Abstract
Purpose
To evaluate morpho-functional outcomes of the intravitreal fluocinolone acetonide (FAc) implant.
Methods
Retrospective, observational, single-center study. Primary endpoint was the mean change in central macular thickness (CMT) from baseline to month 1–3. Secondary endpoints included mean CMT change from baseline to month 4–8 and 9–14 and mean best corrected visual acuity (BCVA), photopic negative response (PhNR) and b-wave of flash full-field electroretinogram (ERG) changes from baseline to month 1–3, 4–8, and 9–14.
Results
Fourteen patients (18 eyes) were included. Mean (standard deviation) CMT decreased from 473 (196) µm at baseline to 371 (163) µm at month 1–3 (mean difference − 102.3 ± 98.35 µm, 95% CI ± 46.4 µm; p < 0.0001) and this decrease tended to endure up to month 9–14. BCVA did not change significantly. There was an improvement in mean PhNR amplitude from 2.76 (1.65) µV at baseline to 3.73 (2.32) µV at month 1–3 (mean difference 0.91 (1.14) µV, 95% CI ± 0.54 µV, p = 0.003); b-wave amplitude improved from 8.83 (4.52) µV at baseline versus 10.05 (5.04) µV at month 1–3 (mean difference 1.22 (2.23) µV, 95% CI ± 1.08 µV, p = 0.0384). These ERG positive changes tended to endure up to month 9–14, although they did not reach statistical significance after month 3.
Conclusions
Intravitreal FAc implant significantly improved anatomic as well as functional outcomes related to middle and inner retinal layers, known to be altered in diabetic retinopathy. Our findings support the hypothesis that intravitreal FAc implant may exert a protective effect in diabetic retinas with diabetic macular edema.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Diabetic macular edema (DME) is accountable for most of the vision loss in patients with diabetes. The intravitreal 0.19 mg fluocinolone acetonide (FAc) implant releases a steady dose of drug for a period of up to 36 months, thus providing a long-term control of macular edema. |
Intravitreal FAc implant significantly improved anatomic as well as functional outcomes related to middle and inner retinal layers. Our findings support the hypothesis of a potential protective effect of FAc implant in retinas with DME. |
Digital Features
This article is published with digital features, including a summary slide to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14414594.
Introduction
In recent decades, we have faced a tremendous increase in the global prevalence of diabetes mellitus, which is expected to increase up to 592 million people by 2035 [1]. Diabetic retinopathy (DR) affects approximately one-third of patients with diabetes and a further one-third of these are affected by vision-threatening DR. Proliferative diabetic retinopathy is the most common vision-threatening lesion; however, diabetic macular edema (DME) is accountable for most of the vision loss [2,3,4].
DME is a consequence of DR and can be present at any stage of retinopathy [4]; it is caused by the rupture of the blood-retinal barrier due to a range of metabolic changes, brought on by hyperglycemia [5]. It appears as a retinal thickening caused by intraretinal fluid accumulation, especially in the outer (OPL) and inner plexiform layers (IPL) [4]. Several treatments for DME have been developed and approved for use, such as laser photocoagulation [6], subthreshold micropulse laser [7, 8], intravitreal administration of anti-vascular endothelial growth factor (anti-VEGF) [9], and sustained release corticosteroids implants [10, 11].
The intravitreal 0.19 mg fluocinolone acetonide (FAc) implant (ILUVIEN® Alimera Sciences Europe Ltd.) has been approved for the treatment of DME. It releases a steady, low dose of corticosteroid (0.2 μg/day) for a period up to 36 months [12]; thus a single injection provides stable and prolonged control of macular edema and reduces the economic and clinical impact of intermittent and repeated injections [13, 14]. Preclinical studies [15, 16] in animal models of retinal degeneration as well as clinical studies [17] in patients with DME have suggested that the intravitreal FAc implant may have a neuroprotective effect on the retina.
The primary aim of our study was to assess, retrospectively, the morpho-functional effect of the intravitreal FAc implant, by assessing best corrected visual acuity (BCVA), central macular thickness (CMT), photopic negative response (PhNR) and b-wave of the electroretinogram (ERG), generated by the inner and middle retinal layers, respectively, and known to be altered in DR [18]. The secondary aim, due to the scarcity of published data in this area, was to investigate positioning and mobility of the FAc implant into the vitreous body, by using B-scan ophthalmic ultrasound.
Methods
We retrospectively studied 18 eyes from 14 consecutive patients (age 64–91 years; six women, eight men) with diabetes mellitus, treated with the intravitreal FAc implant for chronic DME. All patients provided signed informed consent and were observed between 12 June 2018 and 15 May 2020 at the Ophthalmology Department of Fondazione Policlinico Universitario A. Gemelli IRCCS of Rome, Italy. This retrospective study adhered to the tenets of the Declaration of Helsinki and was approved by the ethics committee of Fondazione Policlinico Universitario A. Gemelli IRCCS (11 November 2020, ID 3166).
Inclusion criteria were age 18 years or older, a clinical and instrumental diagnosis of chronic DME, pseudophakia, previous panretinal laser photocoagulation and/or intravitreal injections of anti-VEGF and/or dexamethasone, FAc intravitreal implantation between 12 June 2018 and 14 February 2020, according to the drug reimbursability indication in the country of study.
Exclusion criteria were refusal to sign the informed consent, uncontrolled elevation of intraocular pressure (IOP), and retinal or choroidal disease other than DR that could affect CMT, ERG, and/or BCVA.
The washout period, before FAc implantation, was at least 3 months, regardless of the intravitreal drug previously administered (anti-VEGF or sustained-release dexamethasone implant).
In all patients, no focal laser and/or panretinal laser photocoagulation was performed in the 6 months prior to FAc implantation.
Treatment and Follow-up
The FAc implant was injected by the same physician (AMM) and administered under topical anesthesia and accurate disinfection of conjunctival sac with 5% povidone-iodine. The FAc implant was injected in the inferotemporal quadrant, 3.5–4 mm posterior to the sclerocorneal limbus. Postoperative care included (in all patients) antibiotic (moxifloxacin eye drops) three times daily for 5 days.
All patients underwent a complete ophthalmologic examination at baseline including BCVA, IOP measurement, optical coherence tomography (OCT), and Ganzfeld cone-mediated electroretinograms (according to a published technique) [19,20,21]. At month 1–3 post injection, we acquired data from all the aforementioned testing procedures. In addition, we performed ocular ultrasound.
In 14 patients (14 out of 18 treated eyes), CMT and BCVA were also measured at months 4–8, 9–14 post injection of the FAc implant, in addition to those obtained at baseline and months 1–3. In seven patients (seven treated eyes), the ERGs were recorded at months 4–8 and 9–14 post-FAc implant injection, in addition to the recordings obtained at baseline and month 1–3. In 13 patients, ERG PhNR and b-wave were also recorded in the fellow untreated eyes, taken as control eyes. Ocular ultrasound has been performed only once, in seven eyes from seven patients, during the follow-up.
Outcome Measures
The main outcome was mean change in CMT at month 1–3. Secondary outcomes were changes in CMT and BCVA from baseline to month 4–8 and 9–14 and changes in BCVA as well as components of the cone electroretinogram (cone b-wave and PhNR) from baseline to month 1–3, 4–8, 9–14 and the incidence of adverse events.
For each patient, Ganzfeld cone-mediated electroretinograms (Retimax, CSO, Firenze, Italy) were recorded with a specific, published protocol (employed to isolate and analyze the PhNR from the single flash cone-mediated responses) [19,20,21]. Typical ERG recordings are shown elsewhere [20]. The amplitude of the PhNR and that of the cone b-wave were measured in each recording session. One of the 14 patients was not included in ERG measures analysis owing to his lack of cooperation during testing. For the purposes of the current study, we used normative values collected in age-matched control subjects as reference. The mean normal PhNR amplitude was 8 ± 2 µV; the mean normal b-wave amplitude was 22 ± 3 µV.
All the OCT acquisitions were performed using either an SD-OCT or an SS-OCT: the SD-OCTs were acquired either using a Spectralis OCT (Heidelberg Engineering, Inc.) or a Cirrus HD-OCT (Carl Zeiss, AG.); the SS-OCTs were acquired using a DRI OCT Triton (Topcon, Inc., USA). Each eye was analyzed by the same machine at baseline and during the follow-up period.
BCVAs were determined using ETDRS charts and were expressed as number of letters read.
Tonometry was performed with a Reichert non-contact tonometer (NCT) and confirmed with a Goldmann applanation tonometer (if IOP > 21 mmHg).
B-scan ocular ultrasonography was performed, in seven eyes from seven patients, by using the Optikon Hi Scan (software Optikon 2000, Optikon Rome, Italy) system according to a standardized method. Transpalpebral B-scans of the retina and vitreous body were acquired by orienting the 20-MHz probe at different meridians. B-scans were performed at month 1–3 after intravitreal FAc implant injection in order to define the position and kinetics of the implant.
All the previous mentioned instruments were regularly inspected and maintained by specialized personnel.
Data Registration and Acquisition
Data were extracted from the patients’ medical charts (either electronic or paper ones) and from their IDs in the electroretinographic machine, OCTs, and ultrasound equipment proprietary software.
Statistical Analysis
Data were analyzed by non-parametric Wilcoxon test and Friedman’s non-parametric analysis of variance (ANOVA), comparing the measures obtained at follow-up with those recorded at baseline. Given multiple comparisons, a conservative p value less than 0.05 was considered to represent statistical significance.
Results
The individual data for each patient, including age, visual acuity, CMT, and ERG amplitudes recorded at baseline, are reported in Table 1. All our patients had a long-standing DME (mean 7.72 ± 3.13 years) before FAc implantation.
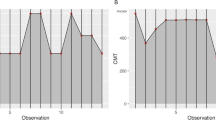
Figure 1a shows a representative change in CMT. DME largely decreased after the FAc implant. Figure 1b shows a scatterplot of individual data for all treated eyes and plots baseline values against those recorded in the same patients at month 1–3. This figure shows that all but two data points lie below the diagonal line indicating an improvement in CMT from baseline. A Wilcoxon test showed a highly significant decrease of CMT from baseline (473 ± 196 µm) to month 1–3 (371 ± 163) (p < 0.0001).
a Fundus image and macular OCT of a patient showing improvement in CMT after FAc intravitreal implant. When the implant was injected, the patient was 74 years old and had diabetes for 25 years and DME for 8 years and was receiving insulin and oral blood glucose-lowering drugs. In the study eye, he had previously undergone one intravitreal ranibizumab and two long-release intravitreal dexamethasone implants. b Scatterplot of CMT recorded at baseline and month 1–3 after FAc intravitreal implantation. The diagonal lines in the plots indicate equivalence values
At month 1–3, ERG b-wave amplitude and PhNR, the ERG component following the b-wave, increased from baseline. The effect was reproducible and was observed in the majority of our patients. Figure 2a shows a scatterplot of individual PhNR data for all treated eyes, comparing values recorded at month 1–3 against baseline values. Except three points, all sat above the diagonal line, indicating an increase of ERG PhNR amplitude from baseline. A Wilcoxon test showed a highly significant increase of PhNR amplitude from baseline (2.76 ± 1.65 µV) to month 1–3 (3.73 ± 2.32) (p = 0.003). The same analysis for the ERG b-wave component is shown in Fig. 2b and shows 12 of the 17 data points above the diagonal line, indicating an improvement post-FAc implant. A Wilcoxon test showed a significant change of b-wave amplitude from baseline (8.83 ± 4.52 µV) to month 1–3 (10.05 ± 5.04 µV) after treatment (p = 0.0384).
Mean BCVA did not show significant changes at month 1–3 versus baseline (p = 0.766, ns) with similar values recorded at baseline (mean 51.47 ± 24.2 ETDRS letters) and month 1–3 (mean 49.44 ± 24.5 ETDRS letters).
ERG PhNR and b-wave were also recorded in a subgroup of the fellow untreated eyes, taken as control eyes. Fellow untreated eyes were later treated with intravitreal anti-VEGF when they developed significant DME, according to good clinical practice and standard of care. The amplitudes of both components did not significantly change 1–3 months post injection compared to baseline (Wilcoxon test, p = 0.6). Figure 3 shows scatterplots of PhNR and b-waves recorded in the untreated fellow eyes of 13 patients at baseline and at month 1–3 follow-up. Note that the data show no clear trend, with both increment and decrement of amplitude post injection.
In follow-ups longer than month 3, in our study population, mean changes in BCVA, PhNR and b-wave amplitudes did not reach statistical significance, while CMT showed a significant trend toward decrease over time (Friedman’s non-parametric ANOVA, p < 0.01, n = 14). Nonetheless PhNR and b-wave amplitudes showed a trend toward improvement (as shown in Fig. 4a, b). Results of these analyses for CMT and BCVA are reported in Fig. 5a, b.
In the seven patients who underwent ocular ultrasound, we were able to identify the implant and it had remained in the inferotemporal quadrant (site of injection). At dynamic evaluation, the implants did not show any movement in relation to the vitreous body kinetics.
Only two (11.1%) eyes developed ocular hypertension over the course of follow-up and, in both cases, IOP was successfully controlled with topical hypotensive medications. Two other eyes were already on therapy with topical hypotensive medications and did not report any increase in IOP after the intravitreal FAc implant.
Discussion
The present retrospective study was designed to evaluate morpho-functional effects of the FAc implant in patients with DME from assessments of CMT and changes in retinal function. We observed that, in association with the expected reduction in CMT due to a regression of DME, cone ERG did show significant changes at month 1–3 and these positive changes tended to endure up to month 9–14, although they did not reach statistical significance after month 3. These changes involved both the b-wave and PhNR component of the ERG. The b-wave and the PhNR are now widely accepted as indicators of middle and inner retinal function, respectively, and can be altered even in early stages of DR [18]. The results obtained in this small pilot study indicate a beneficial effect of the intravitreal FAc implant on middle and inner retinal function of patients with DME that accompanies the reduction in macular edema. The fellow untreated eyes, which served as test controls, did not show the same changes, supporting the hypothesis that the changes we observed were not due to chance or some unknown artifact. Our results suggest that the ERG b-wave and PhNR could be considered new biomarkers in monitoring the response of diabetic retinas to therapies.
There is clinical evidence [17, 22] that the intravitreal FAc implant may decelerate neuroretinal degeneration in DME, as shown by the thickness of inner retinal layers measured over time. Other evidence of the neuroprotective effect of intravitreal FAc implant comes from animal models, where a neuroprotective effect has been shown both anatomically (preserving outer and inner and nuclear layer thickness) and functionally (preserving ERG amplitudes) [15, 16].
Our results are in agreement with this view, suggesting a potential protective role of the drug in addition to its anti-inflammatory activity. In our population we did not observe improvements in mean BCVA over the follow-up period; this finding is not consistent with what we know from literature and could be due both to the small study population and/or to possible selection bias. Other real-world studies, in bigger populations, have reported an increase in mean BCVA of about 5 ETDRS letters that lasted up to 30 months after a single FAc injection [23, 24].
In our study population, CMT significantly decreased from baseline up to month 9–14; this result is consistent with published data that have shown a statistical improvement in CMT up to month 30 after implant [25].
As previously mentioned, in our study population two eyes (11.1%) developed ocular hypertension but had been treated with prior intravitreal steroids. These results are consistent with those reported in literature [23, 24, 26, 27] and show a safety profile consistent with other reports, especially in patients that are not steroid responders, thus a therapeutically controlled ocular hypertension could not be an absolute contraindication to an intravitreal FAc implant.
Another possible ocular complication correlated with steroid use is cataract. We could not evaluate the incidence of this complication because, in the country of study (Italy), eligible patients need to have a pseudophakic lens in order to be treated with a FAc implant. Data from other studies [23, 27] have reported a significant incidence of cataract in phakic patients that underwent FAc implant, but with an overall increase in mean BCVA, upon cataract extraction. These data lead us to believe that a good approach could be to suggest FAc implant to pseudophakic patients or, if phakic, in the same setting of cataract extraction.
Limitations
The major limitation of this study is its retrospective design. A second limitation is the number of patient eyes that were examined. Larger studies are needed to address this issue and to better evaluate the clinical significance. We also reported that some data were lost to follow-up and some visits were missed because of the SARS-CoV-2 outbreak (still ongoing at the time of writing), and this has further reduced the statistical power of the study. Lastly, our report could be influenced by patient selection bias as many patients included in the study had long-standing DME. This could be the reason why, in our population, the improvements in CMT were not reflected in the expected changes in BCVA [26]. Again, further studies should consider this issue when selecting patients.
Conclusion
To our knowledge, this is the first report that provides evidence of an improvement of retinal cone function, in humans, following the administration of the intravitreal FAc implant. Although our results need to be confirmed by prospective controlled studies, they suggest a possible positive effect of the FAc treatment on the neurodegenerative process associated with diabetic retinopathy.
Further studies on a larger patient population and a longer follow-up period are both needed to support this hypothesis and improve our knowledge about this novel drug.
References
Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine (Abingdon). 2014;42:698–702.
Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17.
Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–64.
Musat O, Cernat C, Labib M, et al. Diabetic macular edema. Rom J Ophthalmol. 2015;59(3):133–6.
Romero-Aroca P. Targeting the pathophysiology of diabetic macular edema. Diabetes Care. 2010;33:2484–5.
The Early Treatment Diabetic Retinopathy Study Research Group. Techniques for scatter and local photocoagulation treatment of diabetic retinopathy: Early Treatment Diabetic Retinopathy Study Report no. 3. Int Ophthalmol Clin. 1987;27(4):254–64.
Midena E, Micera A, Frizziero L, Pilotto E, Esposito G, Bini S. Sub-threshold micropulse laser treatment reduces inflammatory biomarkers in aqueous humour of diabetic patients with macular edema. Sci Rep. 2019;9(1):10034. https://doi.org/10.1038/s41598-019-46515-y.
Akhlaghi M, Dehghani A, Pourmohammadi R, Asadpour L, Pourazizi M. Effects of subthreshold diode micropulse laser photocoagulation on treating patients with refractory diabetic macular edema. J Curr Ophthalmol. 2019;31(2):157–60.
Stefanini FR, Badaró E, Falabella P, Koss M, Farah ME, Maia M. Anti-VEGF for the management of diabetic macular edema. J Immunol Res. 2014;2014:632307. https://doi.org/10.1155/2014/632307.
Bonfiglio V, Reibaldi M, Pizzo A, et al. Dexamethasone for unresponsive diabetic macular oedema: optical coherence tomography biomarkers. Acta Ophthalmol. 2019;97(4):e540–4.
Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125–32.
Campochiaro PA, Nguyen QD, Hafiz G, et al. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology. 2013;120(3):583–7.
Sivaprasad S, Oyetunde S. Impact of injection therapy on retinal patients with diabetic macular edema or retinal vein occlusion. Clin Ophthalmol. 2016;10:939–46.
Quhill F, Beiderbeck A. Cost advantage of fluocinolone acetonide implant (ILUVIEN®) versus ranibizumab in the treatment of chronic diabetic macular oedema. Glob Reg Health Technol Assess. 2017. https://doi.org/10.5301/grhta.5000268.
Glybina IV, Kennedy A, Ashton P, Abrams GW, Iezzi R. Photoreceptor neuroprotection in RCS rats via low-dose intravitreal sustained-delivery of fluocinolone acetonide. Invest Ophthalmol Vis Sci. 2009;50(10):4847–57. https://doi.org/10.1167/iovs.08-2831.
Glybina IV, Kennedy A, Ashton P, Abrams GW, Iezzi R. Intravitreous delivery of the corticosteroid fluocinolone acetonide attenuates retinal degeneration in S334ter-4 rats. Invest Ophthalmol Vis Sci. 2010;51(8):4243–52.
Lynch SK, Lee K, Chen Z, et al. Intravitreal fluocinolone acetonide may decelerate diabetic retinal neurodegeneration. Invest Ophthalmol Vis Sci. 2019;60(6):2134–9. https://doi.org/10.1167/iovs.18-24643.
Chen H, Zhang M, Huang S, Wu D. The photopic negative response of flash ERG in nonproliferative diabetic retinopathy. Doc Ophthalmol. 2008;117(2):129–35.
Abed E, Piccardi M, Rizzo D, et al. Functional loss of the inner retina in childhood optic gliomas detected by photopic negative response. Invest Ophthalmol Vis Sci. 2015;56(4):2469–74. https://doi.org/10.1167/iovs.14-16235.
Abed E, Placidi G, Campagna F, et al. Early impairment of the full-field photopic negative response in patients with Stargardt disease and pathogenic variants of the ABCA4 gene. Clin Exp Ophthalmol. 2018;46(5):519–30.
Falsini B, Chiaretti A, Rizzo D, et al. Nerve growth factor improves visual loss in childhood optic gliomas: a randomized, double-blind, phase II clinical trial. Brain. 2016;139(Pt 2):404–14.
Pessoa B, Coelho J, Marta A, et al. Evaluation of retinal ganglionar cell using optical coherence tomography in patients with DME treated with the 0.19 mg fluocinolone acetonide implant. Invest Ophthalmol Vis Sci. 2019;60(9):2613.
Bailey C, Chakravarthy U, Lotery A, Menon G, Talks J. Real-world experience with 0.2 μg/day fluocinolone acetonide intravitreal implant (ILUVIEN) in the United Kingdom. Eye (Lond). 2017;31(12):1707–15.
Augustin AJ, Bopp S, Fechner M, et al. Three-year results from the Retro-IDEAL study: real-world data from diabetic macular edema (DME) patients treated with ILUVIEN® (0.19 mg fluocinolone acetonide implant). Eur J Ophthalmol. 2020;30(2):382–91.
Fusi-Rubiano W, Mukherjee C, Lane M, et al. Treating diabetic macular oedema (DMO): real world UK clinical outcomes for the 0.19 mg fluocinolone acetonide intravitreal implant (Iluvien™) at 2 years. BMC Ophthalmol. 2018;18(1):62.
Chakravarthy U, Taylor SR, Koch FHJ, Castro de Sousa JP, Bailey C. Changes in intraocular pressure after intravitreal fluocinolone acetonide (ILUVIEN): real-world experience in three European countries. Br J Ophthalmol. 2019;103(8):1072–7.
Mansour SE, Kiernan DF, Roth DB, et al. Two-year interim safety results of the 0.2 µg/day fluocinolone acetonide intravitreal implant for the treatment of diabetic macular oedema: the observational PALADIN study. Br J Ophthalmol. 2020;105:414–9.
Acknowledgements
We thank the participants of the study.
Funding
The journal’s Rapid Service Fee was funded by Alimera Sciences. Open access funding was provided by the Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement. No funding was received for the study itself.
Medical Writing and Editorial Assistance
Medical writing services has been provided by Alimera Sciences. Alimera Sciences did not participate in the design of the study, the collection and analysis of data or interpretation of the findings. Editorial assistance in the preparation of this article was provided by Dr Chris Wright of Alimera Sciences. Support for this assistance was funded by Alimera Sciences.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Authorship Contributions
Conceptualization, Angelo Maria Minnella, Stefano Maria Picardi, Martina Maceroni, Benedetto Falsini; methodology, Angelo Maria Minnella, Stefano Maria Picardi, Benedetto Falsini; formal analysis, Stefano Maria Picardi, Benedetto Falsini; investigation, Angelo Maria Minnella, Stefano Maria Picardi, Martina Maceroni, Francesca Albanesi, Elisa De Siena, Giorgio Placidi, Carmela Grazia Caputo, Umberto De Vico and Benedetto Falsini; data curation, Stefano Maria Picardi, Martina Maceroni, Francesca Albanesi, Elisa De Siena, Giorgio Placidi; writing—original draft preparation, Angelo Maria Minnella, Stefano Maria Picardi, Martina Maceroni, Benedetto Falsini; writing—review and editing Angelo Maria Minnella, Stefano Maria Picardi, Martina Maceroni, Benedetto Falsini; supervision, Angelo Maria Minnella, Benedetto Falsini, Stanislao Rizzo; funding acquisition, Angelo Maria Minnella.
Disclosures
Angelo Maria Minnella, Stefano Maria Picardi, Martina Maceroni, Francesca Albanesi, Elisa De Siena, Giorgio Placidi, Carmela Grazia Caputo, Umberto De Vico, Stanislao Rizzo and Benedetto Falsini have nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committee of Fondazione Policlinico Universitario A. Gemelli (11 November 2020, ID 3166) and all patients signed an informed consent.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Minnella, A.M., Picardi, S.M., Maceroni, M. et al. Retinal Morpho-Functional Changes Following 0.19 mg Fluocinolone Acetonide Intravitreal Implant for Chronic Diabetic Macular Edema. Adv Ther 38, 3143–3153 (2021). https://doi.org/10.1007/s12325-021-01751-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01751-5