Abstract
Introduction
Baricitinib–remdesivir (BARI-REM) combination is superior to remdesivir (REM) in reducing recovery time and accelerating clinical improvement among hospitalized patients with coronavirus disease 2019 (COVID-19), specifically those receiving high-flow oxygen/noninvasive ventilation. Here we assessed the cost-effectiveness of BARI-REM versus REM in hospitalized patients with COVID-19 in the USA.
Methods
A three-state model was developed addressing costs and patient utility associated with COVID-19 hospitalization, immediate post hospital care, and subsequent lifetime medical care. Analysis was performed from the perspective of a payer and a hospital. Both perspectives evaluated two subgroups: all patients and patients who required oxygen. The primary measures of benefit in the model were patient quality-adjusted life years (QALYs) accrued during and after hospitalization, cost per life years gained, cost per death avoided, and cost per use of mechanical ventilation avoided.
Results
In the base-case payer perspective with a lifetime horizon, treatment with BARI-REM versus REM resulted in an incremental total cost of $7962, a gain of 0.446 life years and gain of 0.3565 QALYs over REM. The incremental cost-effectiveness ratios of using BARI-REM were estimated as $22,334 per QALY and $17,858 per life year. The base-case and sensitivity analyses showed that the total incremental cost per QALY falls within the reduced willingness-to-pay threshold of $50,000/QALY applied under health emergencies. In all hospitalized patients, treatment with BARI-REM versus REM reduced total hospital expenditures per patient by $1778 and total reimbursement payments by $1526, resulting in a $252 reduction in net costs per patient; it also resulted in a net gain of 0.0018 QALYs and increased survival of COVID-19 hospitalizations by 2.7%.
Conclusion
Our study showed that BARI-REM is cost-effective compared to using REM for hospitalized patients with COVID-19. The base-case results of this cost-effectiveness model were most sensitive to average annual medical costs for recovered patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
COVID-19 pandemic continues to impose substantial economic and heath care resource burden on payers and hospitals globally and in the USA. |
As the treatment landscape continues to evolve, there is a need to assess cost-effectiveness of various therapeutic options available for the treatment of patients hospitalized as a result of COVID-19. |
This study assesses the cost-effectiveness of combination of baricitinib and remdesivir versus remdesivir alone in hospitalized patients with COVID-19 in the USA. |
What was learned from the study? |
Our study showed that the combination of baricitinib and remdesivir is cost-effective compared to using remdesivir alone for patients hospitalized as a result of COVID-19. |
Introduction
Since the first identified case in China, in December 2019, coronavirus disease 2019 (COVID-19) has grown into a global pandemic with 206.9 million confirmed global cases of COVID-19 until August 2021; this number also includes 4.3 million deaths [1, 2]. As of August, 2021, the USA reported 36.3 million confirmed cases of COVID-19 with 615,747 deaths [2]. The Centers for Disease Control and Prevention noted a total of 194,642 laboratory-confirmed hospitalizations in the USA as of August 2021 [3].
Cost impacts and capacity constraints place a large burden on hospitals and health care systems during the global pandemic [4]. Vaccinations are demonstrated to be clinically effective and cost-effective. However, the time taken to vaccinate a sufficient proportion of the population and the uncertainty associated with the emergence of multiple variants of the virus call for more and improved treatment options [5].
Recently, baricitinib (BARI) was granted an emergency use authorization (EUA) by the US Food and Drug Administration (FDA) for the treatment of hospitalized adult and pediatric patients with COVID-19 aged 2 years and more requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) [6]. Earlier, BARI was granted an EUA in combination with remdesivir (REM) by the FDA for the treatment of hospitalized adult patients with COVID-19 [7]. Results of the second stage Adaptive COVID-19 Treatment Trial 2 (ACTT-2) showed superiority of BARI-REM over REM alone for reducing recovery time and accelerating clinical improvement in hospitalized patients with COVID-19. This benefit was specifically among patients receiving high-flow oxygen or noninvasive ventilation [8].
The Institute for Clinical and Economic Review (ICER) performed an assessment of REM for hospitalized patients with COVID-19 using clinical efficacy data from ACTT-1 and the report separately evaluated REM plus standard of care in the mild and moderate-to-severe populations on the basis of clinical evidence from several trials [9,10,11,12,13,14]. However, the ICER model did not consider any COVID-19-specific post hospital care of patients nor the detailed (i.e., diagnostic related group [DRG related]) economics associated with a hospital perspective. For these reasons, we evaluated cost-effectiveness of BARI-REM versus REM using data from the ACTT-2 trial by creating a more detailed version of the cost-effectiveness (CE) model to accommodate these important features of COVID-19 treatment and reimbursement.
Methods
Model Overview
A pharmacoeconomic model was developed to estimate the cost-effectiveness of BARI-REM as a treatment for hospitalized adults with COVID-19 (aged ≥ 18 years) in the USA. The primary perspective of the analysis used was from the payer or partial societal (Medicare, Medicaid, private payer, uninsured/self-insured patients), where costs to payers were defined as reimbursements paid to hospitals, to post-acute discharge care providers, for long-term post recovery costs, and the indirect costs due to missed work during the inpatient hospital stay to account for the number of working days lost. Inpatient hospital expenditures were based on Medicare Severity DRGs (MS-DRGs) for COVID-19 admissions. A lifetime horizon was used in the base-case analysis. The robustness of the base-case results was evaluated using one-way and probabilistic sensitivity analyses, where key model parameters were varied. This manuscript does not contain any new studies with human or animal subjects performed by any of the authors.
The measure of benefit in the model was quality-adjusted life years (QALYs) that were accrued during and after discharge of hospitalized patients. QALYs and costs were discounted at 3% per year [15]. Health outcomes represented in the model were based on the ordinal scale (OS) used to measure efficacy in the ACTT-2 trial [11]. Inpatient hospital outcomes in the model included medical care without oxygen (OS 4), supplemental oxygen (OS 5), noninvasive ventilation (OS 6), mechanical ventilation (OS 7), and death (OS 8). Mortality, overall time to recovery, duration of treatment with mechanical ventilation/noninvasive ventilation/supplementary oxygen, proportion of patients who progressed to new use of mechanical ventilation/noninvasive ventilation/supplementary oxygen, and impact of treatment on discharge status of patients were included in the model as treatment effects. For different mortality assumptions, incremental cost-effectiveness ratio of BARI-REM versus REM was assessed.
In a secondary analysis, we focused on the hospital perspective, whereby the net hospital cost impact (hospital costs less DRG reimbursement) was used with the time horizon set to be within the hospital stay period.
Model Framework
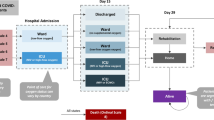
A schematic representation of the model used for this analysis is shown in Fig. 1. The model consists of three functional components addressing inpatient hospitalization, immediate post discharge patient care, and patient life after hospitalization or post discharge care.
Model structure. *Patients were tracked after discharge to self-care or custodial care, home health care, inpatient rehab, skilled nursing facility, short-term hospital, long-term acute care hospital, hospice; the color coding is as follows: blue, model stages; gray, health states in the Markov submodel; red, top-line results; green, interim results passed to and from other submodels
The inpatient hospitalization and post discharge portions of the model estimate QALYs and costs for patients based on (treatment-related) hospital survival and disease severity (e.g., use of mechanical ventilation) and the use of acute post hospital discharge care. After a patient left post hospital discharge care (if used), their remaining life medical costs, QALYs, and mortality were based on age-specific inputs (e.g., standard population life tables) adjusted for treated patient comorbidities. The discharge submodel assumed that all patients who were discharged to hospice died at the end of their stay, and all patients discharged to any other type of post-acute care were assumed to survive and proceeded to the recovered submodel.
Patients who entered the recovered submodel were followed until death or the end of the simulation time horizon. The recovered submodel tracked patients surviving hospitalization and immediate post-hospitalization, estimating remaining all-cause mortality over a lifetime horizon. Mortality of patients who recovered from COVID-19 was based on the general population that has not had COVID-19. The model included an upward adjustment to mortality rates to reflect higher rates of comorbidities associated with the hospitalized COVID-19 population as seen in the ACTT-2 trial [8].
In the analysis from hospital perspective, only costs and QALYs incurred during the inpatient submodel are included, since the costs after discharge are not covered by the hospital.
Model Inputs
Population: The key population variables are demographics, level of care at baseline, severe comorbidities, and distribution of patients by payer type (Table 1). The modeled population consisted of 35.6% female patients with an average age of 58.9 years at hospital admission [11]. The average age of patients who recovered was 57.5 years and was sourced from ICER’s REM model report [10]. The level of care required by the cohort at baseline—such as use of mechanical (10.7%) or noninvasive ventilation (20.9%), supplemental oxygen (54.7%), or medical care without oxygen (13.7%) on an OS—was sourced from Kalil et al. [8]. A total of 34.2% patients modeled in the study had severe comorbidities requiring adjustments to both costs and utilities associated with these patients [8]. The post-discharge cost-multiplier was set at 1.601 [16], the utility multiplier was set at 0.962 [17], and post-discharge mortality multiplier at 1.37 [18] for these patients. The model sourced the distribution of patients by payer type from the Premier Healthcare Database, which is a large and all-payer US hospital database of detailed information on inpatient discharges (henceforth referred to as the Premier cost analysis) [19]. The majority of the modeled population were enrolled in Medicare (52.9%) or had private payer coverage (27.9%) [19].
Treatment efficacy: Clinical efficacy data of BARI-REM and REM were sourced from the ACTT-2 trial, Kalil et al., and Beigel et al. [8, 11, 20] and divided into two overlapping subgroups; (1) all hospitalized patients and (2) patients who required use of oxygen at admission for analysis. The efficacy parameters used in the model were incidence of new ventilation or oxygen (after initial hospital admission), duration of care, and probability of recovery (patients that did not die) (Table 2). For all hospitalized patients, there were 10.0%, 20.0%, and 23.0% patients in the BARI-REM group and 15.0%, 24.0%, and 40.0% patients in the REM group who required new mechanical ventilation, new noninvasive ventilation, and new supplemental oxygen, respectively. The time to recovery was 10.6 days in the BARI-REM treatment arm versus 11.2 days in the REM treatment arm. The probability of recovery with mechanical ventilation was 76.8% with BARI-REM versus 77.1% with REM.
Costs: The cost of acquiring 100 mg REM was priced at $390 for Medicaid/Medicare patients and $520 for private payer or uninsured patients [21]. The cost for a 2-mg BARI tablet was $75.50 [22]. The inputs for hospital costs were obtained from Premier cost analysis [19]. The average reimbursement per patient by payer type and highest level of care during the inpatient stay in the base case is presented in Supplementary Table S1. The inpatient cost incurred by payers was based on MS-DRG reimbursement amounts (Supplementary Table S2). For Medicare patients, special additional payments were made by Medicare (a 20% extra payment under the CARES act). BARI and REM were also eligible for potential new COVID-19 treatments add-on payment (NCTAP) (Supplementary Table S3). The indirect costs were estimated by multiplying the percentage of patients employed (32.4%) [23, 24] by missed workdays and cost per workday ($218.63) and were sourced from the Bureau of Labor Statistics [25] (Table 3). The proportion of patients with full-time employment was calculated by weighting the employment percentages for each payer type by the distribution of payers estimated from the Premier cost analysis [19]. The proportion of Medicare patients with full-time employment (11.6%) was assumed to be equal to the proportion of the population aged 65 and older with full-time employment [23]. The proportion of Medicaid patients with full-time employment (48.0%) was sourced from a report by the Kaiser Family Foundation [24]. The proportion of commercially insured patients with full-time employment was assumed to be the proportion of the US population aged 20–64 years with full-time employment (61%) [23]. The cost per workday missed was based on employer costs for employee compensation from the Bureau of Labor Statistics ($38.26/h) and the assumption of 40 h/week for full-time employment [25]. These indirect costs were only accounted for in the payer perspective.
Health utilities: Health utilities were based on US age-adjusted general population utilities derived from Sullivan et al. [26]. The disutility associated with COVID-19 symptoms and additional disutility associated with each level of oxygen support were based on Campbell et al., Sullivan et al., Barbut et al., and Sackett et al. [10, 26,27,28] using the assumption that the illnesses in the publications were similar to the corresponding severity of COVID-19. These are presented in Table 4. Our model used the same disutilities as the ICER model for COVID-19 symptoms and levels of care provided during the inpatient stay. The ICER model did not contain a corresponding health state for supplemental oxygen; therefore, the disutility of this health state in our model was interpolated as the midpoint between noninvasive ventilation and medical care without oxygen.
The discount rates for costs and QALY were set to 3% and were sourced from Sanders et al. [29] and the ICER 2020–23 value assessment framework [29, 30]. The life tables were sourced from the Social Security Administration [31].
Base-Case Results and Sensitivity Analyses
Base-case results are presented from both the payer perspective and the hospital perspective. Deterministic sensitivity analysis and probabilistic sensitivity analysis results are presented from the payer perspective. All the analyses in this study were done using Microsoft® Excel® for Microsoft 365 MSO.
Results
Payer Perspective
Base-Case Results
The treatment with BARI-REM versus REM for all hospitalized patients resulted in an incremental total cost of $7962, a total life-year gain of 0.446, and a total QALY gain of 0.3565. For all patients, the incremental cost-effectiveness was $22,334 per QALY gained and $17,858 per life year gained (Table 5). In patients with oxygen use at baseline, treatment with BARI-REM versus REM resulted in an incremental total cost of $8960, a total life year gain of 0.513, and a total QALY gain of 0.4107. The incremental cost per QALY gained was $21,818 and cost per life-year gained was $17,458 (Table 5). The disaggregated total costs and QALY results for all hospitalized patients and patients with oxygen use are presented in Supplementary Table S4.
Deterministic (One-Way) Sensitivity Analysis
From the payer perspective, the ten most sensitive inputs identified through one-way sensitivity analysis are shown in Fig. 2. In all hospitalized patients, the incidence of new mechanical ventilation in the REM treatment arm had the greatest effect on the cost-effectiveness ratio, while average annual medical costs for patients that recover from the COVID-19 hospitalization and variations in percentage recovery from supplemental oxygen, non-mechanical and mechanical ventilation in the BARI-REM treatment arms have smaller effects on the cost-effectiveness ratio. The post recovery medical cost multiplier for patients with severe comorbidities was also among the ten inputs that most influenced the output of the model. A similar pattern of the most influential parameters was observed in patients with oxygen use at baseline. The incremental cost-effectiveness ratio lies between $15,000 and $30,000 for all variables, which falls well below the threshold of $50,000 per QALY gained.
Deterministic sensitivity analysis tornado diagram: payer perspective. a One-way sensitivity analysis—10 most sensitive inputs outcome measure: ICER base case = $22,334 —all hospitalized patients. b One-way sensitivity analysis—10 most sensitive inputs outcome measure: ICER base case = $21,818—patients with oxygen use at baseline
Probabilistic Sensitivity Analysis (PSA)
PSA results were generated by jointly varying key model parameters over 5000 replications for the model base case. Depending on the variable, normal, Dirichlet, and Beta distributions were used to generate the variable samples (Tables 1, 2, 3, 4; Supplementary Tables S5, S6). The rationale of the PSA was to understand the robustness of model conclusions when varying all model inputs, given the uncertainty around model input estimates and the assumptions. The payer perspective PSA results for both patient subgroups (all patients and patients with oxygen use on admission) are shown in Fig. 3. The results from the analysis showed that BARI-REM was associated with both increased cost (incremental cost $7962 [95% CI − $14,055 to $23,880]) and benefit (incremental QALY 0.356 [95% CI − 0.416 to 0.952]) versus REM. Similarly, in patients with oxygen use, BARI-REM was associated with both increased cost (incremental cost $8960 [95% CI − $16,637 to $27,276]) and benefit (incremental QALY 0.4107 [95% CI − 0.485 to 1.096]) versus REM. The incremental cost was below the “willingness to pay” threshold for both, all hospitalized patients, and patients with oxygen use.
Treatment Mortality Assumptions
The incremental cost-effectiveness of BARI-REM compared with REM for different mortality assumptions is presented in Table 6. Specifically, two scenarios were evaluated: (a) adding a survival benefit to dexamethasone in the SOC treatment arm, and (b) removing the survival benefit from BARI-REM.
When a survival benefit was added to dexamethasone, the total incremental costs were $6030 and the total incremental QALYs 0.287 in the all-patient group. In the subgroup of patients using oxygen at baseline, the total incremental costs were $6724 and the total incremental QALYs 0.330. When the survival benefit for BARI-REM was removed, the total incremental costs were − $1711 and the total incremental QALYs 0.009 in the all patient group. In the subgroup of patients using oxygen at baseline, the total incremental costs were − $2237 and the total incremental QALYs 0.008. The detailed results of these scenarios are presented in Supplementary Tables S7, S8, S9, and S10.
Hospital Perspective
Base-Case Results
We also evaluated the use of BARI-REM from a hospital perspective for the base-case scenario. The rationale for the hospital perspective is to examine the effect of US reimbursement policies and how they impact hospital reimbursement and net income. In all hospitalized patients, treatment with BARI-REM versus REM reduced total hospital expenditures by $1778 and total reimbursement payments by $1526, resulting in a $252 reduction in net costs per patient. Treatment with BARI-REM versus REM also resulted in a net gain of 0.0018 QALYs and increased survival by 2.7% (Supplementary Table S11). Similarly, in patients with oxygen use at baseline, treatment with BARI-REM versus REM reduced total hospital expenditures by $2709 and total reimbursement payments reduced by $1961, resulting in a $748 reduction in net costs per patient. Treatment with BARI-REM versus REM also resulted in a net gain of 0.0023 QALYs and increased survival by 3.2% (Supplementary Table S11).
Net Impact on Clinical Outcomes
Net impacts on clinical outcomes are presented in Supplementary Figs. S1 and S2. The net number of patients who progressed to new use of oxygen was lower in BARI-REM versus REM (per 1000 patients, difference: mechanical ventilation, − 45; noninvasive ventilation, − 27; supplemental oxygen, − 23). Similarly, the net impact on total hospital days was lower with BARI-REM versus REM (per 1000 patients, difference: − 600 days), with the difference mainly driven by the mechanically ventilated group (− 1260 days). In patients with oxygen use at baseline, the net number of those who progressed to new use of oxygen was lower in BARI-REM versus REM (per 1000 patients, difference: mechanical ventilation, − 53; noninvasive ventilation, − 32; supplemental oxygen, 0). The net impact on total hospital days was lower with BARI-REM versus REM (per 1000 patients, difference: − 950 days), with the difference mainly driven by the mechanically ventilated group (− 1460 days) (Supplementary Figs. S1 and S2).
Discussion
We conducted an economic evaluation on the use of BARI-REM versus REM among hospitalized patients with COVID-19 in a US setting. A cost-effectiveness model was developed to capture the costs and QALYs of patients with COVID-19, during the hospital stay, as well as for any post-acute care settings. In the base-case scenario, it was observed that a combination of BARI-REM was cost-effective relative to REM alone for treatment of patients hospitalized as a result of COVID-19. Most of the incremental cost and QALYs were accrued during the recovered time period owing to more patients surviving the hospitalization. A small portion of the incremental QALY gained was due to the greater efficacy of BARI-REM versus REM in reducing time to recovery as well as reducing transitions to worse health states (e.g., fewer patients requiring mechanical ventilation), and thus more QALYs after hospital discharge. The reduction in the number of patients who require mechanical ventilation contributed to reduced inpatient costs, since patients requiring mechanical ventilation are the most resource-intensive and costly to treat. Also, from the payer perspective the savings in inpatient costs are offset by higher costs post discharge because of the higher survival with BARI-REM versus REM. Most of the cost difference and QALY gains arise from lifetime costs and benefits due to the higher survival. BARI-REM combination remains cost-effective at less than $50,000 per QALY gained despite including lifetime all-cause healthcare costs in the analysis.
Viewing these results in aggregate, we find that treatment with BARI-REM resulted in an incremental cost per QALY gained of $22,334 and cost per life year gained of $17,858. The sensitivity analysis also showed that the total incremental cost per QALY gained falls well below commonly used willingness to pay thresholds, as well as the lowest $50,000/QALY threshold proposed by ICER for public health emergency conditions, and shows the robustness of our model [32].
Our findings from the hospital perspective suggest that treatment with BARI-REM reduces total hospital expenditures versus REM alone, primarily by reducing the proportion of patients who require mechanical ventilation. Since patients requiring mechanical ventilation are reimbursed via more expensive DRGs, corresponding reimbursements are also reduced. Treatment with BARI-REM combination versus REM leads to a reduction in hospital expenses, which is estimated to be larger than the reduction in reimbursements received, yielding net cost savings (reduced expenses minus reduced reimbursement). In addition, the greater number of QALYs accrued to BARI-REM due to increased survival during the hospital stay demonstrates a dominant case of cost-effectiveness (greater benefits and lower costs) for BARI-REM compared to REM in treating patients with COVID-19.
In the second update by ICER, they investigated the effects of remdesivir on mortality in patients with COVID-19; their report did not support a survival benefit with remdesivir. However, data from the US-based ACTT-1 trial suggested that remdesivir treatment might result in savings on insurer payments for hospital services. In the time since ACTT-1, the label of remdesivir has been expanded to include hospitalized patients with moderate-to-severe disease; ICER’s final report suggested a health-benefit price benchmark of $2470 for remdesivir assuming no survival benefit. If a survival benefit was assumed, the updated ICER COVID CE model suggested a CE price range of $3980–4140 for patients hospitalized with moderate-to-severe disease [33]. However, ICER’s model did not consider any discharge status or post-acute care of patients, nor did it consider the hospital perspective [33]. Another limitation of the ICER model was simulation of a lifetime horizon using mortality and cost and QALY estimations for the general population, whereas hospitalized patients with COVID-19 tend to have more comorbidities and generally poorer health than the general population.
Our model overcomes those limitations by considering total hospital expenditures, reimbursement payments, and the net costs per QALY gained in the model. Our model also considered the health outcomes and costs by discharge status (self-care or custodial care, home healthcare, inpatient rehabilitation, skilled nursing facilities, short-term hospitalization, long-term acute care hospitalization, and hospice status) to provide a broader picture of post-acute hospital consequences. Including indirect costs in our model to factor in the costs per workday missed by hospitalized patients gives a holistic view of the payer’s perspective. In addition, our model does estimate the potential impact of efficacious therapies that reduce progression to higher levels of oxygen care. Therefore, there is a reduction in the use of hospital days and intensive care unit days. This results in more available resources for hospitals for care of other patients without COVID-19, though these potential benefits are not directly quantified. The adjustment of cost, mortality, and utilities for the higher prevalence of comorbidities makes our analysis more conservative by yielding higher incremental lifetime all-cause costs and lower incremental lifetime QALYs. Also, the mortality calculations in our model accommodated a wide range of age groups. Another major strength of our model is the use of Premier cost analysis, which provides detailed data from real-world COVID-19-affected patients. Using the Premier cost analysis also facilitated the tracking of discharge status of patients and provided highly precise estimates of post-acute care costs in the model. The structure of the model allows for adaptations to other countries using local data inputs.
Data on anticipated long-term burden of COVID-19 have just started surfacing [34, 35]. At present, none of the existing CE models including ours considers long-term consequences of COVID-19 because of lack of data and the very dynamic situation at hand [36]. Further, our model does not factor in any resource constraints that hospitals might experience, such as lack of beds in intensive care units because of more patients with COVID-19 progressing towards requirements for ICU-level care. This might translate to more lives lost as a result of other patients not being able to receive critical care. The data on disutilities is limited in the existing ICER model and our model, which accounts for one of the limitations of this model. Another limitation specific to the secondary analysis from the hospital perspective is that our estimates of hospital expenditures and DRG payments are national averages. Therefore, it may not be representative of the average expenses and reimbursement payments experienced by a specific hospital. Also, the model did not take hospital readmissions into account.
The healthcare situation with COVID-19 is still evolving rapidly and continues to impose substantial burden on the healthcare system. Tocilizumab was also granted an EUA by the FDA for the treatment of COVID-19 in hospitalized adult and pediatric patients receiving systemic corticosteroids and requiring supplemental oxygen, noninvasive or invasive mechanical ventilation, or ECMO [37]. Recently, the FDA has issued an EUA to permit the use of BARI for treatment of COVID-19 in hospitalized adults and pediatric patients 2 years of age or older requiring supplemental oxygen, noninvasive or invasive mechanical ventilation, or ECMO [6]. Because of the increasing burden and emerging treatment options, there is an elevated need to assess the value of these treatments to improve patient outcomes and effectively utilize healthcare resources. The assessment of the clinical and economic value of these emerging treatments will help decision-makers to make informed evidence-based pricing and treatment access decisions [38].
Conclusion
Our study showed that baricitinib in combination with remdesivir is more cost-effective than using remdesivir alone for patients hospitalized because of COVID-19 in the USA. The sensitivity of this cost-effectiveness model was mainly driven by average annual costs of recovered patients, incidence of new mechanical ventilation after admission, and the mechanical ventilation days on REM and BARI-REM. Effective COVID-19 treatments for hospitalized patients may not only reduce disease burden but also represent good value for the healthcare system.
References
Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33.
WHO. WHO Coronavirus (COVID-19) Dashboard: World Health Organization. 2021. https://covid19.who.int/. Accessed 20 Aug 2021.
Laboratory-confirmed COVID-19 associated hospitalizations: COVID-NET: COVID-19 associated hospitalization surveillance network: Centers for Disease Control and Prevention. 2021. https://gis.cdc.gov/grasp/COVIDNet/COVID19_5.html. Accessed 20 Aug 2021.
Di Fusco M, Shea KM, Lin J, et al. Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. J Med Econ. 2021;24(1):308–17.
Kohli M, Maschio M, Becker D, Weinstein MC. The potential public health and economic value of a hypothetical COVID-19 vaccine in the United States: use of cost-effectiveness modeling to inform vaccination prioritization. Vaccine. 2021;39(7):1157–64.
FDA. Fact sheet for healthcare providers Emergency Use Authorization (EUA) of baricitinib U.S. Food and Drug Administration. 2021. https://www.fda.gov/media/143823/download. Accessed 19 Aug 2021.
FDA. Coronavirus (COVID-19) update: FDA authorizes drug combination for treatment of COVID-19. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-combination-treatment-covid-19. Accessed 20 Aug 2021.
Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2020;384(9):795–807.
Whittington MD, Campbell J. Alternative pricing models for remdesivir and other potential treatments for COVID-19. June 24 ICER; 2020. https://icer.org/news-insights/press-releases/updated_icer-covid_models_june_24/. Accessed 29 April 2021.
Campbell JD, Whittington MD, Rind DM, Pearson SD. Alternative pricing models for remdesivir and other potential treatments for COVID-19; updated report. Institute for Clinical and Economic Review. 2020. http://icer.org/wp-content/uploads/2020/11/ICER-COVID_Updated_Report_11102020.pdf. Accessed 10 Nov 2020.
Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med. 2020;383(19):1813–26.
Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–57.
Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2020;384(8):693–704.
Pan H, Peto R, Karim QA, et al. Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results. medRxiv. 2020:2020.10.15.20209817.
Halpern MT, Luce BR, Brown RE, Geneste B. Health and economic outcomes modeling practices: a suggested framework. Value Health. 1998;1(2):131–47.
Boudreau DM, Malone DC, Raebel MA, et al. Health care utilization and costs by metabolic syndrome risk factors. Metab Syndr Relat Disord. 2009;7(4):305–14.
Vetter ML, Wadden TA, Lavenberg J, et al. Erratum: Relation of health-related quality of life to metabolic syndrome, obesity, depression, and comorbid illness. Int J Obes. 2012;36(2):325–6.
Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28(7):1769–78.
Premier Applied Sciences®. Premier healthcare database white paper: data that informs and performs. 2020. https://learn.premierinc.com/white-papers/premierhealthcaredatabase. Accessed 20 Aug 2021.
Data on file. A multicenter, adaptive, randomized blinded controlled study of the safety and efficacy of investigational therapeutics for the treatment of COVID-19 in hospitalized adults. ACTT-2: Baricitinib/Remdesivir vs Remdesivir. Final Integrated Clinical/Statistical Report. 2020.
O’Day D. An open letter from Daniel O’Day, Chairman & CEO, Gilead Sciences. Gilead Sciences. 2020. https://www.gilead.com/news-and-press/press-room/press-releases/2020/6/an-open-letter-from-daniel-oday-chairman--ceo-gilead-sciences. Accessed 27 Apr 2021.
Olumiant Price Information 2020. http://www.lillypricinginfo.com/olumiant. Accessed 27 Apr 2021.
Labor Force Statistics from the Current Population Survey: Bureau of Labor Statistics. 2021. https://data.bls.gov/PDQWeb/ln. Accessed 26 June 2021.
Garfield RRR, Guth M, Orgera K, Hinton E, Neuman T. Work among medicaid adults: implications of economic downturn and work requirements. Kaiser Family Foundation. 2021. https://www.kff.org/report-section/work-among-medicaid-adults-implications-of-economic-downturn-and-work-requirements-issue-brief/. Accessed 20 Aug 2021.
Employer Costs for Employee Compensation. Series ID: CMU1010000000000D (C). All civilian total compensation for all occupations; cost per hour worked, 2010 to 2020: Bureau of Labor Statistics. 2021. https://www.bls.gov/data/. Accessed 20 Aug 2021.
Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Mak. 2006;26(4):410–20.
Barbut F, Galperine T, Vanhems P, et al. Quality of life and utility decrement associated with Clostridium difficile infection in a French hospital setting. Health Qual Life Outcomes. 2019;17(1):6.
Sackett DL, Torrance GW. The utility of different health states as perceived by the general public. J Chronic Dis. 1978;31(11):697–704.
Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–103.
2020–2023 Value Assessment Framework. Institute for Clinical and Economic Review. 2020. https://icer.org/wp-content/uploads/2020/10/ICER_2020_2023_VAF_102220.pdf. Accessed 29 Apr 2021.
Social Security Actuarial Life Table. Period Life Table, 2017. https://www.ssa.gov/oact/HistEst/PerLifeTables/2017/PerLifeTables2017.html. Accessed 26 June 2021.
Sarah K, Emond SDP. Alternative policies for pricing novel vaccines and drug therapies for COVID-19. 2020 July 1.
ICER update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;857(1):2.
del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA. 2020;324(17):1723–4.
Miller IF, Becker AD, Grenfell BT, Metcalf CJE. Disease and healthcare burden of COVID-19 in the United States. Nat Med. 2020;26(8):1212–7.
Taribagil P, Creer D, Tahir H. ‘Long COVID’ syndrome. BMJ Case Rep. 2021;14(4):e241485.
FDA. Fact sheet for healthcare providers: emergency use authorization for Actemra® (Tocilizumab) U.S. Food and Drug Administration; 2021. https://www.fda.gov/media/150321/download. Accessed 19 Aug 2021.
Sheinson D, Dang J, Shah A, Meng Y, Elsea D, Kowal S. A cost-effectiveness framework for COVID-19 treatments for hospitalized patients in the United States. Adv Ther. 2021;38(4):1811–31. https://doi.org/10.1007/s12325-021-01654-5.
Acknowledgements
Funding
The study was funded by Eli Lilly and Company. Eli Lilly and Company also funded the journal’s rapid publication charges.
Medical Writing, Editorial, and Other Assistance
Suchita Dubey, an employee of Eli Lilly Services India Pvt. Ltd., provided writing support.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
RB, TS, MB, KK, PLM, and TK contributed to conception of the work. TH, MB, KK, PLM, and TK contributed to design of the study. RB, KK, PLM, and TK contributed to acquisition of the data. KK, TK, DM, and EH contributed to analysis of data and all authors contributed to data interpretation. KK, DM, EH, and RB contributed to drafting of the manuscript and all authors contributed to critical revision of the manuscript for important intellectual content.
Disclosures
Patrick L McCollam, Theodore Spiro, Russel Burge, and Mark Belger are employees of Eli Lilly and Company. Kari Kelton, Tim Klein, Dan Murphy, and Erik Hille are employees of MDM Inc., who received funding from Eli Lilly and Company to conduct the study.
Compliance with Ethics
This manuscript does not contain any new studies with human or animal subjects performed by any of the authors.
Data Availability
The data that support the findings of this study were provided by MDM Inc. A part of the data used in this study is owned by Eli Lilly and Company or NIAID, USA. Restrictions may apply to the availability of these data, which were used under agreement for this study and therefore, not publicly available. Requests may be sent to Eli Lilly and Company for more information on data availability and licensing.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kelton, K., Klein, T., Murphy, D. et al. Cost-Effectiveness of Combination of Baricitinib and Remdesivir in Hospitalized Patients with COVID-19 in the United States: A Modelling Study. Adv Ther 39, 562–582 (2022). https://doi.org/10.1007/s12325-021-01982-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01982-6