Abstract
Most patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) have exhausted their treatment options and are deemed palliative. CD19-directed chimeric antigen receptor (CAR) T-cell therapy has recently been introduced as a new option for these patients. Lisocabtagene maraleucel (liso-cel) is an investigational CAR T-cell therapy that has shown promising activity in this setting. We used an unanchored matching-adjusted indirect comparison (MAIC) methodology to compare liso-cel, using individual patient-level data from the TRANSCEND NHL 001 (TRANSCEND; NCT02631044) trial, to salvage chemotherapy, using summary-level data from the SCHOLAR-1 study, for the treatment of patients with R/R LBCL. Standardized mean differences were used to evaluate imbalances between the TRANSCEND and SCHOLAR-1 studies. MAIC was conducted to determine the relative efficacy of liso-cel vs. salvage chemotherapy with regard to overall survival, complete response rate, and objective response rate. For all efficacy outcomes assessed, comparisons of clinical factors before MAIC showed that five of seven baseline characteristics were similar between the TRANSCEND and SCHOLAR-1 studies; however, age and R/R to last therapy status differed between studies, thus requiring matching and adjusting to ensure the validity of this analysis. The base case analyses demonstrated a significantly lower risk of mortality (hazard ratio, 0.5; 95% confidence interval [CI] 0.4–0.6; p < 0.001) with significantly higher rates of complete response (odds ratio, 12.9; 95% CI 8.0–20.7) and objective response (odds ratio, 7.0; 95% CI 4.6–10.5) for patients treated with liso-cel than patients treated with salvage chemotherapy. MAIC comparisons demonstrated favorable efficacy for liso-cel compared with salvage chemotherapy in the treatment of patients with R/R LBCL.
Trial Registration ClinicalTrials.gov identifier: NCT02631044.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Treatment options are limited for patients with third-line or later relapsed/refractory (R/R) diffuse large B-cell lymphoma (LBCL), and chimeric antigen receptor (CAR) T-cell therapy has shown promise for treating this patient population. |
Comparative effectiveness of the CAR T-cell therapy lisocabtagene maraleucel (liso-cel) to salvage chemotherapy in R/R LBCL has not yet been established. |
Unanchored matching-adjusted indirect comparison (MAIC) analyses, which are commonly used to compare interventions across studies, can reduce many biases associated with naive comparisons between trials by adjusting for differences in patient and study characteristics. |
What was learned from this study? |
For all efficacy outcomes, comparisons of clinical characteristics before MAIC showed that most factors were balanced between TRANSCEND NHL 001 and SCHOLAR-1, though notable differences were observed for age and R/R to last therapy status. |
Following matching/adjusting, an indirect treatment comparison showed favorable efficacy for liso-cel vs. salvage chemotherapy. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.14452953.
Introduction
Non-Hodgkin lymphoma (NHL) is one of the most common forms of cancer worldwide, with incidence rates of 6.7 and 4.7 per 100,000 male and female individuals, respectively [1]. Approximately 85–90% of all NHL cases are categorized as B-cell lymphomas, which include both aggressive and indolent forms [2]. Diffuse large B-cell lymphomas (DLBCLs) represent the most common NHL subtype worldwide, accounting for about 30% of all adult NHL cases. Additional aggressive large B-cell lymphoma (LBCL) subtypes include high-grade B-cell lymphoma (HGBCL) with MYC and BCL2 and/or BCL6 rearrangements, follicular lymphoma grade 3B (FL3B), and primary mediastinal B-cell lymphoma (PMBCL). Although first-line standard of care (SOC) treatment with rituximab in combination with an anthracycline-based chemotherapy regimen is curative in > 50% of patients with LBCL [3], 10–15% of patients are refractory and about one-third relapse after responding to their initial treatment [4,5,6,7]. Patients with relapsed or refractory (R/R) LBCL often have a poor clinical prognosis [8].
Chimeric antigen receptor (CAR) T-cell therapy, which involves modifying a patient’s own T cells to recognize the cancer, is a new treatment option that has shown promise for patients with R/R LBCL. Three CD19-directed CAR T-cell therapies are currently approved by the US Food and Drug Administration for patients with R/R DLBCL: axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel (liso-cel). Liso-cel, a CD19-directed, defined composition, 4-1BB CAR T-cell product administered at equal target doses of CD8+ and CD4+ CAR+ T cells, was studied in the phase 1, single-arm TRANSCEND NHL 001 trial (TRANSCEND). Of 344 patients who underwent leukapheresis, 269 received liso-cel infusion, and 256 patients formed the efficacy analysis set [9]. Based on independent review committee assessment, the objective response rate (ORR) was 73% (95% confidence interval [CI] 67–78), and the complete response rate (CRR) was 53% (95% CI 47–59) with a median study follow-up of 12 months (range 0–35 months); duration of response at 12 months was 55% (95% CI 46.7–62.0). Median progression-free survival was 6.8 months (95% CI 3.3–14.1), and median overall survival was 21.1 months (95% CI 13.3–not reached) [9].
Assessing comparative effectiveness of CAR T-cell therapy and chemotherapy is difficult without head-to-head randomized controlled trials. Naive, indirect comparisons may be inappropriate, as the study designs and patients included could be heterogeneous, thus invalidating any conclusions drawn. Unanchored matching-adjusted indirect comparison (MAIC) analyses, which are commonly used to compare interventions across studies, can reduce many biases associated with naive comparisons between trials by adjusting for differences in patient and study characteristics. MAICs are increasingly being included in submissions to reimbursement agencies, such as the National Institute for Health and Care Excellence [10]. This study used MAIC to compare efficacy of liso-cel from the TRANSCEND trial to salvage chemotherapy from the SCHOLAR-1 study in the treatment of patients with R/R LBCL.
Methods
Data Sources
Individual patient-level data (IPD) for liso-cel–infused efficacy analysis set from the TRANSCEND trial were used for this analysis, with a data cutoff of August 12, 2019. TRANSCEND (ClinicalTrials.gov identifier: NCT02631044) was a phase 1, single-arm, multicenter, open-label trial that sought to investigate the efficacy and safety of liso-cel in patients with third-line or later R/R LBCL [9]. TRANSCEND was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice guidelines, and applicable regulatory requirements. Institutional review boards at participating institutions approved the study protocol and amendments. All patients provided written informed consent.
Summary-level data (SLD) for salvage chemotherapy were derived from the SCHOLAR-1 study [8]. SCHOLAR-1 was a retrospective database study consisting of patients from four sources: two real-world databases and two follow-up phase 3 randomized-controlled trials. A systematic literature review identified the SCHOLAR-1 study as a key publication evaluating salvage chemotherapies in the treatment of refractory large B-cell NHL, as the database included a relatively large cohort of patients and a broad range of salvage chemotherapy regimens commonly used in real-world clinical settings (Table 1). SCHOLAR-1 was the first and largest patient-level pooled retrospective analysis to characterize response rates and survival in patients with refractory large B-cell NHL, which potentially reduced bias relative to having used individual prospective or retrospective studies.
Study Comparisons
Patient characteristics from the TRANSCEND and SCHOLAR-1 studies were sufficiently compatible to enable application of MAIC methodology using these studies (Table 1). Overall, seven baseline characteristics (NHL subtype, sex, age, prior autologous hematopoietic stem cell transplantation [HSCT], R/R status to last therapy, disease stage, and International Prognostic Index [IPI] score) were reported in both TRANSCEND and SCHOLAR-1. Slight differences in the categorization of variables and extent of missing data were noted between studies for NHL subtype and IPI score (Table 2). Those differences were adjusted by reclassifying variables or collapsing variables into a narrower set of categories. For covariates with missing values, the proportion of patients in each non-missing category was recalculated based on the remaining total number of patients after excluding patients from the missing category. For example, in SCHOLAR-1, 49% of patients had an IPI score of 0–2 and 18% had a missing IPI score (Table 2). After removing patients with missing IPI scores, 59.8% (49% of 82% of patients with non-missing IPI scores) were determined to have an IPI score of 0–2 (Table 3). Patients were removed from the TRANSCEND data set if data were missing for any of the seven clinical factors reported in SCHOLAR-1. As a result, eight patients with missing/indeterminate values were removed from the TRANSCEND data set, leaving 248 patients remaining for efficacy analysis (Table 3).
Standardized mean differences (SMDs) were used to describe imbalances among baseline patient characteristics between TRANSCEND and SCHOLAR-1. Typically, SMDs exceeding 0.10 are indicative of statistically meaningful imbalances [11, 12].
Efficacy outcomes included OS, CRR, and ORR. While the definition of OS was the same between the TRANSCEND and SCHOLAR-1 studies, disease assessment criteria differed for CRR and ORR: SCHOLAR-1 used the revised International Working Group 1999 guidelines [13], whereas TRANSCEND used the Lugano classification [14]. Investigator-assessed CRR and ORR were reported in SCHOLAR-1, whereas both investigator- and independent review committee–assessed responses were reported in TRANSCEND. Thus, investigator-assessed CRR and ORR were used to compare response rates between liso-cel and salvage chemotherapy. Progression-free survival was not reported in SCHOLAR-1 and was therefore not included in this analysis. The base case analysis of efficacy outcomes included seven clinical factors: age, disease histology, disease stage, IPI score, prior autologous HSCT, relapsed or refractory status to last therapy, and sex. No comparisons of safety outcomes were performed in this analysis because salvage chemotherapy interventions are associated with a significant degree of hematologic toxicities, infections, and withdrawals due to adverse events, whereas CAR T-cell therapies are associated with cytokine release syndrome and neurotoxicity, which often require pharmacologic interventions, cytopenias, and infections. Additionally, SCHOLAR-1 did not report safety outcomes, which further prohibited any comparison between the two treatment modalities.
Statistical Analysis
In the absence of a common comparator, unanchored MAICs were conducted to determine the relative efficacy of liso-cel vs. salvage chemotherapy with regard to OS, CRR, and ORR [10]. Indirect treatment comparisons were formed by “matching” and “adjusting” patients from TRANSCEND to the marginal distribution (e.g., mean and variance) of clinical factors in patients from SCHOLAR-1 who received salvage chemotherapy. Specifically, patients from TRANSCEND were adjusted using inverse probability of treatment weights as obtained from method-of-moments propensity score estimation. Method of moments was chosen to accommodate the availability of only SLD for SCHOLAR-1 and because it guarantees an exact balancing of clinical factors between the interventions of interest. After matching and adjusting patients from TRANSCEND, predictions of the outcome of interest may be viewed as an estimate of the outcome that would have occurred had the comparison population (from SCHOLAR-1) received liso-cel instead of salvage chemotherapy. Point estimates for liso-cel vs. salvage chemotherapy were derived as the difference between this prediction and the estimated outcome using published SLD from the comparator trials.
For CRR and ORR, after matching patients from TRANSCEND to patients from SCHOLAR-1, estimates were derived by fitting an intercept-only logistic regression model with MAIC adjustment weights. The estimated intercept represents a prediction of the log odds of the outcome of interest if a typical patient from SCHOLAR-1 had received liso-cel. Robust standard errors were estimated using the sandwich estimator [15]. An estimate of the log odds ratio (OR) for liso-cel vs. salvage chemotherapy was then derived by taking the difference between this predicted log odds and the log odds based on SLD from SCHOLAR-1. The variance of the log OR between liso-cel vs. salvage chemotherapy was estimated as the sum of the variance of the log odds for each treatment included in the comparison.
For the time-to-event end point of OS, weighted IPDs from TRANSCEND were combined with pseudo-IPD (setting weights for pseudo-observations equal to 1) that represented patients from SCHOLAR-1. This data set was then used to fit a weighted Cox proportional hazard regression with a binary treatment indicator (i.e., liso-cel vs. salvage chemotherapy). The estimated regression coefficient for the treatment indicator was used to represent the log hazard ratio (HR) for liso-cel vs. salvage chemotherapy. OS pseudo-IPDs for salvage chemotherapy were derived by numerical inversion of digitized survival curves from SCHOLAR-1 [16].
All analyses were conducted using R software (R Core Team, Vienna, Austria; https://www.r-project.org/), largely based on the code outlined in the National Institute for Health and Care Excellence Evidence Synthesis Technical Support Document Series [10, 17].
Results
Comparison of Baseline Characteristics Before and After Matching and Adjusting
For all efficacy outcomes, comparisons of clinical characteristics before MAIC showed that most factors were balanced (5 of 7 clinical factors with SMDs < 0.1) between TRANSCEND and SCHOLAR-1 (Table 3). Notable differences were observed for age and R/R to last therapy status. After matching and adjusting patients from TRANSCEND to the SCHOLAR-1 population, all clinical factors showed a high degree of balance (SMD < 0.001 for all factors).
Response Rates
Overall, the results of the MAIC analyses showed significantly higher response rates for patients in TRANSCEND compared with SCHOLAR-1 (Table 4). Unadjusted ORR was higher for patients in TRANSCEND (73%) vs. SCHOLAR-1 (26%). The base case analysis that adjusted for seven clinical factors (effective sample size [ESS] of 142 for liso-cel) showed that the adjusted ORR for patients receiving liso-cel was 71%, and the matched and adjusted treatment effect for ORR also showed a statistically significant advantage for patients in TRANSCEND compared with SCHOLAR-1 (OR, 7.0; 95% CI 4.64–10.54; p < 0.001). Unadjusted CRR was higher for patients in TRANSCEND (52%) vs. SCHOLAR (7%). In the base case analysis that adjusted for the seven clinical factors, the adjusted CRR for liso-cel was 49% and was associated with increased odds of response compared with SCHOLAR-1 (OR, 12.9; 95% CI 8.0–20.7; p < 0.001).
Note that the mean and standard deviation of age for the SCHOLAR-1 population was imputed using the reported median and range. Adjustment for the proportion of patients aged ≥ 65 years, instead of the mean and standard deviation of age, generated similar effect estimates for CRR (OR, 12.6; 95% CI 8.0–19.8; p < 0.001; ESS = 167) and ORR (OR, 6.3; 95% CI 4.2–9.2; p < 0.001; ESS = 167). Furthermore, scenario analyses for response outcomes were conducted by iteratively removing one clinical factor at a time in reverse order of clinical importance. The results of each scenario analysis were similar to the base case analyses. For CRR, the OR ranged from 12.9 (95% CI 8.0–20.7) to 13.8 (95% CI 9.0–21.1) across scenarios. For ORR, the OR ranged from 6.9 (95% CI 4.6–10.4) to 7.5 (95% CI 5.3–10.7) across scenarios.
Overall Survival
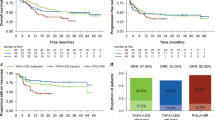
Before conducting the MAIC, unadjusted median OS was 21.1 months (95% CI 13.3–not reached) for patients in TRANSCEND (N = 256) compared with 6.0 months (95% CI 5.6–6.8) for SCHOLAR-1 (N = 603), with the latter estimates derived from digitized Kaplan-Meier curves. The naive, unadjusted HR indicated a significantly lower rate of mortality for patients in TRANSCEND compared with SCHOLAR-1 (HR 0.4; 95% CI 0.4–0.5; p < 0.001). In the base case analysis that adjusted for seven clinical factors, median OS for patients in TRANSCEND was 20.5 months (95% CI 12.1–not reached), with an ESS of 142. The matched and adjusted treatment effect again showed a significantly lower rate of mortality for patients in TRANSCEND compared with SCHOLAR-1 (HR 0.5; 95% CI 0.4–0.6; p < 0.001). A sensitivity analysis adjusting for the reported proportion of patients aged ≥ 65 years, instead of the imputed mean and standard deviation of age, generated similar effect estimates (HR 0.46; 95% CI 0.36–0.59, p < 0.001; ESS = 167). After matching and adjusting, Kaplan-Meier estimates showed consistently increased survival probabilities through 3 years of follow-up for patients in TRANSCEND compared with SCHOLAR-1 (Fig. 1). Note that the small number of patients in the remaining risk set post-18 months contributed to notable drops in the Kaplan-Meier curves for TRANSCEND after 18 months.
Kaplan-Meier curve of overall survival comparison between the TRANSCEND and SCHOLAR-1 studies from MAIC. Both before and after matching and adjusting, Kaplan-Meier estimates showed consistently increased survival probabilities (hazard ratio [HR] 0.4; 95% CI 0.4–0.5; p < 0.001 and HR 0.5; 95% CI 0.4–0.6; p < 0.001, respectively) through 3 years of follow-up for liso-cel compared with salvage chemotherapy. aP values for the naive and base case comparisons of each outcome were based on two-tailed t tests of the null hypothesis of no difference in the log hazard of mortality
Sensitivity Analysis
A sensitivity analysis was conducted using all patients who underwent leukapheresis (n = 344). This comparison adjusted for five of the seven characteristics used in the base case MAIC, as two factors (IPI score and disease stage) were missing for 77 of 344 patients who received leukapheresis. Compared to the base case MAIC, this analysis had a greater ESS (209) and numerically smaller, but statistically significant, point estimates of relative efficacy for CRR (OR, 8.6; 95% CI 5.5–13.3; p < 0.001), ORR (OR, 3.6; 95% CI 2.6–5.1; p < 0.001), and OS (HR 0.6; 95% CI 0.5–0.7; p < 0.001).
Discussion
The initial feasibility assessment that identified three outcomes (OS, CRR, and ORR) and seven covariates that were reported in both TRANSCEND and SCHOLAR-1 showed that imbalances between studies were small to moderate. Therefore, it was determined that MAIC would achieve reductions in between-study imbalances without significant loss in ESS. The base case analysis that adjusted for all seven covariates reported in both studies showed a significantly favorable efficacy profile for TRANSCEND compared with SCHOLAR-1. Notably, patients in TRANSCEND achieved significantly higher ORR and CRR and significantly longer OS compared with patients in SCHOLAR-1. The base case analysis for patients in TRANSCEND vs. SCHOLAR-1 had an OR of 7.0 (95% CI 4.7–10.5; p < 0.001) for the ORR and an OR of 12.9 (95% CI 8.0–20.7; p < 0.001) for the CRR. For OS, the base case analysis showed a significantly lower risk of mortality for patients in TRANSCEND vs. SCHOLAR-1 (HR 0.5; 95% CI 0.4–0.6; p < 0.001). Results from the sensitivity analysis were similar to those from the base case analysis favoring liso-cel compared with salvage chemotherapy.
TRANSCEND was a single-arm trial as no standard of care was available at the time of protocol writing. The absence of a common comparator between TRANSCEND and SCHOLAR-1 necessitated use of this unanchored MAIC. Unanchored MAICs have several limitations, particularly that they require adjustment for all clinically relevant prognostic factors and treatment effect modifiers [10]. MAICs typically adjust to reported baseline characteristics for the enrolled population, which is not always the same as the efficacy evaluable set. This may be considered a general limitation of MAIC methodology that depends on summary level data. Specific to this study, due to a lack of reporting of baseline characteristics in SCHOLAR-1, it was only possible to adjust for seven covariates in this MAIC. It was not possible to adjust for Eastern Cooperative Oncology Group performance status, lactate dehydrogenase levels, or prior lines of therapy, since the ability to match patients from TRANSCEND to the SCHOLAR-1 population before adjustment was limited by a possible lack of reporting of eligibility criteria, lack of reporting patient or disease characteristics, or abstraction guidelines in the SCHOLAR-1 publication. However, it is worth noting that both Eastern Cooperative Oncology Group performance status and lactate dehydrogenase levels were indirectly reflected in the adjustment through the composite score IPI. It was also not possible to compare progression-free survival since those data were not reported in SCHOLAR-1. In addition, response assessment criteria differed between SCHOLAR-1 and TRANSCEND. Although it is virtually impossible to match and adjust for all known or unknown differences in prognostic factors and assessment criteria, it is reassuring that similar efficacy results were observed across outcomes of interest before and after adjusting for the seven baseline characteristics as well as in scenario analyses. Nevertheless, a strength of the analysis was the large number of patients in TRANSCEND that contributed to the IPD, as the ability to adjust for multiple baseline factors is dependent on having a sufficient number of patients [18]. Because of the limited follow-up beyond 24 months for TRANSCEND at the time of this analysis, conclusions cannot be made for longer time points.
Notably, a comparison between axicabtagene ciloleucel, using the pivotal phase 2 ZUMA-1 study, and salvage chemotherapy, using SCHOLAR-1, was recently performed, although the methodology used to reduce between trial differences differs from what was employed in this study [19]. The authors adjusted only for refractory status, leaving age as an unadjusted factor. In the comparison of ZUMA-1 and SCHOLAR-1 analysis, potential imbalances between studies were addressed by equally weighting the proportions of patients through use of refractory categorization and receipt of post-refractory HSCT. Furthermore, the authors included a subset of chemotherapy-treated patients than was originally reported in the SCHOLAR-1 primary publication. In contrast, the results of this study leveraged the entire SCHOLAR-1 cohort, which may be more representative of patients eligible for salvage chemotherapy regimens. The standardized ORR and CRR for patients in ZUMA-1 were higher than in SCHOLAR-1 (Cochran-Mantel–Haenszel test p < 0.0001 for both). The standardized 2-year OS rate was higher for ZUMA-1 vs. SCHOLAR-1, which represented a 73% reduction in the risk of death with axicabtagene ciloleucel compared with salvage chemotherapy (p < 0.0001). These results, which are similar to what this analysis found, confirm the potential use of CAR T-cell therapy for patients with DLBCL as preferable to salvage chemotherapy.
Conclusion
In conclusion, this analysis presents a comparison of the efficacy of liso-cel with salvage chemotherapy in the treatment of R/R LBCL. Adjusted comparisons, which accounted for between-study differences in eligibility criteria and baseline characteristics, demonstrated favorable efficacy for liso-cel compared with salvage chemotherapy in this treatment setting. Overall, these results provide consistent evidence supporting the effectiveness of liso-cel for the treatment of patients with R/R LBCL.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, et al.: SEER cancer statistics review (CSR) 1975–2017. 2020. https://seer.cancer.gov/csr/1975_2017/. Accessed 20 July 2020.
Chao MP. Treatment challenges in the management of relapsed or refractory non-Hodgkin’s lymphoma—novel and emerging therapies. Cancer Manag Res. 2013;5:251–69.
Chaganti S, Illidge T, Barrington S, McKay P, Linton K, Cwynarski K, et al. Guidelines for the management of diffuse large B-cell lymphoma. Br J Haematol. 2016;174(1):43–56.
Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v116–v25.
Gonzalez-Barca E, Coronado M, Martin A, Montalban C, Montes-Moreno S, Panizo C, et al. Spanish Lymphoma Group (GELTAMO) guidelines for the diagnosis, staging, treatment, and follow-up of diffuse large B-cell lymphoma. Oncotarget. 2018;9(64):32383–99.
National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). B-cell lymphomas. 2020. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf. Version 4.2020. Accessed 12 Jan 2021.
Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–8.
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–52.
Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Mak. 2018;38(2):200–11.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107.
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–79.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–86.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68.
White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48(4):817–38.
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9.
National Institute for Health and Care Excellence (NICE) Decision Support Unit: Population-adjusted indirect comparisons (MAIC and STC). 2016. http://nicedsu.org.uk/technical-support-documents/population-adjusted-indirect-comparisons-maic-and-stc/. Accessed 20 July 2020.
Signorovitch JE, Sikirica V, Erder MH, Xie J, Lu M, Hodgkins PS, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–7.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Reagan PM, Miklos DB, et al. A comparison of 2-year outcomes in ZUMA-1 (axicabtagene ciloleucel [axi-cel]) and SCHOLAR-1 in patients (pts) with refractory large B cell lymphoma (LBCL). Biol Blood Marrow Transplant. 2020;26(suppl 3):S232.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Acknowledgements
Funding
Funding for this study and the journal’s Open Access and Rapid Service fees were provided by Bristol Myers Squibb (Princeton, NJ).
Medical Writing and Editorial Assistance
Medical writing and/or editorial assistance was provided by Meredith Rogers, MS, CMPP, and Jeremy Henriques, PhD, of The Lockwood Group (Stamford, CT), funded by Bristol Myers Squibb.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Paul Spin and Fei Fei Liu. The first draft of the manuscript was written by Paul Spin, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Gilles Salles reports consultancy fees from AbbVie, Allogene, Autolus Therapeutics, BeiGene, Celgene, a Bristol-Myers Squibb Company, Debiopharm, Genmab, Janssen, Kite Pharma, a subsidiary of Gilead Sciences, Miltenyi Biotec, MorphoSys, Novartis, Roche, and Velosbio and financial compensation for educational events from AbbVie, Celgene, a Bristol-Myers Squibb Company, Gilead Sciences, Janssen, Kite Pharma, a subsidiary of Gilead Sciences, MorphoSys, Novartis, and Roche. Paul Spin is an employee of EVERSANA. Fei Fei Liu and Yeonhee Kim are employees of Bristol Myers Squibb and may own stock in Bristol Myers Squibb. Jacob Garcia was an employee of Bristol Myers Squibb at the time of this analysis and may own stock in Bristol Myers Squibb; he is currently employed by Umoja Biopharma (Seattle, WA). Jens Hasskarl is an employee of Celgene, a Bristol-Myers Squibb Company, and may own stock in Bristol Myers Squibb.
Compliance with Ethics Guidelines
TRANSCEND was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice guidelines, and applicable regulatory requirements. Institutional review boards at participating institutions approved the study protocol and amendments. All patients provided written informed consent. The authors had permission to use data from TRANSCEND for this analysis.
Data Availability
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Garcia: Affiliation at the time the research was conducted.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Salles, G., Spin, P., Liu, F.F. et al. Indirect Treatment Comparison of Liso-Cel vs. Salvage Chemotherapy in Diffuse Large B-Cell Lymphoma: TRANSCEND vs. SCHOLAR-1. Adv Ther 38, 3266–3280 (2021). https://doi.org/10.1007/s12325-021-01756-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01756-0