Abstract
Introduction
Results from the open-label extension of the phase 3b CONQUER trial are presented to evaluate the effectiveness and safety of galcanezumab, a monoclonal antibody targeting calcitonin gene-related peptide, for up to 6 months in patients with multiple prior migraine preventive treatment failures.
Methods
Patients were 18–75 years old with episodic or chronic migraine and 2–4 standard-of-care migraine preventive medication category failures. After 3 months of randomized treatment with galcanezumab (120 mg/month with 240 mg loading dose; n = 232) or placebo (n = 230), patients entered a 3-month open-label extension (120 mg/month galcanezumab with a blinded 240 mg loading dose for previous-placebo patients). Primary efficacy measure was mean change from double-blind baseline in monthly migraine headache days.
Results
A total of 432/449 patients (96%) who entered open-label treatment completed the study. Mean change in monthly migraine headache days in the total population, which was − 1.3 for placebo and − 4.4 for galcanezumab patients at the end of double-blind treatment (p < 0.001), was − 5.2 and − 5.6, respectively, at the end of open-label treatment with galcanezumab. Among patients with episodic migraine, mean change in monthly migraine headache days had been − 0.6 for placebo and − 2.8 for galcanezumab after double-blind treatment (p < 0.001) and was − 4.5 and − 3.8, respectively, after open-label treatment. Among patients with chronic migraine, mean change in monthly migraine headache days had been − 2.5 for placebo and − 6.6 for galcanezumab after double-blind treatment (p < 0.001) and was − 6.5 and − 8.2, respectively, after open-label treatment. Adverse events were similar to those observed during double-blind placebo treatment. Review of data in elderly patients (65–75 years of age) indicated that galcanezumab was well tolerated in this age group, with no safety issues identified.
Conclusions
Galcanezumab was effective and safe during open-label treatment in patients who had experienced failures of previous migraine preventives.
Clinical Trial Registration
ClinicalTrials.gov identifier NCT03559257.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the open-label period of the CONQUER trial, galcanezumab, a monoclonal antibody specifically developed for prevention of migraine by targeting calcitonin gene-related peptide, continued to show effectiveness in patients who have experienced multiple previous migraine preventive treatment failures. |
Galcanezumab appeared safe and well tolerated with up to 6 months of treatment, including in patients 65 to 75 years of age. |
Adherence to galcanezumab treatment was very high in this population of patients with a history of multiple treatment discontinuations. |
Consistent with phase 3 data, the longer that patients are on galcanezumab treatment, the greater the benefit they may experience. |
Introduction
Migraine preventive medications are more likely to be prescribed for patients with more severe headache-related disability [1]. However, persistence on traditional standard-of-care migraine preventive medications is quite poor, and the primary reasons for treatment discontinuation are lack of efficacy, presence of side effects, or both [1, 2]. Studies have found that approximately half of all patients discontinued oral standard-of-care preventive treatments within 2 or 3 months after initiating [3,4,5,6], and that 75% of patients discontinued oral migraine preventive treatment within 6 months [3]. The Hepp et al. (2017) study [3] also found that treatment persistence worsened further as patients switched to different preventive medications; by the third preventive medication switch, only 13–20% of patients were still adherent to treatment at 6 months. Thus, patients with a history of having tried and discontinued multiple migraine preventives present the clinician with a challenge to find a treatment with a higher likelihood of success for that patient.
Starting in 2018, monoclonal antibody (mAb) therapies targeting the calcitonin gene-related peptide (CGRP) signaling pathway became available for prescription in many countries [7]. Galcanezumab is a humanized CGRP mAb with demonstrated safety and efficacy in multiple double-blind clinical trials for the preventive treatment of episodic and chronic migraine [8,9,10,11,12]. Current guidance from the European Headache Federation and American Headache Society recommends that CGRP mAbs be offered for migraine prevention only after at least two standard-of-care preventive treatments have shown inadequate efficacy for or cannot be used by the patient [13, 14].
In post hoc analyses of three phase 3 double-blind trials, galcanezumab was effective in patient subgroups (N = 188 episodic migraine, N = 356 chronic migraine) with at least two prior treatment failures [15, 16]. The CONQUER trial was specifically designed to assess galcanezumab use in patients who had experienced 2–4 prior standard-of-care migraine preventive medication category failures due to inadequate efficacy or tolerability. Results of the previously published 3-month double-blind period of CONQUER demonstrated the safety and efficacy of galcanezumab in this population [11]. We now present results from the open-label extension of the CONQUER study. A 3-month open-label extension was included to give patients on placebo an opportunity to receive active treatment and to provide up to a total of 6 months of data to assess longer-term safety and treatment effectiveness, including maintenance of response and adherence to treatment, in patients for whom multiple treatments have failed. It was expected that efficacy gains from the double-blind treatment period would be maintained for those patients previously randomized to galcanezumab and that patients newly started on galcanezumab after previous treatment with placebo would show similar efficacy gains as had been previously seen in the double-blind galcanezumab group. Additionally, efficacy, safety, and tolerability in the elderly subpopulation (65–75 years of age) was assessed.
Methods
Study Design and Participants
The CONQUER study (ClinicalTrials.gov NCT03559257) was a phase 3b trial with four study periods: screening (3–30 days), prospective baseline (1 month), randomized double-blind (3 months), and open-label treatment (3 months). The primary report of the double-blind period provides details regarding study sites and enrollment/exclusion criteria [11]. Patients were 18–75 years old with a diagnosis of migraine with or without aura, or chronic migraine, as defined by the International Classification of Headache Disorders, third edition (ICHD-3) [17]. Patients had to have experienced prior failures of 2–4 standard-of-care migraine preventive medication categories in the past 10 years. Treatment failure was defined as discontinuation due to inadequate efficacy after at least 2 months at the maximum tolerated dose or due to inadequate safety/tolerability. Contraindication against use was not considered treatment failure. Migraine preventive medication categories were (a) propranolol or metoprolol, (b) topiramate, (c) valproate or divalproex, (d) amitriptyline, (e) flunarizine, (f) candesartan, (g) botulinum toxin A or B if used for chronic migraine, and (h) medications approved locally for migraine prevention. Patients also had to have averaged at least four monthly migraine headache days over the past 3 months with at least one headache-free day per month and then demonstrate the same during the 1-month prospective baseline period on an electronic daily diary (eDiary) while achieving at least 80% compliance with eDiary entries.
Acute headache medications were allowed throughout the trial, although opioid and barbiturate use was restricted to a maximum of 4 days per month. Use of migraine preventive treatments during the trial was not permitted. All patients provided informed, written consent before participation. Institutional review boards at each site approved the study protocol, and the study was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki guidelines.
Participants in the open-label period had previously been randomized 1:1 in the double-blind treatment period to subcutaneous injection of either galcanezumab (120 mg/month with a 240-mg loading dose) or placebo. Patients who completed the 3-month double-blind period were eligible to enter the 3-month open-label period. Dosing in the open-label period was 120 mg/month galcanezumab, with a blinded loading dose of 240 mg for previous-placebo patients. Thus, all patients received two injections at their first open-label dose visit (either two 120-mg galcanezumab injections, or one 120-mg galcanezumab injection plus one placebo injection) and a single injection of galcanezumab 120 mg at all subsequent monthly dosing visits.
Assessments
Starting at the prospective baseline period, patients recorded daily headache occurrence, duration, features, and severity as well as any acute headache medication use. Primary outcome measure was mean change in monthly migraine headache days, derived from patients’ eDiary entries. Patients also completed the Migraine-Specific Quality-of-Life Questionnaire (MSQ, version 2.1) monthly, and the Migraine Disability Assessment (MIDAS) and the Patient Global Impression of Severity (PGI-S) every 3 months. Safety assessments included collection of treatment-emergent adverse events (AEs), serious AEs, discontinuations due to AEs, and vital signs at every visit, and weight, electrocardiograms (ECGs), and laboratory analytes every 3 months. A number of other patient-reported health outcome measures were also assessed but will be reported in separate publications; those measures include the 4-item Migraine Interictal Burden Scale, the European Quality of Life 5-Dimensions 5-Levels, the Work Productivity and Activity Impairment Questionnaire, the 7-item Generalized Anxiety Disorder Scale, the Patient Health Questionnaire-9, and healthcare resource utilization.
Statistical Analyses
The total analysis population included all patients who were randomized and received at least one dose of study drug. Within the total population, the episodic migraine subpopulation included patients with low frequency episodic migraine (4 to < 8 monthly migraine headache days) or high frequency episodic migraine (8–14 monthly migraine headache days and < 15 monthly headache days) in the prospective baseline period, and the chronic migraine subpopulation included patients with at least 8 monthly migraine headache days and at least 15 monthly headache days in the prospective baseline period.
Patients were included in analyses only if they had a baseline and at least one post-baseline assessment. For efficacy analysis, patients were considered assessable for a given month only if they had more than 50% compliance with the eDiary in that month. Patients who did not have at least 1 month of assessable post-baseline data were excluded from the analyses.
Efficacy analyses included both the double-blind and open-label periods, with changes calculated from double-blind baseline. Efficacy analyses were performed on the total population as well as the episodic migraine and chronic migraine subgroups. Changes from baseline in continuous variables with repeated measures were analyzed using a restricted maximum likelihood-based mixed model repeated measures (MMRM) technique. The primary efficacy model included fixed, categorical effects of treatment, pooled country, month, and treatment-by-month interaction, and continuous, fixed covariates of baseline value and baseline value-by-month interaction. Analysis of continuous secondary efficacy measures used the same model as the primary but also included baseline migraine frequency category in the model when applicable (i.e., low frequency episodic, high frequency episodic, and chronic for the total population analyses; low frequency episodic and high frequency episodic for the episodic migraine subgroup analyses). A common unstructured covariance structure was used to model the within-patient errors. MMRM models analyzing safety variables included the same categorical effects but excluded country and baseline migraine frequency category. Mean change analyses for those outcomes assessed only at baseline and endpoint (MIDAS and PGI-S) employed an analysis of covariance, with main effects of treatment, baseline migraine frequency category, pooled country, and baseline value. Type III sum-of-squares was used for statistical comparison of least square means.
Between-group comparisons for categorical variables used Fisher’s exact test. Binary variables with repeated measures, including response rates, were analyzed using a generalized linear mixed model as pseudo-likelihood mixed effects repeated measures analysis, with fixed, categorical effects of treatment, month, and treatment-by-month interaction, and continuous covariate of baseline value. Because the chronic migraine 100% response rate model did not converge because of zero counts for placebo, analysis was limited to months 4–6, and comparison at month 3 used Fisher’s exact test.
Response rates were the estimated percentage of patients with at least 30%/at least 50%/at least 75%/100% reduction from double-blind baseline in monthly migraine headache days. Maintenance of at least 50% response was defined as a reduction from baseline to month 3 of at least 50%, with continued maintenance of at least 50% response throughout the open-label period, and was analyzed using logistic regression.
Adverse events were defined as treatment-emergent if they emerged or worsened post-baseline, with baseline defined as the period prior to first injection with study drug for the double-blind period, and prior to first open-label dose for the open-label period.
All statistical analyses were done using SAS Enterprise Guide 7.2 (SAS Institute Inc., Cary, NC, USA). Determination of significance was conducted using a two-sided alpha of 0.05 for all analyses.
Results
Of the 462 patients randomized to treatment, 451 completed the double-blind period and were eligible to enter the open-label extension (Fig. 1). Two patients decided not to enter the open-label period: one because of moderate constipation and one because of concerns about risks. Of the 449 who entered the open-label period, 432 (96%) completed the study. Among patients randomly assigned to galcanezumab in the double-blind period (n = 232), adherence to galcanezumab treatment at 6 months was 94%, compared with 93% for double-blind placebo followed by open-label galcanezumab.
Compliance with daily eDiary entries was high throughout the study, with an average of 97% of daily entries completed at month 3 and 91% at month 6. The number of patients in the total population whose compliance was at most 50% in a given month (thus requiring exclusion of that month’s data from the eDiary analyses) ranged from 4 to 6 patients per month. There was no statistically significant difference in eDiary compliance between treatment groups.
Table 1 presents baseline demographics and disease characteristics for the total population as well as the episodic and chronic migraine subpopulations. Baseline characteristics were similar for placebo- and galcanezumab-treated groups. Mean baseline number of monthly migraine headache days was 13.2 for the total population, 9.3 for the episodic migraine subpopulation, and 18.7 for the chronic migraine subpopulation. Patients had discontinued an average of 3.3 migraine preventive drugs in the past 10 years because of insufficient efficacy or tolerability. Baseline demographics for the open-label only population can be found in Supplementary material (Table S1) but did not differ substantially from those of the total population.
Efficacy
Figure 2 shows mean change from baseline in monthly migraine headache days at each month of the treatment periods. In the total population (Fig. 2a), mean changes from baseline for both treatment groups became similar after placebo-treated patients switched to galcanezumab at the start of the open-label period. After their first month of open-label galcanezumab at month 4, the previous-placebo group experienced a mean decrease of 3.9 monthly migraine headache days, similar to the reduction observed in the previous-galcanezumab group in the double-blind period at month 1, thus catching up to the previous-galcanezumab group. Thereafter, both groups showed similar reductions.
Mean change from baseline in monthly migraine headache days: Mean changes in monthly migraine headache days for the 3-month double-blind and 3-month open-label periods are shown for all patients (a), patients with episodic migraine (b), and patients with chronic migraine (c). GMB galcanezumab, GMB/GMB galcanezumab treatment in double-blind and open-label periods, LS least squares, PBO/GMB placebo treatment in double-blind and galcanezumab treatment in open-label periods, SE standard error. **p < 0.001, *p < 0.05 compared to PBO/GMB
In the episodic migraine population (Fig. 2b), the placebo-treated group showed minimal response during double-blind treatment but experienced a large mean decrease from baseline once on open-label galcanezumab, thus surpassing the previous-galcanezumab group (p = 0.005). In contrast, in the chronic migraine population, the placebo-treated group showed a small response in the double-blind period, then improved significantly once on open-label galcanezumab, but never completely caught up with the previous-galcanezumab group (Fig. 2c).
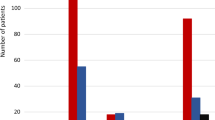
Percentages of patients experiencing decreases from baseline in monthly migraine headache days of at least 50%, at least 75%, and 100% are shown in Fig. 3 and Table 2. In the total population (Fig. 3a), galcanezumab was superior to placebo for all three response thresholds at the end of the double-blind period (month 3) (p < 0.001 for at least 50%, p = 0.006 for at least 75%, and p = 0.009 for 100%). Response rates at all threshold levels continued to increase for the previous-galcanezumab group and were similar for the two groups when all patients were receiving open-label galcanezumab (month 6).
Patients with ≥ 50%, ≥ 75%, and 100% reductions from baseline in monthly migraine headache days at months 3 and 6: Bars show model-estimated rates of patients experiencing ≥ 50%, ≥ 75%, and 100% decreases from baseline in monthly migraine headache days for the total population (a), patients with episodic migraine (b), and patients with chronic migraine (c) at month 3 (end of double-blind period) and month 6 (end of open-label period) for both treatment arms. For the chronic migraine population with 100% decrease, raw rates are shown at 3 months as a result of non-convergence of the model because zero placebo-treated patients met the response threshold for this time point. GMB/GMB galcanezumab treatment in double-blind and open-label periods, PBO/GMB placebo treatment in double-blind and galcanezumab treatment in open-label periods, standard error. ***p < 0.001, **p < 0.01, *p < 0.05 compared to PBO/GMB
Patterns of response rates in the episodic (Fig. 3b) and chronic (Fig. 3c) migraine populations were generally similar to those seen in the total population, but with higher rates observed in the episodic group than the chronic group, as would be expected. Among patients treated with galcanezumab for 6 months (previous-galcanezumab group), 57% of patients with episodic migraine and 48% of patients with chronic migraine had achieved at least a 50% reduction in their monthly migraine headache days at month 6.
For patients with chronic migraine, a reduction in monthly migraine headache days of at least 30% from baseline is considered a clinically meaningful response [22]. At the end of the double-blind period, 58% of galcanezumab-treated patients versus 28% of placebo-treated patients (p < 0.001) had at least a 30% reduction from baseline in monthly migraine headache days. At the end of the open-label period, these percentages increased to 68% for the previous-galcanezumab group and 57% for the previous-placebo group, with the between-group difference not statistically significant (p = 0.15).
Maintenance of response was assessed by analysis of the 87 of 224 previous-galcanezumab patients (39%) who had achieved at least a 50% response at month 3. Of these patients, 45 (52%) maintained that decrease from baseline of at least 50% in monthly migraine headache days in all 3 months of the open-label period. Among all patients who entered open-label treatment, 33% maintained at least 50% response at every month throughout the open-label period.
Table 2 shows results for the efficacy and quality-of-life outcomes at the end of double-blind (month 3) and open-label (month 6) periods. Mean changes from baseline in MSQ-RFR, MIDAS, PGI-S, monthly headache days, and monthly migraine headache days with acute headache medication use followed a similar pattern. For all of these outcomes, at month 3, mean improvement was statistically significantly greater in the galcanezumab-treated group than placebo, and then not statistically significantly different between groups after all were treated with galcanezumab. The only exceptions were in the chronic migraine population, in which reductions in PGI-S and monthly migraine headache days with acute medication use remained statistically significantly greater in the previous-galcanezumab group relative to previous-placebo.
Safety and Tolerability
Adverse events (AEs) in the double-blind and open-label periods are summarized in Table 3. There were no deaths. Nine of 449 patients (2%) experienced serious AEs during the open-label period, with none considered related to treatment by the investigator, and none resulting in discontinuation from the trial. Five of 449 patients (1%) discontinued the open-label period because of an AE, three of which were considered related to treatment (induration, rash, and injection site erythema). Of the five most common treatment-emergent AEs in the open-label period (at least 1.5% of patients), nasopharyngitis and back pain occurred at a similar or lower rate than in the double-blind placebo group and were not considered related to treatment; the remaining three common AEs related to injection site. Most AEs were mild or moderate in severity. There were no clinically meaningful changes in laboratory analytes, vital signs, weight, or ECGs.
Safety and Efficacy in Older Patients
Of the 462 patients enrolled in the CONQUER trial, 29 were 65–75 years old (defined here as the elderly subpopulation). All 29 entered the open-label period, and 13 received galcanezumab during both periods of the trial. There were no clinically significant safety findings for AEs, laboratory analytes, vital signs, or ECGs in this older population. In the double-blind period, 2/13 galcanezumab-treated patients reported treatment-emergent AEs versus 7/16 for placebo, and incidence in the open-label period (48%) was similar to that observed in the wider adult population (43%). However, none of the open-label period AEs in the elderly patients were considered related to treatment by the investigator. All AEs in the elderly were mild or moderate in severity, with no AEs related to injection site or hypersensitivity. One patient in this group (3%) experienced a serious AE of pneumonia associated with aspiration during a planned surgery that was not considered related to treatment. No elderly patients discontinued because of an AE. Four of the 29 (14%) experienced treatment-emergent high diastolic blood pressure, but these elevations were transient, and all four patients had experienced previous elevations and/or had a diagnosis of hypertension prior to starting galcanezumab. These results are summarized in Table 4.
Although the number of elderly patients was too small for a formal efficacy analysis, and patients were not stratified by age to ensure even distribution of elderly patients across the two treatment groups, a post hoc analysis was conducted to explore the primary efficacy measure in this subpopulation using the same model as for the total population. Mean change from baseline in the number of monthly migraine headache days was numerically greater in the 13 elderly patients receiving galcanezumab than for the 16 elderly patients receiving placebo (− 3.1 [SE = 1.4] versus − 2.1 [SE = 1.2], respectively, between-group p = 0.57) at the end of the double-blind treatment period (month 3). At the end of the open-label period (month 6), previous-galcanezumab elderly patients maintained their change in monthly migraine headache days from double-blind baseline (− 3.2 [SE = 1.2]) while previous-placebo elderly patients now receiving galcanezumab had a mean change of − 2.8 (SE = 1.5; between-group p = 0.88).
Discussion
In the open-label period of CONQUER, galcanezumab continued to be effective, safe, and well tolerated in patients with episodic or chronic migraine who had experienced treatment failures of multiple previous preventive medications. Moreover, despite patients’ history of discontinuing multiple previous treatments, adherence to galcanezumab at 6 months was 94%. Note that real-world adherence could differ from that observed in a clinical trial setting.
Results from this trial are supportive of the durable effect of galcanezumab in the reduction of monthly migraine headache days. Patients previously treated with galcanezumab in the double-blind period continued to show further mean reductions in monthly migraine headache days, headache days, migraine headache days with acute medication use, disease severity, and disability, as well as further improvements in functioning during their subsequent 3 months of galcanezumab treatment in the open-label period. Patients previously treated with placebo during the double-blind period showed rapid mean improvement in all these measures after the first open-label dose of galcanezumab, consistent with the rapid improvements previously seen with galcanezumab during the double-blind treatment period.
Patients also achieved clinically meaningful levels of response during the open-label period, with 54% of patients achieving at least 50% response, 30% achieving at least 75% response, and 8% achieving 100% response at month 6. Maintenance of response was also demonstrated. In the total, episodic, and chronic migraine populations, percentages of patients experiencing response rates of at least 50%, at least 75%, and 100% were higher at the end of the open-label period (month 6) than at the end of the double-blind period (month 3) for both the previous-placebo and previous-galcanezumab groups.
Safety and tolerability results were consistent with the known safety profile of galcanezumab as demonstrated in earlier double-blind and open-label studies [8,9,10,11,12]. Inclusion of patients more than 65 years up to 75 years of age did not result in any new safety findings, and patients in this age range appeared to tolerate the drug very well. An exploratory post hoc analysis suggested that galcanezumab treatment was effective in this elderly subpopulation, although the very small sample size severely limits the conclusions which can be drawn from that analysis.
Overall, the results of the CONQUER study have several clinical health implications. First, adherence to treatment was very high. Poor adherence has been a serious problem with oral standard-of-care migraine preventive treatments, particularly in patients who have discontinued multiple treatments [3], and the high completion rate for the CONQUER trial indicates that patients found the treatment tolerable and suggests that patient adherence in a clinical setting could also be high. Second, the finding that galcanezumab-treated patients with episodic and especially chronic migraine experienced further gains during the open-label period indicates that patients may continue to see improvement in monthly migraine headache days with galcanezumab treatment for at least 6 months, although lack of a placebo comparator in the open-label period may limit interpretability. Third, the observation that 52% of all patients who responded by the end of the double-blind period maintained that response throughout all 3 months of the open-label period indicates that patients with initial response to galcanezumab have a good chance of continuing to respond in subsequent months of treatment.
Another finding of interest was the difference observed between episodic and chronic migraine patients in how previous-placebo patients responded to treatment in the open-label period relative to the previous-galcanezumab patients. While all patients with multiple previous treatment failures may have lower expectations for future such treatments compared with patients who have not had multiple treatments fail for them, this expectation may be even lower among patients with chronic migraine, who continue to carry a high disease state burden when a treatment fails. Patients with episodic migraine, however, may have had a more positive expectation upon entering the open-label period, which may have provided a boost to their results.
Other studies examining CGRP mAbs in patients with multiple treatment failures include the LIBERTY and FOCUS phase 3b trials of erenumab and fremanezumab, respectively [23, 24]. The differences in methodology and populations across these studies have previously been described [11], with the CONQUER study using a somewhat stricter definition of treatment failure (contraindications were not counted as treatment failures) and including patients up to 75 years of age, with approximately 40% of patients having chronic migraine. All three trials included an open-label extension, with the LIBERTY trial in episodic migraine patients including a 3-year open-label period [23], and the FOCUS trial in both episodic and chronic migraine patients including a 3-month open-label period [24]. Similar to the CONQUER study, results from the 3-month FOCUS open-label period [25] and the 12-month interim results from the LIBERTY open-label period [26] suggest very good tolerability and adherence to treatment, with no new safety signals identified during the open-label period. Indirect efficacy comparisons between the studies should be made with caution because of methodological differences.
There are some limitations to consider for the open-label period of the CONQUER study. Patients in the open-label period, by definition, are aware of receiving active treatment, and this knowledge may influence patient response. However, the rapid improvements in the previous-placebo group after starting open-label galcanezumab were generally consistent with those seen in the previous-galcanezumab group during the double-blind period, supporting the overall validity of the results. Another possible limitation was the 6-month treatment duration (3 months double-blind and 3 months open-label), which may be insufficient to characterize the full measure of benefit or risk that could arise with longer-term use of galcanezumab.
Conclusions
Galcanezumab was effective and safe after 3 months of double-blind treatment and an additional 3 months of open-label treatment in patients with episodic or chronic migraine who had experienced 2–4 prior standard-of-care migraine preventive medication category failures. Galcanezumab was also very well tolerated in the subset of patients who were 65–75 years of age. Results indicated durable efficacy and excellent treatment adherence in this population of patients with a history of discontinuing previous migraine preventive medications because of lack of efficacy or tolerability.
References
Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53(4):644–55.
Ford JH, Jackson J, Milligan G, Cotton S, Ahl J, Aurora SK. A real-world analysis of migraine: a cross-sectional study of disease burden and treatment patterns. Headache. 2017;57(10):1532–44.
Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia. 2017;37(5):470–85.
Woolley JM, Bonafede MM, Maiese BA, Lenz RA. Migraine prophylaxis and acute treatment patterns among commercially insured patients in the United States. Headache. 2017;57(9):1399–408.
Meyers JL, Davis KL, Lenz RA, Sakai F, Xue F. Treatment patterns and characteristics of patients with migraine in Japan: a retrospective analysis of health insurance claims data. Cephalalgia. 2019;39(12):1518–34.
Berger A, Bloudek LM, Varon SF, Oster G. Adherence with migraine prophylaxis in clinical practice. Pain Pract. 2012;12(7):541–9.
Raffaelli B, Neeb L, Reuter U. Monoclonal antibodies for the prevention of migraine. Expert Opin Biol Ther. 2019;19(12):1307–17.
Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–8.
Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–54.
Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91(24):e2211–21.
Mulleners WM, Kim BK, Lainez MJA, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2020;19(10):814–25.
Camporeale A, Kudrow D, Sides R, et al. A phase 3, long-term, open-label safety study of galcanezumab in patients with migraine. BMC Neurol. 2018;18(1):188.
American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59(1):1–18.
Sacco S, Bendtsen L, Ashina M, et al. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20(1):6.
Ruff DD, Ford JH, Tockhorn-Heidenreich A, et al. Efficacy of galcanezumab in patients with chronic migraine and a history of preventive treatment failure. Cephalalgia. 2019;39(8):931–44.
Ruff DD, Ford JH, Tockhorn-Heidenreich A, et al. Efficacy of galcanezumab in patients with episodic migraine and a history of preventive treatment failure: results from two global randomized clinical trials. Eur J Neurol. 2020;27(4):609–18.
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31(3):301–15.
Jhingran P, Davis SM, LaVange LM, Miller DW, Helms RW. MSQ: Migraine-Specific Quality-of-Life Questionnaire. Further investigation of the factor structure. Pharmacoeconomics. 1998;13(6):707–17.
Bagley CL, Rendas-Baum R, Maglinte GA, et al. Validating Migraine-Specific Quality of Life Questionnaire v2.1 in episodic and chronic migraine. Headache. 2012;52(3):409–21.
Guy W. ECDEU assessment manual for psychopharmacology. Rockville: National Institute of Mental Health, Psychopharmacology Research Branch. 1976. p. 217–22. https://archive.org/details/ecdeuassessmentm1933guyw.
Silberstein S, Tfelt-Hansen P, Dodick DW, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia. 2008;28(5):484–95.
Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392(10161):2280–7.
Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. 2019;394(10203):1030–40.
Ashina M, Cohen J, Galic M, et al. Efficacy and safety of fremanezumab in patients with episodic and chronic migraine and documented inadequate response to 2–4 classes of migraine preventive medications during the open-label period of the phase 3b FOCUS study (4379). Neurology. 2020;94(15 Supplement):4379.
Goadsby PJ, Reuter U, Lanteri-Minet M, et al. Long-term efficacy and safety of erenumab: results from 64 weeks of the LIBERTY study. Neurology. 2021;96(22):e2724–e2735.
Acknowledgements
The authors thank all the study participants, site investigators, and personnel involved in the CONQUER study. A full list of site investigators can be found in Supplementary material. The authors also thank Janet Ford (Eli Lilly), for her contributions to study design, implementation, and interpretation, Phebe Kemmer (Eli Lilly), Mallikarjuna Rettiganti (Eli Lilly) and Sarah Lipsius (Syneos Health) for their assistance with statistical analyses.
Funding
The CONQUER study and all associated publication costs, including the journal’s Rapid Service and Open Access Fees, were funded by Eli Lilly and Company.
Medical Writing Assistance
We thank Deirdre Hoban (Eli Lilly) for her contribution to the writing and editing of this manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
UR, CL, and DD were investigators and involved in data collection. ALH was involved in data analysis. MDP drafted the manuscript. RMN, AT-H, and HCD were involved in study design. CS and HCD were involved in study implementation, monitoring, and oversight. All authors were involved in data interpretation, provided critical input and review of drafts, and approved the final version of the manuscript for submission.
Prior presentation
The CONQUER open-label results have previously been presented at the following congresses: Journees de Neurologie de Langue Francaise—2021 Congress, Headache and Pain Studies Association—18th Basagrisi Kis Okulu (Headache Winter School), Emirates Neurology Society—8th International Congress, American Academy of Neurology 2020—72nd Annual Meeting, American Association of Nurse Practitioners—AANPconnect 2020, Swiss Headache Society—2020, European Academy of Neurology—6th Congress, and European Headache Federation—14th European Headache Federation Congress.
Disclosures
Uwe Reuter has received financial compensation for the participation in advisory boards and scientific presentations of Abbvie, Amgen, Allergan, Eli Lilly, Lundbeck, Novartis, Perfood, TEVA. Uwe Reuter has received research support from Novartis and the German BMBF. Christian Lucas has received financial compensation for participation in advisory boards and as investigator or coordinator for clinical trials from Allergan, Amgen, Novartis, TEVA, Sos oxygene, Grunenthal, Eli Lilly, Lundbeck, and Ethypharm. David Dolezil has received financial compensation for consulting, speaking and/or teaching from Allergan, Amgen, Biogen Idec, Novartis, Bayer, Eli Lilly, and Teva. Holland C. Detke, Martha D. Port, Russell M. Nichols, CS and Antje Tockhorn-Heidenreich are full-time employees and minor stockholders of Eli Lilly and Company. Austin L. Hand has nothing to disclose.
Compliance with Ethics Guidelines
Appropriate institutional review boards reviewed and approved this study, which was conducted according to the Declaration of Helsinki, and all subjects provided informed consent to participate in the study. The ethical review boards included Quorum Review, Inc; IRB Services; NRES Committee London—City and East Bristol REC Centre; Egeszsegugyi Tudomanyos Tanacs; Bimetra—UZ Gent; CPP Tours—Region Céntre (Ouest-1); Hopital Universitario La Fe de Valencia Bulevar Sur, S/N; Eticka komise IKEM a Thomayerova nemocnice Multicentricka a localni eticka komise; Landesamt für Gesundheit und Soziales (LAGeSo) Geschäftsstelle der Ethik-Kommission des Landes; Isala Klinieken METC; Tatsuoka Neurology Clinic; Dr. Mano Medical Clinic; Nihonbashi Sakura Clinic; Sugiura Clinic Institutional Review Board; Oita Central Institutional Review Board; Tominaga Hospital; Dokkyo Medical University Hospital; Tokyo-Eki Center-Building Clinic; NPO Clinical research promotion network Japan IRB; Severance Hospital Yonsei University Health System; Nowon Eulji Medical Center, Eulji University; Seoul National University Hospital; Kangbuk Samsung Hosp.
Data Availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Reuter, U., Lucas, C., Dolezil, D. et al. Galcanezumab in Patients with Multiple Previous Migraine Preventive Medication Category Failures: Results from the Open-Label Period of the CONQUER Trial. Adv Ther 38, 5465–5483 (2021). https://doi.org/10.1007/s12325-021-01911-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01911-7