groups [20]. Clinical equipoise was quantified using a preference score, derived from a propensity score, to assess potential overlap of key clinical variables between the ibrutinib and CIT groups. The variables included demographics (age at CLL diagnosis, gender, year of index date, race), clinical characteristics (including family history of CLL, body mass index at diagnosis, Rai stage at diagnosis, comorbidities, Charlson Comorbidity Index score), and cytogenetic/molecular testing status (TP53 mutation, del(17p), del(11q), unmutated IGHV). If more than half of the patients in each group had preference scores between 0.3 and 0.7, the two groups would be considered sufficiently similar for comparative effectiveness research [20]. The equipoise assessment confirmed that comparative analysis was suitable between the high-risk ibrutinib and CIT patient groups. The non-high-risk groups did not reach the acceptable variation between ibrutinib and CIT and were considered not sufficiently similar.
In real-world studies, the treatment chosen for each patient is usually not entirely random, and patient characteristics may be potential confounders in head-to-head comparisons. To minimize the effect of confounders in the comparison between the high-risk ibrutinib and CIT groups, we used inverse probability of treatment weighting (IPTW) [21] to balance key characteristics. First, a logistic regression model was developed to generate a propensity score to reduce the selection bias on the estimates of the treatment effect. Subsequently, the propensity score weight was calculated as the inverse of the propensity score. Each patient was assigned a weighting coefficient, which was the inverse of the propensity score for the ibrutinib group and the inverse of (1 − propensity score) for the CIT group. As a result, the sample size in each weighted treatment group, generated from the IPTW process, was different from the original dataset, even though the same patients contributed to the dataset. Once the IPTW dataset was generated, standardized differences in means (SDM) were used to evaluate the balance in the key characteristics between the weighted high-risk ibrutinib and CIT groups (see Table S1 in the electronic supplementary material for details). When the SDM was less than 10%, a good balance between the groups for a given baseline characteristic was confirmed. P values from propensity score-weighted t tests were also generated to further evaluate the balance in baseline characteristics.
The outcome TTNT was used as a surrogate for progression-free survival and was defined as the duration from the date of first-line treatment initiation (either CIT or ibrutinib) to the start date of the second-line treatment for CLL. TTNT was compared between the following paired groups: (1) weighted high-risk ibrutinib versus weighted high-risk CIT, (2) high-risk versus non-high-risk ibrutinib, and (3) high-risk versus non-high-risk CIT. The comparisons between high-risk and non-high-risk patients for either treatment did not involve weighted analysis, because high-risk and non-high-risk patients were prescribed the same medication of interest (CIT or ibrutinib).
Patients who did not initiate a second-line treatment by the data cutoff date (December 31, 2018) were censored at the date of their last follow-up visit or date of death, whichever was later. TTNT was evaluated using Kaplan–Meier curves, and median durations with 95% confidence intervals (CIs) are reported. Paired comparisons in TTNT between the previously specified groups were conducted using Cox proportional hazards regression analyses. Log-rank tests were used to assess statistical significance in the comparisons between groups. The Kaplan–Meier curves were truncated at the time point when the number of patients at risk (patients who had not reached second-line treatment) dropped below 10% in either of the compared groups [22]. Median follow-up duration was calculated using the reverse Kaplan–Meier method [23].
Results
Study Patients
Physicians at 40 clinical practices (68% community and 32% academic practices) participated in this study and provided data on 516 patients with CLL, including 271 high-risk patients and 245 non-high-risk patients (see Fig. S1 in the Electronic Supplementary Material for details). Of the high-risk patients, 175 received ibrutinib and 96 received CIT as first-line therapy. Of the non-high-risk patients, 82 received ibrutinib and 163 received CIT. The demographic and baseline characteristics of patients in the unweighted ibrutinib and CIT groups are displayed in Table 1.
On the basis of the equipoise assessment, 50.9% (85 of 167, excluding 8 patients with missing data) of patients in the high-risk ibrutinib group and 52.1% (50 of 96) in the high-risk CIT group had preference scores between 0.3 and 0.7. As more than 50% in each group met this criterion, the two high-risk groups were considered appropriate for IPTW. The non-high-risk ibrutinib and CIT groups had 39% and 38% patients with preference scores between 0.3 and 0.7, respectively. As such, these two groups were not appropriate for comparative analysis.
For the high-risk datasets, applying IPTW produced a weighted high-risk ibrutinib group of 244 and a weighted high-risk CIT group of 229. The demographic and baseline clinical characteristics were well balanced between the weighted high-risk ibrutinib and high-risk CIT groups (Table 1). The mean age was 65.9 years in the weighted high-risk ibrutinib group and 65.4 years in the weighted high-risk CIT group. In the weighted high-risk ibrutinib and CIT groups, 54.0% and 51.2% of the patients, respectively, had Rai stage 3 or 4 CLL. The high-risk cytogenetic features were similarly distributed between the weighted high-risk ibrutinib and CIT groups (Table 2). The most common high-risk feature was del(17p).
Treatment Patterns
The first- and second-line treatment patterns for weighted and non-weighted groups are presented in Table 3. The high-risk ibrutinib group had a median (range) treatment duration of 28.6 (1.0–58.1) months. The weighted high-risk CIT group had a median (range) treatment duration of 5.5 (0.5–38.4) months. Of the CIT regimens initiated for high-risk patients, the most common were bendamustine + rituximab (46.3%), fludarabine + cyclophosphamide + rituximab (26.6%), and chlorambucil + obinutuzumab (23.6%).
During the available follow-up period, 74.7% patients in the weighted high-risk ibrutinib group received only one line of treatment for CLL, compared with 47.2% patients in the high-risk CIT group. Within the CIT group, a larger proportion of non-high-risk patients (69.9%) had only one line of treatment, compared with high-risk patients (45.8%). However, within the ibrutinib group, very few patients required a second-line treatment regardless of risk status (high-risk, 18.3%; non-high-risk, 8.5%).
The documented second-line treatment choices are summarized in Table 3 for weighted and unweighted groups by risk status. The most common second-line treatment was single-agent ibrutinib (81.7%) for the weighted high-risk CIT group and venetoclax monotherapy or in combination with rituximab (62.3%) as the second line for the weighted high-risk ibrutinib group.
Time to Next Treatment
Within the index period, the median (95% CI) duration of follow-up was 34.0 (31.8–38.8) months for the weighted high-risk ibrutinib group and 35.1 (33.1–45.1) months for the weighted high-risk CIT group and were not significantly different (Table 4).
The Kaplan–Meier curves demonstrate that the weighted high-risk ibrutinib group had significantly longer TTNT compared with the weighted high-risk CIT group (P < 0.010, Fig. 1a). The median duration of TTNT was not reached in the weighted high-risk ibrutinib group by the end of the study, as fewer than half of the patients had started a new treatment (Table 4). In comparison, the median TTNT in the weighted high-risk CIT group was 34.4 (95% CI 32.4–43.1) months. The weighted high-risk ibrutinib group was 54% less likely to start a new treatment than the weighted high-risk CIT group (hazard ratio [95% CI] 0.46 [0.3–0.6]).
For ibrutinib-treated patients, the median TTNT was not reached, and the difference between the high-risk and non-high-risk groups was not significantly different (hazard ratio [95% CI] 2.2 [1.0–5.0]; P = 0.060) (Fig. 1b, Table 4). Among patients treated with CIT, the high-risk group had significantly shorter median TTNT (median 38.8 months [95% CI 33.4–47.1]) than non-high-risk group (median 52.8 months [95% CI 45.5–not reached]) (Table 4); the hazard ratio for new treatment was 2.4 (95% CI 1.6–3.5) for the high-risk group compared with the non-high-risk group (P < 0.010) (Fig. 1c).
Discussion
In randomized clinical trials, ibrutinib, as a single-treatment or in combination with a CD20 monoclonal antibody, has shown significant benefits in overall survival and progression-free survival over chemotherapy and/or immunotherapy in patients with previously untreated or relapsed/refractory CLL or small lymphocytic lymphoma [13,14,15,16, 24]. Recent studies have confirmed that first-line treatment with ibrutinib results in excellent long-term progression-free survival and overall survival rates in patients with high-risk genomic features, including TP53 aberrations, unmutated IGHV, BIRC3 mutation, and other mutations associated with poor responses to chemotherapy and/or CIT regimens [25, 26]. The 7-year overall survival rate of patients treated with first-line ibrutinib (median age 71 years), which exceeded 75%, was comparable to those of the age-matched US and UK general populations (see Fig. S2 in the Electronic Supplementary Material) [27, 28].
Previous real-world studies, which did not stratify by risk levels, support the benefits of ibrutinib in CLL, including longer TTNT and lower healthcare resource utilization than first-line CIT regimens [29, 30]. To the best of our knowledge, this study is the largest US real-world study to date that compares the clinical outcomes of first-line ibrutinib and CIT treatments for CLL in patients stratified by risk status. Consistent with the clinical trial results [13, 31, 32], we found that high-risk patients treated with single-agent ibrutinib had significantly longer TTNT and lower likelihood of requiring a second-line treatment than high-risk patients receiving CIT. To date, ibrutinib is the only BTK inhibitor with real-world comparison of clinical outcomes with CIT in patients with high-risk CLL.
In addition, we compared the outcomes of high-risk and non-high-risk patients within each treatment group. As expected, CIT-treated high-risk patients had significantly shorter TTNT than non-high-risk patients, which highlights the importance of cytogenetic/molecular testing before initiating CIT for all patients with CLL, as recommended by clinical guidelines [17, 33]. Real-world data from the informCLL Prospective Observational Registry suggested that FISH testing was performed in 28% of the patients with CLL/small lymphocytic lymphoma (SLL) in the registry during the period of October 2015–June 2019, and TP53 and IGHV tests were performed in 11% and 12% of the patients, respectively [34]. Among patients receiving ibrutinib, the findings support the use of ibrutinib as first-line treatment for CLL regardless of risk status.
In this retrospective chart review study, the treatment patterns of CLL in the real-world setting reflected the shifting trends in clinical practice from 2014 through 2018. Before 2015, the most common first-line treatments for CLL were chemotherapy agents and rituximab [35,36,37], as recommended by treatment guidelines at the time [38]. However, the emergence of small-molecule inhibitors (SMIs) led to a shift in treatment guidelines [7, 19], and our findings confirm this shift in clinical practice. Novel agents were preferred for second-line treatment, with the most common choices being ibrutinib after CIT and venetoclax after ibrutinib.
One of the strengths of this study was the collection of extensive information on the treatment pattern and response data by experienced oncologists/hematologists, yielding a substantial sample size. We utilized the IPTW approach to balance potential confounding factors, including demographics, disease stage, and comorbidities, to isolate the treatment effect. We took additional steps to ensure that the datasets were appropriately comparable.
The study is limited by its retrospective design and the availability of medical record data. Potential confounders remain (e.g., tumor burden, unreported comorbidities, socioeconomic status, clinician preference) despite the efforts to balance the groups. The study only included patients with known risk status, classified by the relevant cytogenetic/molecular testing. Therefore, the generalizability of the results may be limited for patients who do not have complete risk data, as a significant patient population in the real world may not receive all relevant tests [34]. Another limitation of this study is that the non-high-risk ibrutinib and CIT groups were not directly compared, as these groups did not meet the prespecified level of comparability in preference scores and were deemed not appropriate for comparative analysis. In addition, external factors such as cost and access could have influenced clinical decisions such as treatment initiation and discontinuation. In the index period of treatment (2014–2016), the CLL treatment landscape was different from the current clinical practice, which may limit the generalizability of the findings. Finally, only records through December 31, 2018 were extracted, limiting the follow-up information for some patients. As a result of this limited follow-up period for some patients and the difficulties in obtaining death records, we did not evaluate overall survival.
Conclusions
In this real-world study, first-line treatment with single-agent ibrutinib provided sustained clinical benefits over CIT regimens for the treatment of CLL, as evidenced by significantly longer time to the next treatment, which is consistent with randomized clinical trials. The favorable outcomes of ibrutinib were evident regardless of cytogenetic/molecular risk status, with the majority of patients not requiring second-line treatment. Finally, the findings highlight the importance of risk assessment through cytogenetic/molecular testing before choosing to treat with CIT, as a risk-based approach is recommended by clinical guidelines for high-risk patients.
References
Jemal A, Siegel R, Xu J, et al. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. https://pubmed.ncbi.nlm.nih.gov/20610543/.
Watson L, Wyld P, Catovsky D. Disease burden of chronic lymphocytic leukaemia within the European Union. Eur J Haematol. 2008;81:253–8.
National Cancer Institute. Chronic lymphocytic leukemia—Cancer Stat Facts. Surveillance, Epidemiology, and End Results Program, Cancer Statistics. 2017. https://seer.cancer.gov/statfacts/html/clyl.html. Accessed 17 Nov 2020.
Molica S. Sex differences in incidence and outcome of chronic lymphocytic leukemia patients. Leuk Lymphoma. 2006;47:1477–80.
Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–6.
Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. http://ashpublications.org/blood/article-pdf/94/6/1840/1657568/1840.pdf.
Voorhies BN, Stephens DM. What is optimal front-line therapy for chronic lymphocytic leukemia in 2017? Curr Treat Opt Oncol. 2017;18:12. https://pubmed.ncbi.nlm.nih.gov/28243993/.
Delgado J, Espinet B, Oliveira AC, et al. Chronic lymphocytic leukaemia with 17p deletion: a retrospective analysis of prognostic factors and therapy results. Br J Haematol. 2012;157:67–74.
Hamblin TJ, Davis Z, Gardiner A, et al. Unmutated Ig V H genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. http://ashpublications.org/blood/article-pdf/94/6/1848/1658108/1848.pdf.
Rigolin GM, Cavallari M, Quaglia FM, et al. In CLL, comorbidities and the complex karyotype are associated with an inferior outcome independently of CLL-IPI. Blood. 2017;129:3495–3498. https://pubmed.ncbi.nlm.nih.gov/28446433/.
Blachly JS, Byrd JC, Grever M. Cyclin-dependent kinase inhibitors for the treatment of chronic lymphocytic leukemia. Semin Oncol. 2016;43:265–273. https://pubmed.ncbi.nlm.nih.gov/27040705/.
Hallek M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am J Hematol. 2019;94:1266–1287. https://pubmed.ncbi.nlm.nih.gov/31364186/.
Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381:432–43.
Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223. https://pubmed.ncbi.nlm.nih.gov/24881631/.
Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–2437. https://pubmed.ncbi.nlm.nih.gov/26639149/.
Munir T, Brown JR, O’Brien S, et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94:1353–1363. https://pubmed.ncbi.nlm.nih.gov/31512258/.
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Version 2.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org. Accessed 21 Mar 2022.
Rigolin GM, Cibien F, Martinelli S, et al. Chromosome aberrations detected by conventional karyotyping using novel mitogens in chronic lymphocytic leukemia with “normal” FISH: correlations with clinicobiologic parameters. Blood. 2012;119:2310–2313. https://pubmed.ncbi.nlm.nih.gov/22246039/.
Osterweil N. NCCN guide on CLL to shift toward targeted therapies. Medscape Medical News. 2015. https://www.medscape.com/viewarticle/841541. Accessed 24 Nov 2020.
Walker AM, Patrick AR, Lauer MS, et al. A tool for assessing the feasibility of comparative effectiveness research. Comp Eff Res. 2013;3:11–20.
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79.
Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: Good practice and pitfalls. Lancet. 2002;359:1686–1689. https://pubmed.ncbi.nlm.nih.gov/12020548/.
Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Controlled Clinical Trials. 1996;17:343–346. https://pubmed.ncbi.nlm.nih.gov/8889347/.
Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34:787–798. https://pubmed.ncbi.nlm.nih.gov/31628428/.
Allan JN, Shanafelt T, Wiestner A, et al. Long-term efficacy of first-line ibrutinib treatment for chronic lymphocytic leukemia (CLL) with 4 years of follow-up in patients with TP53 aberrations (del(17p) or TP53 mutation): a pooled analysis from 4 clinical trials. ASH Annual Meeting and Exposition. 2020. https://ash.confex.com/ash/2020/webprogram/Paper134431.html. Accessed 21 Mar 2022.
Burger JA, Robak T, Demirkan F, et al. Outcomes of first-line ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and high-risk genomic features with up to 6.5 years follow-up: Integrated analysis of two phase 3 studies (RESONATE-2 and iLLUMINATE). ASH Annual Meeting and Exposition. 2020. https://ash.confex.com/ash/2020/webprogram/Paper134437.html. Accessed 21 Mar 2022.
Byrd JC, Furman RR, Coutre S, et al. Up to 7 years of follow-up of single-agent ibrutinib in the phase 1b/2 PCYC-1102 trial of first line and relapsed/refractory patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Blood. 2018;132:3133–3133.
Huang Q, Deering KL, Harshaw Q, et al. Clinical outcomes among real-world patients with chronic lymphocytic leukemia (CLL) initiating first-line ibrutinib or chemoimmunotherapy (CIT) stratified by risk status: results from a US retrospective chart review study. Blood. 2020;136:16–8.
Huang Q, Borra S, Li J, et al. Time to next treatment, health care resource utilization, and costs associated with ibrutinib use among U.S. veterans with chronic lymphocytic leukemia/small lymphocytic lymphoma: a real-world retrospective analysis. J Manag Care Spec Pharm. 2020;26:1266–1275. .
Emond B, Sundaram M, Romdhani H, et al. Comparison of time to next treatment, health care resource utilization, and costs in patients with chronic lymphocytic leukemia initiated on front-line ibrutinib or chemoimmunotherapy. Clin Lymphoma Myeloma Leuk. 2019;19:763-775.e2.
Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517–28.
Salles G, Bachy E, Smolej L, et al. Single-agent ibrutinib in RESONATE-2TM and RESONATETM versus treatments in the real-world PHEDRA databases for patients with chronic lymphocytic leukemia. Ann Hematol. 2019;98:2749–2760. https://pubmed.ncbi.nlm.nih.gov/31745601/.
Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131:2745–2760. https://pubmed.ncbi.nlm.nih.gov/29540348/.
Mato AR, Barrientos JC, Sharman JP, et al. Real-world prognostic biomarker testing, treatment patterns and dosing among 1461 patients (pts) with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) from the informCLL Prospective Observational Registry. In: 62nd ASH Annual Meeting and Exposition. American Society of Hematology; 2020. https://ashpublications.org/blood/article/136/Supplement%201/42/470309/Real-World-Prognostic-Biomarker-Testing-Treatment. Accessed 21 Mar 2022.
Mato A, Nabhan C, Lamanna N, et al. The Connect CLL Registry: final analysis of 1494 patients with chronic lymphocytic leukemia across 199 US sites. Blood Adv. 2020;4:1407–18.
Seymour EK, Ruterbusch JJ, Beebe-Dimmer JL, et al. Real-world testing and treatment patterns in chronic lymphocytic leukemia: a SEER patterns of care analysis. Cancer. 2019;125:135–43.
Kabadi SM, Goyal RK, Nagar SP, et al. Treatment patterns, adverse events, and economic burden in a privately insured population of patients with chronic lymphocytic leukemia in the United States. Cancer Med. 2019;8:3803–10.
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Version 1.2015. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org. Accessed 21 Mar 2015.
Acknowledgements
Funding
This study was sponsored by Janssen Scientific Affairs, LLC. Additionally, Janssen Scientific Affairs, LLC provided funding for the journal’s Rapid Service and Open Access fees for this manuscript.
Medical Writing, Editorial, and Other Assistance
Jun Yan, PharmD, provided medical writing assistance in the preparation of this manuscript. The authors would like to thank Panagiotis Mavros, PhD, from Janssen Scientific Affairs, LLC, for providing statistical methodological advice and Tarun Bhagnani, MS for providing support on the conduct of the study. All assistance was funded by Janssen Scientific Affairs, LLC.
Prior Presentation
This study was presented as a podium presentation at the 2020 ASH Annual Meeting (Abstract #372).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Drs. Huang, Deering, Harshaw, and Leslie all contributed to the design, conduct, and analysis of the study and manuscript preparation and review.
Disclosures
Dr. Huang is an employee of Janssen Scientific Affairs. Drs. Deering and Harshaw are employees of EPI-Q Inc., which received payment from Janssen Scientific Affairs associated with the development and execution of this study. Dr. Leslie is on the Speaker’s Bureaus of Seagen, Celgene/BMS, KitePharma, BeiGene, Pharmacyclics/Janssen, AstraZeneca, Epizyme, and Karyopharm; and has advisory board participation and/or consultancy role with Bayer, Seattle Genetics, ADC therapeutics, Abbvie, Janssen, Pharmacyclics, Kite, AstraZ eneca, and TG Th erapeutics.
Compliance with Ethics Guidelines
The study was approved by the New Englan d Institutional Review Board, a WIRB Copernicus Group Company (#120190010).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
12325_2021_1991_MOESM1_ESM.docx
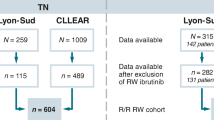
Supplementary Fig. 1. Number of patients in each analyzed group. Abbreviations: 1L=first line, CIT=chemoimmunotherapy, CLL=chronic lymphocytic leukemia
12325_2021_1991_MOESM2_ESM.tif
Supplementary Fig. 2. Seven-year survival rate of ibrutinib treatment comparable to age-matched general population. Source: Huang Q, Deering KL, Harshaw Q, Bhagnani T, Leslie LA. Clinical outcomes among real-world patients with chronic lymphocytic leukemia (CLL) initiating first-line ibrutinib or chemoimmunotherapy (CIT) stratified by risk status: results from a US retrospective chart review study. 2020 American Society of Hematology Annual Meeting. Oral presentation. Ibrutinib data from: Byrd et al. Up to 7 years of follow-up of single-agent ibrutinib in the phase 1b/2 PCYC-1102 trial of first-line and relapsed/refractory patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Presented at 60th ASH Annual Meeting & Exposition; December 1–4, 2018; San Diego, CA. Mortality data from The Human Mortality Database (www.mortality.org; US 2017 and UK 2014)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Huang, Q., Deering, K.L., Harshaw, Q. et al. Real-world Clinical Outcomes of First-Line Ibrutinib or Chemoimmunotherapy in Patients with Chronic Lymphocytic Leukemia by Risk Status. Adv Ther 39, 3292–3307 (2022). https://doi.org/10.1007/s12325-021-01991-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01991-5