Abstract
Background
Targeted treatment of rheumatoid arthritis (RA) includes biological DMARDs (bDMARDs) and JAK inhibitors (JAKi). These agents are recommended at the same level on the basis of their efficacy and safety data. However, no local evidence of the impact of RA treatment regimens on total budget spending is available to date. This study aimed to explore the budget impact of different sequential targeted treatments in Thai patients with RA who failed at least three conventional synthetic DMARDs.
Methods
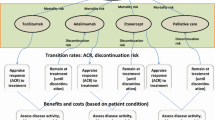
We used the adapted model to evaluate the budget impact of adding tofacitinib in different order to RA targeted treatment regimens. The Thai RA population eligible for treatment was assessed on the basis of local prevalence and experts’ opinion. Cost-impact analysis was evaluated for the treatment sequences of four different lines of targeted therapies using inputs like clinical efficacy, safety, and costs. The model used a decision tree structure with treatment nodes corresponding to treatment response outcomes for a cohort of patients. The comparisons included five bDMARDs [etanercept (ETN), infliximab (IFX), golimumab (GOL), rituximab (RTX), tocilizumab (TCZ) intravenous formulation], two JAKi [tofacitinib (TOF) and baricitinib (BAR)], and two IFX biosimilars (PF-06438179/GP1111 and CT-P13). A total of 80 treatment sequences within each containing four sequential first-, second-, third-, and fourth-line options were generated.
Results
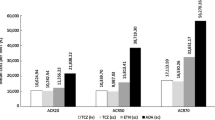
The findings of the base case scenario indicated the treatment sequence with RTX as first-line, followed by IFX biosimilar (PF-06438179/GP1111), TOF, and TCZ, respectively, produced the lowest budget impact of US $693.54 million. Sensitivity analyses confirmed the robustness of our findings.
Conclusion
The order of targeted therapy starting with RTX, then IFX biosimilar, TOF, and finally TCZ incurred the lowest budget impact over a 5-year time horizon for treating moderate to severe RA. Our findings may help payers and policy makers consider appropriate budget allocation on chronic non-communicable diseases, especially RA.


Similar content being viewed by others
References
Sparks JA. Rheumatoid arthritis. Ann Intern Med. 2019;170(1):ITC1–16.
Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78(11):1463–71.
Taylor PC, Moore A, Vasilescu R, Alvir J, Tarallo M. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int. 2016;36(5):685–95.
Lau CS, Chia F, Harrison A, et al. APLAR rheumatoid arthritis treatment recommendations. Int J Rheum Dis. 2015;18(7):685–713.
Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26.
Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99.
Thai Rheumatism Association. 2010 Guideline for Biological Therapy in Rheumatoid Arthritis. (Thai language) [Internet] Available at: https://www.thairheumatology.org/wp-content/uploads/2016/07/guideline-biologics-final-17-dec-10.pdf. Accessed 10 Aug 2020.
Claxton L, Jenks M, Taylor M, et al. An economic evaluation of tofacitinib treatment in rheumatoid arthritis: modeling the cost of treatment strategies in the United States. J Manag Care Spec Pharm. 2016;22(9):1088–102.
Narongroeknawin P, Chevaisrakul P, Kasitanon N, et al. Drug survival and reasons for discontinuation of the first biological disease modifying antirheumatic drugs in Thai patients with rheumatoid arthritis: analysis from the Thai Rheumatic Disease Prior Authorization registry. Int J Rheum Dis. 2018;21:169–77.
Kamal KM, Madhavan SS, Hornsby JA, Miller LA, Kavookjian J, Scott V. Use of tumor necrosis factor inhibitors in rheumatoid arthritis: a national survey of practicing United States rheumatologists. Jt Bone Spine. 2006;73(6):718–24.
Hazlewood GS, Barnabe C, Tomlinson G, Marshall D, Devoe D, Bombardier C. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta-analysis. BMJ. 2016;353:i1777.
Drug and Medical Supply Information Center, Ministry of Public Health, Thailand. [Internet] Available at: http://dmsic.moph.go.th/index/dataservice/90/0. Accessed 26 Aug 2020.
PPP conversion factor, GDP (LCU per international $)—Thailand | Data (worldbank.org) https://data.worldbank.org/indicator/PA.NUS.PPP?locations=TH. Accessed 8 Mar 2021.
Alten R, Batko B, Hala T, et al. Randomised, double-blind, phase III study comparing the infliximab biosimilar, PF-06438179/GP1111, with reference infliximab: efficacy, safety and immunogenicity from week 30 to week 54. RMD Open. 2019;5(1):e000876.
Emery P, Deodhar A, Rigby WF, et al. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab’s Efficacy in MTX iNadequate rEsponders (SERENE)). Ann Rheum Dis. 2010;69(9):1629–35.
Keystone EC, Genovese MC, Klareskog L, et al. Golimumab, a human antibody to tumour necrosis factor alpha given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis. 2009;68(6):789–96.
Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354(9194):1932–9.
Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371(9617):987–97.
van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65(3):559–70.
Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340(4):253–9.
Wells AF, Greenwald M, Bradley JD, Alam J, Arora V, Kartman CE. Baricitinib in patients with rheumatoid arthritis and an inadequate response to conventional disease-modifying antirheumatic drugs in united states and rest of world: a subset analysis. Rheumatol Ther. 2018;5(1):43–55.
Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72(10):1613–20.
Cohen SB, Radominski SC, Kameda H, et al. Long-term efficacy, safety, and immunogenicity of the infliximab (IFX) biosimilar, PF-06438179/GP1111, in patients with rheumatoid arthritis after switching from reference IFX or continuing biosimilar therapy: week 54–78 data from a randomized, double-blind, phase III trial. BioDrugs. 2020;34(2):197–207.
Genovese M, Smolen J, Takeuchi T, et al. Safety profile of baricitinib for the treatment of rheumatoid arthritis up to 7 years: an updated integrated safety analysis 2019 ACR/ARP annual meeting. Arthritis Rheumatol. 2019;71(suppl 10):1461–3.
Keystone EC, Genovese MC, Hall S, et al. Safety and efficacy of subcutaneous golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: final 5-year results of the GO-FORWARD trial. J Rheumatol. 2016;43(2):298–306.
Urata Y, Abe S, Denvers B, Nakamura Y, Takemoto H, Furukawa K-I. OP0104-Tocilizumab: dose reduction or interval spacing—which proves a better tapering strategy for rheumatoid arthritis in clinical remission?. EULAR. Ann Rheum Dis. 2017;76:95.
van Vollenhoven RF, Fleischmann RM, Furst DE, Lacey S, Lehane PB. Longterm safety of rituximab: final report of the rheumatoid arthritis global clinical trial program over 11 years. J Rheumatol. 2015;42(10):1761–6.
Vander Cruyssen B, Durez P, Westhovens R, De Keyser F. Seven-year follow-up of infliximab therapy in rheumatoid arthritis patients with severe long-standing refractory disease: attrition rate and evolution of disease activity. Arthritis Res Ther. 2010;12(3):R77.
Weinblatt ME, Bathon JM, Kremer JM, et al. Safety and efficacy of etanercept beyond 10 years of therapy in North American patients with early and longstanding rheumatoid arthritis. Arthritis Care Res (Hoboken). 2011;63(3):373–82.
Wollenhaupt J, Lee EB, Curtis JR, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther. 2019;21(1):89.
Yoo DH, Prodanovic N, Jaworski J, et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis. 2017;76(2):355–63.
Institute of Clinical and Economic Review. Targeted immune modulators for rheumatoid arthritis: effectiveness and value. ICER. 2017. http://icerorg.wpengine.com/wp-content/uploads/2020/10/NE_CEPAC_RA_Evidence_Report_FINAL_040717.pdf. Accessed 5 May 2021.
Malottki K, Barton P, Tsourapas A, et al. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor: a systematic review and economic evaluation. Health Technol Assess 2011;15:1–278.
Patel D, Shelbaya A, Cheung R, Aggarwal J, Park SH, Coindreau J. Cost-effectiveness of early treatment with originator biologics or their biosimilars after methotrexate failure in patients with established rheumatoid arthritis. Adv Ther. 2019;36(8):2086–95.
Chaiamnuay P, Darmawan J, Muirden KD, Assawatanabodee P. Epidemiology of rheumatic disease in rural Thailand: a WHO-ILAR COPCORD study. Community oriented programme for the control of rheumatic disease. J Rheumatol. 1998;25(7):1382–7.
Acknowledgements
The authors would like to acknowledge Ms. Vaidehi Wadhwa (Senior Scientific Communications Specialist, Medical Excellence, Emerging Markets, Pfizer) for her medical writing and editorial support for developing this manuscript.
Funding
The study process and the Rapid Service Fee were funded by Pfizer (Thailand) Limited. The funder has no role in the data interpretation, results presentation, and discussion parts of this study.
Disclosures
Prof. Manathip Osiri receives honoraria from Novartis, Pfizer, Sandoz, Viatris and Zuellig Pharma. Piyameth Dilokthornsaku received an honorarium from Pfizer (Thailand) according to this project. Sasitorn Chokboonpium is a Pfizer employee. Pichaya Suthipinijtham is an ex Pfizer employee (during this study developed, the one who created the model for simulation) and a current Boehringer-Ingelheim employee. Ajchara Koolvisoot has nothing to disclose.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
All the authors have substantially contributed in development of the manuscript by providing critical insights. Study concept and design: MO, AK, SC, PS. Model generation and adaptation: PS, MO, AK, SC, PD. Cost data acquisition: MO, AK. Statistical analysis: PS, PD, SC. Manuscript drafting: MO, AK, SC, PS, PD. Manuscript editing and review: MO, AK, PD, SC.
Compliance with Ethics Guidelines
The IRB review was waived for this study as it involved in open data from published RCTs and costs input from tertiary care hospitals in Bangkok, Thailand, which can be accessible from general search engines. There was no contact, interview, survey, specimens taken from real patients in this study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author or author S Chokboonpium (Sasitorn.Chokboonpium@pfizer.com) on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Osiri, M., Dilokthornsakul, P., Chokboonpium, S. et al. Budget Impact of Sequential Treatment with Biologics, Biosimilars, and Targeted Synthetic Disease-Modifying Antirheumatic Drugs in Thai Patients with Rheumatoid Arthritis. Adv Ther 38, 4885–4899 (2021). https://doi.org/10.1007/s12325-021-01867-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01867-8